Abstract
We present three patients with aggressive non-Hodgkin’s B-cell lymphoma (NHL) who received anti-CD19 chimeric antigen receptor T (CAR T) cells therapy after failure of several lines of chemotherapy that developed pseudo-progression. One-week clinical and radiological findings were consistent with tumor progression. Positron emission tomography-computed tomography (PET-CT) at 1 month post CAR T cells administration was consistent with treatment response. The rapid tumor growth and subsequent resolution are suggestive of tumor pseudo-progression mediated secondary to infiltration and immune activation of CAR T cells. Overall, 56 adult patients with NHL were enrolled in a phase 1b/2 in house clinical study with CD19 CAR T cells. Out of them 22/56 patients progressed as per PET-CT the 1 month post CAR T cells. In 14 patients, signs of progression started 7–10 days after CAR T cells infusion. In 11/14 patients, it was true progression, while in 3 it was pseudo-progression. Additional studies are warranted to describe the extent of this phenomenon and evaluate correlation with the CAR T activity and long-term disease control.
Similar content being viewed by others
Introduction
Immunotherapies have considerably changed treatment strategies in advanced cancers and lymphomas [1, 2]. The evaluation and understanding of antitumor responses in the era of immuno-oncology are becoming increasingly important with the rapid expansion of indications and approvals of checkpoint inhibitors and chimeric antigen receptor T cells (CAR T cells). These immunotherapies might enhance the accumulation of inflammatory cells, which may translate into the enlargement of a target lesion [3,4,5,6,7,8]. Enlargement of a primary or new region in the positron emission tomography-computed tomography (PET-CT) scans after chemotherapy is interpreted as a progressive disease, where a treatment change is usually recommended [9].
However, with the advent of immunotherapy, tumor growth is no longer straightforward and requires an individual approach [4]. Pseudo-progression following immunotherapy has been described in some oncological diseases, including high-grade glioblastomas, non-small-cell lung carcinoma, and melanoma [10,11,12,13,14,15,16,17], but less commonly in hematological malignancies as chronic lymphocytic leukemia (CLL), lymphomas, and Waldenstrom macroglobulinemia [13, 14, 18,19,20]. Table 1 summarizes previous publications of pseudo-progression for solid tumors and lymphomas. As a result, modified response criteria were proposed for use when assessing the response to ipilimumab: the immune-related response criteria [6], Lugano criteria were adapted to immune-based therapy in lymphoma patients [4].
Therapy with CAR T cells targeting CD19 has been approved for NHL, following several institutional and international clinical trials [21,22,23,24]. The optimal timing for imaging post-CAR T has yet to be determined and differs across trials from 1 to 3 months after CAR T infusion [25,26,27]. Until recently, it was not clear if CAR T cells could cause pseudo-progression. We report here three patients having early imaging findings indicating progression of disease, later determined as pseudo-progression.
Unusual response patterns during immunotherapy make it difficult to differentiate responders from nonresponders early on in treatment. Interleukin-8 (IL-8) is a member of the CXC chemokine family originally identified as a chemotactic factor for neutrophils [28]. IL-8 is secreted by malignant cells and tumor stroma cells across many different tumor types including solid tumors (brain, breast, cervical, colon, gastric, lung, melanoma, mesothelioma, ovarian, prostate, renal, and thyroid) and hematological malignancies (acute myeloid leukemia, CLL, and Hodgkin’s lymphoma) [29]. Serum IL-8 levels reflect tumor burden and allow to monitor response to BRAF inhibitors (vemurafenib), an anti-cytotoxic T lymphocyte antigen-4 monoclonal antibodies (ipilimumab), anti-programmed cell death protein 1 in metastatic melanoma and non-small lung carcinoma patients [30, 31]. Interestingly, despite imaging-assessed increases in the target lesion size during pseudo-progression, serum IL-8 levels decreased and remained lower than baseline at subsequent imaging evaluations. Moreover, serum IL-8 levels steadily increased when patients eventually developed true progression [31]. We advocate that lymphoma patients who develop immune imitation of progression (pseudo-tumor progression) after CAR T cells therapy are behaving similarly to the previously reported cancer patients following immunotherapy. Probably IL-8 level decreases during pseudo-progression unlike true progression. In order to verify this hypothesis, we retrospectively assessed serum IL-8 levels in the three patients who developed immune imitation of progression during CAR T cells therapy compared to the IL-8 levels in the patients with true progression for whom we could allocate blood samples.
Methods
Patients included in the analysis were part of a phase 1b/2 trial (NCT02772198) conducted at the Sheba Medical Center, Tel-Hashomer Israel. The study was approved by the Institutional Review Board and the Israeli Ministry of Health in accordance with the Declaration of Helsinki. The full protocol is described elsewhere [32]. Briefly, patients underwent a single leukapheresis procedure; peripheral blood mononuclear cells were isolated, activated, and transduced with a gamma retrovirus encoding for a CD19 CAR (based on an FMC63-derived ScFv, a CD28 co-stimulatory domain, and CD3-zeta signaling domain). Lymphodepletion included fludarabine 25 mg/m2 × 3 days (days −4 to −2) and cyclophosphamide 900 mg/m2 × 1 day (day −2), followed by infusion (day 0) of 1 × 106 CAR+ transduced cells per kilogram recipient.
Neurologic adverse events and cytokine release syndrome (CRS) were graded initially according to the CARTOX-10 guidelines [33] and then according to the American Society for Blood and Cellular Therapy (ASTCT) [34] consensus grading once they were published. This applies only for the first patient with pseudo-tumor progression who received CAR T cells before the ASTCT guidelines were published. The conversion was possible as he had grade IV central nervous system (CNS) toxicity.
Serum IL-8 measurement from frozen samples was performed in total of 12 patients using the Luminex MAGPIX system (Luminex Corp, Texas, USA) and analyzed with Milliplex analysis software (Millipore, MA, USA).
Results
Between January 2017 and December 2019, 56 adult patients with aggressive non-Hodgkin’s B-cell lymphoma were treated in our phase 1b/2 in house clinical study with CD19 CAR T cells (NCT02772198). Twenty-two patients had disease progression 1 month after CAR T cells infusion according by PET-CT. In total, 14 patients (3 with pseudo-progression and 11 patients with true progression) developed signs of progression rather early within 7–10 days post CAR T cells infusion (Figs. 1–3). All these 14 patients developed rapid clinical deterioration during hospitalization. Out of them, in three it turns out it was not true progression but immune imitation of progression (pseudo-tumor progression). In the three patients we checked retrospectively from frozen samples IL-8 serum levels at day of CAR T cells infusion (day 0) and subsequent weekly until 2 months (Fig. 4). In 1/3 only three samples were available. Compared with three naive controls two patients had similar low IL-8 serum levels after CAR T-cell therapy that corresponds to response to therapy. While the third patient had initially higher IL-8 level that decline (this patient had only three time points’ samples).
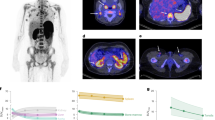
A, B PET-CT before and after CAR T cells. FDG-PET/CT: maximum intensity projection (MIP) (a), representative PET (b), and CT (c) axial slices with 21 days/2 months/2 months interval (A: row—before and B: row—after treatment). C–F X-ray imaging during CAR T cells. C Before CAR T cell. D, E Pseudo-progression. D Day 6 after CAR T cells. E Day 8 after CAR T cells. F Day 22 after CAR T cells—resolution of previous findings.
A, B PET-CT before and after CAR T cells.FDG-PET/CT: maximum intensity projection (MIP) (a), representative PET (b), and CT (c) axial slices with 21 days/2 months/2 months interval (A: row—before and B: row—after treatment). C–F X-ray imaging during CAR T cells. C Before CAR T cell. D, E Pseudo-progression. D Day 7 after CAR T cells. E Day 9 after CAR T cells. F Day 16 after CAR T cells—improvement of previous findings.
A, B PET-CT before and after CAR T cells. FDG-PET/CT: maximum intensity projection (MIP) (a), representative PET (b), and CT (c) axial slices with 21 days/2 months/2 months interval (A: row—before and B: row—after treatment). C–F X-ray imaging during CAR T cells. C Before CAR T cell. D, E Pseudo-progression. D Day 2 after CAR T cells. E Day 8 after CAR T cells. F Day 55 after CAR T cells—improvement of previous findings.
We could allocate frozen serum samples from 5/11 patients who showed high IL-8 level through the weekly follow-up until day 30 post CAR T cells infusion (Fig. 4B). These patients had early disease progression as per PET-CT that was performed 28–30 days following the CAR T cells infusion. In addition, we could allocate samples for four additional patients. These four patients showed early response in their days 28–30 PET-CT but then progressed (>day 60). In these four patients the early (during the 1st month after CAR T cells infusion) IL-8 levels were relatively low (Fig. 4C), correlating with our hypothesis of low IL-8 level in responding patients. Thereby we concluded that the IL-8 level could be a marker of true immediate progression and not the marker of future progression. It helps to separate the rapid immune response from the early true progression and in some cases, it can prevent unnecessary and even dangerous chemotherapy intervention. Further studies will show if it could be a marker of coming progression for patients with late relapse and if the IL-8 level will increase before clinical deterioration and PET-CT changes.
Case presentation
Case report 1
A 23-year-old man presented with weight loss, malaise, fever, itching, and lymphadenopathy in February 2017. A comprehensive diagnostic workup including PET-CT and lymph node biopsy revealed germinal center B-cell-type diffuse large B-cell lymphoma (DLBCL), stage 4B with extensive nodal and extranodal involvement (liver, stomach, lung, and pancreas). The disease was resistant both to three cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), which were followed by one cycle of dexamethasone, cytarabine, and cisplatin (Fig. 1A–C). In June 2017, the patient received 1.5 × 106/kg autologous transduced CAR T cells following lymphodepletion. Six days after CAR T cells infusion he developed severe dyspnea. Chest X-rays on days 6 and 8 showed rapid extension of preexisting masses in the right upper lobe of lung and mediastinum with the evolution of left-sided pleural effusion (Fig. 1D, E). Consistent findings were seen in day 9 by CT, with rapid enlargement of the mediastinal mass, a mediastinal shift, enlargement of lung and liver masses, new pleural effusions, and expansion of bone lytic lesions (Fig. 1D, E). Concomitantly, the patient developed grade 3 CRS with fever and hypotension and grade 4 CNS toxicity characterized by convulsions and stupor, which lasted 7 days. He was treated with broad-spectrum antibiotics, low-dose norepinephrine, three doses of tocilizumab, and dexamethasone 10 mg ×4/day [33].
We deferred a tissue biopsy since the patient was unstable, assuming that the radiographic progression is consistent with a phenomenon of immune activation and tumor infiltration by effector immune cells rather than lymphoma cells [7]. The patient’s condition gradually improved; 3 weeks after CAR T cells infusion, he was afebrile and free of hemodynamic and respiratory support. Furthermore, the pleural effusion had resolved and the mediastinal mass regressed to their pretreatment size (Fig. 1B, F) without any additional lymphoma-directed therapy. PET-CT at 28 days post CAR T showed significant improvement with partial resolution of previous FDG-avid lesions: mediastinal mass decreased from 8.9 to 5.8 cm, left upper lung nodule from 3.2 until 2.5 cm, with only peripheral FDG uptake in these findings, consistent with a partial metabolic response (PMR). Per institutional protocol, the patient underwent an allogeneic stem cell transplantation 2 months following CAR T infusion. He received a peripheral blood stem cell graft from a matched unrelated donor following conditioning with fludarabine and treosulfan. The transplant course was uneventful and PET-CT 40 days after stem cell infusion was consistent with a complete remission (CR). Unfortunately, 7 months later his lymphoma progressed again, the patient received multiple chemotherapy and immunotherapy salvage treatments including second haploidentical transplantation. Eight months after the second transplantation the patient died from the disease progression.
Case 2
A previously healthy 27-year-old male presented with dyspnea, chest pain, and cough. PET-CT showed a localized mediastinal mass and the biopsy was consistent with primary mediastinal B-cell lymphoma. The patient received six cycles of R-CHOP and attained a CR. Six months following completion of therapy relapse in the mediastinum was documented. The disease was refractory to chemotherapy-based salvage therapies, including ifosfamide, carboplatin, etoposide and hyper-cyclophosphamide, vincristine, adriamycin, dexamethasone. He had a short-lived response to pembrolizumab and was referred to CAR T therapy at our institution. Due to rapid progression, which presented by pericardial tamponade, urgent pericardiocentesis was performed, draining 800 ml of effusion, containing numerous lymphoma cells. Patient received two cycles of rituximab, bendamustine, and ibrutinib resulted in clinical improvement and PMR (Fig. 2A, C). Following lymphodepletion, the patient received an infusion of 1.0 × 106/kg autologous CAR T cells. Six days after infusion, he developed hypoxic respiratory failure and atrial fibrillation. Chest X-rays on day 7 showed a rapid extension of preexisting mediastinum mass and worsening of right-sided pleural effusion (Fig. 2D). A total of 3000 ml of pleural fluid was drained in 2 consecutive days (Fig. 2E). The fluid was brown colored with exudative features. Gram stain and cultures were unremarkable. Numerous T cells, mostly cytotoxic type (CD19 and CD20 negative, CD3—95%, CD4—17%, CD8—83%), were identified by flow cytometry test in the pleural effusion. There was no evidence of lymphoma cells by cytology and flow cytometry examinations. Concomitantly, the patient developed grade 3 CRS with fever and hypotension, and grade 3 CNS toxicity characterized by convulsions and stupor. He was treated with broad-spectrum antibiotics, low-dose norepinephrine, two doses of tocilizumab (8 mg/kg per dose), dexamethasone 10 mg ×4/day for 1 day. Due to a persistent confusional state, he was switched to high-dose methylprednisolone 500 mg IV every 12 h for 3 days, followed by a quick taper. His mental status and dyspnea gradually improved. CT scan of the chest carried out on day 19 after CAR T cells infusion showed marked improvement of pleural effusion and mediastinal mass (from 12 to 9 cm on diameter). PET-CT scan done 28 days after infusion of CAR T cells, the residual mass was not FDG avid and the amount of pleural effusion decreased significantly (Fig. 2B). Given the fact that lymphoma cells were absent in the pleural effusion coupled with the follow-up imaging studies, the patient was considered to have pseudo-progression and was followed closely. Unfortunately, PET-CT scan carried out 2 months after the infusion of CAR T cells showed new FDG uptake (SUV 4.8) in a 1.4 cm residual mediastinal mass. The patient was referred directly to an allogeneic stem cell from an HLA-matched sister, which was performed 80 days after the infusion of CAR T cells. One month after the transplantation PET-CT scan was compatible with complete metabolic response (CMR).
Case 3
A 37-year-old female with refractory relapsed primary mediastinal large B-cell lymphoma, treated with multiple immunochemotherapeutic agents, including R-CHOP, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin, high-dose cytosine arabinoside and mitoxantrone, and autologous stem cell transplantation. PET-CT before CAR T-cell therapy showed a large mediastinal mass with high FDG uptake (12.3 SUV). An additional nodule was noted in the right lung with low FDG uptake 1.9 SUV (Fig. 3A, B). The patient received 1.0 × 106/kg autologous transduced CAR T cells after standard lymphodepletion. Two days after CAR T cells infusion, she developed dyspnea and chest pain. An anterior chest wall mass extending from the mediastinum was noted (Fig. 3C, D). The findings significantly worsened until day 16 after the infusion of CAR T cells. An X-ray on day 8 and chest CT on day 10 showed enlargement of the mediastinal tumor from 7 × 4 cm to 11.4 × 5 cm, with involvement of the pericardium, brachiocephalic, and superior vena cava veins. The tumor mass penetrated through the chest wall to subcutaneous layer. Multiple new lung nodules and ground glass appearance were also noted (Fig. 3D, E).
Concomitantly, the patient developed grade 1 CRS grade with fever and weakness. She was treated with broad-spectrum antibiotics and antipyretics. Her clinical condition gradually improved and she was discharged from the hospital. PET-CT scan, carried out 30 days after CAR T cells infusion showed a CMR. The mediastinal mass regressed from 14.7 × 9 cm to 9 × 3 cm, the pericardial effusion resolved and there was no evidence for tumor extension to the chest wall (Fig. 3B, F). A repeated PET-CT scan done 3 months after the infusion of CAR T cells was compatible with CMR.
Discussion
The advent of targeted immunotherapies into oncology has brought new hope to patients with chemo-resistant diseases. Toxicities of checkpoint blockade, including tumor flares, are well characterized [35]. However, while the features of CRS and neurotoxicity related to CAR T-cell therapy are well known, the characterization of tumor pseudo-progression associated with CAR T-cell therapy is lacking. Fifty-six adult patients with NHL received locally produced CAR T cells. Twenty-two patients had disease progression 1 month after infusion as per PET-CT. In total, 14 patients developed the signs of progression 7–10 days after CAR T cells infusion. We report here a case series of 3 patients out of these 14 adult patients with DLBCL and mediastinal involvement, developing rapid tumor enlargement and regression following infusion of CAR T cells, concomitant with an immune activation phenotype. Overall, this sequence of events seems to define a syndrome of “tumor pseudo-progression,” resembling tumor flares seen with checkpoint blockade.
Tumor pseudo-progression is a term that comes from the treatment of solid tumors and lymphomas with other immunological agents, such as checkpoint inhibitors [1]. CD19 CAR T-cell therapy has shown impressive efficacy in patients with relapsed/refractory NHL. However, it has been associated with severe immune-related adverse effects, such as CRS and neurotoxicity [33]. Tumor pseudo-progression post-CAR T-cell therapy is also likely to be an immunologic complication. Though histologic confirmation is not available, we assume that similar to checkpoint blockade, CAR T cells infiltrate the tumor and evoke systemic and local inflammation, resulting in tumor inflation and regression, once malignant cells volume is reduced [7].
In a literature review, we found only one previous description of tumor pseudo-progression post-CAR T-cell therapy for NHL. Wang et al. [36] reported three NHL patients with tumor flare and local immune activation on FDG-PET-CT scanning 1 week post CAR T-cell therapy. Clinical features of patients and their PET/CT results from our and Wang et al. case series are summarized in Table 2. Co-stimulatory domains differed (CD28 vs. 4–1BBB, respectively), suggesting that pseudo-progression is a class effect rather than dependent on a specific construct. From the available information CRS seems to be a common feature. Of the four patients with CRS two had grade 3 and were treated with tocilizumab and the other two had grade 1.
An alternative mechanism for the tumor enlargement and subsequent regression observed in our case series would be frank progression immediately after infusion of CAR T cells with a later response to therapy or simply response to steroids. Delayed responses to CAR T-cell therapy have been reported up to 17 months post infusion [26]. However, the typical kinetics of response in such patients would be an initial achievement of a partial response with evolution to a complete response later in the course. The rapidity of tumor inflation and resolution would suggest a “tumor flare” rather than progression. Furthermore, the demonstration of T cells, without malignant cells, in the pleural effusion of patient number 2 coupled with radiologic enlargement of tumor, would also support an immune-based phenomenon. Tumor response to steroids is also not likely, since patients have been exposed to steroids before, without any significant response. In addition, the responses reported here were observed nearly 1 month post infusion, exceeding the typical time frame for steroid activity. In the setting of checkpoint blockade, pseudo-tumor progression usually occurs after more than 3 weeks from treatment [37], while in the six cases reported to date (Table 2), it was earlier (range 2–6 days). Differences are likely related to the distinct mechanisms of action. While radiologic follow-up with chest x-ray and CT is readily available, other unknown reliable biomarkers distinguishing between pseudo-progression and actual progression may be beneficial for early interventions. Furthermore, revision of early response criteria may be required to account for tumor flares.
Our findings are similar in part to those described in patients with solid tumors [29,30,31], somewhat strengthening our assumption that in the three described patients the early tumor progression we observed is an immune imitation of progression, and not a true progression as we observed low IL-8 levels in 2/3 patients with pseudo-tumor progression and in the third patient the initially relatively high IL-8 level decreased on day 14 in parallel with the beginning of the clinical response. In contrast, in patients with true early but not late tumor progression with available samples we observed high IL-8 levels.
In addition to the 3 patients with pseudo-progression (or immune mediated semi tumor progression), we had serum frozen samples of 5 from the 11 patients who developed early progression after CAR T cells infusion; all demonstrating increased levels of IL-8 through the weekly follow-up until day 30 post CAR T cells infusion (Fig. 4B). In contrast, relatively low IL-8 levels post CAR T cells infusion were demonstrated in patients with late progression (Fig. 4C). These data support the hypothesis that serum IL-8 levels may serve as surrogate marker of lymphoma response to CAR T cells, being low in responding patients and high in true progressing patients, respectively. Furthermore, it may help in separating true progression from the rapid immune response, which is clinical importance as it may help preventing unnecessary intervention.
Unfortunately, IL-8 measurements were performed retrospectively on stored frozen samples serum samples and we do not have samples pre lymphodepletion. It is advisable that future prospective randomized studies will monitor IL-8 levels to help to differentiate true vs. pseudo-progression of lymphoma in NHL patients treated with CAR T cells.
In conclusion, we described a new syndrome of tumor pseudo-progression after CAR T-cell treatment. From our data, it seems that this syndrome is expected in patients with high disease burden, mediastinal mass, and concomitant CRS. The syndrome usually manifests itself several days after the infusion of CAR T cells, and in some cases, the patient’s condition may rapidly deteriorate. Clinically, the patients suffer from dyspnea, chest pain, and hypoxia. These hazardous symptoms may be misdiagnosed as true progression and lead to emergency treatment with steroids or chemotherapy, potentially impeding CAR T-cell activity. While tumor pseudo-progression is not common, it should certainly be considered upon rapid decompensation of patients following infusion of CAR T cells. Given the lack of reliable markers for establishing the diagnosis of tumor pseudo-progression and the risks of obtaining tissue biopsies at the time of symptoms, clinical reasoning remains critical in managing these patients. With the expansion of CAR T-cell therapies, we expect an increase in the number of pseudo-progression observed. Therefore, clear criteria for diagnosis and guidelines for monitoring and treatment are warranted.
References
Kwak JJ, Tirumani SH, Van den Abbeele AD, Koo PJ, Jacene HA. Cancer immunotherapy: imaging assessment of novel treatment response patterns and immune-related adverse events. Radiographics. 2015;35:424–37.
Di Giacomo AM, Danielli R, Guidoboni M, Calabro L, Carlucci D, Miracco C, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58:1297–306.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–52.
Cheson BD, Ansell S, Schwartz L, Gordon LI, Advani R, Jacene HA, et al. Refinement of the Lugano classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128:2489–96.
Hoos A, Wolchok JD, Humphrey RW, Hodi FS. CCR 20th anniversary commentary: immune-related response criteria–capturing clinical activity in immuno-oncology. Clin Cancer Res. 2015;21:4989–91.
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20.
Wang Q, Gao J, Wu X. Pseudoprogression and hyperprogression after checkpoint blockade. Int Immunopharmacol. 2018;58:125–35.
Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34:1510–7.
Nishino M, Jackman DM, Hatabu H, Yeap BY, Cioffredi LA, Yap JT, et al. New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. Am J Roentgenol. 2010;195:W221–8.
Beer L, Hochmair M, Prosch H. Pitfalls in the radiological response assessment of immunotherapy. Memo. 2018;11:138–43.
O’Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–7.
Danielli R, Ridolfi R, Chiarion-Sileni V, Queirolo P, Testori A, Plummer R, et al. Ipilimumab in pretreated patients with metastatic uveal melanoma: safety and clinical efficacy. Cancer Immunol Immunother. 2012;61:41–8.
Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24:5343–9.
Ferrajoli A, Lee BN, Schlette EJ, O’Brien SM, Gao H, Wen S, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–7.
Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–30.
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33.
Nishino M, Ramaiya NH, Chambers ES, Adeni AE, Hatabu H, Janne PA, et al. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer. 2016;4:84.
Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for relapsed/refractory classic hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36:1428–39.
Han HS, Escalon MP, Hsiao B, Serafini A, Lossos IS. High incidence of false-positive PET scans in patients with aggressive non-Hodgkin’s lymphoma treated with rituximab-containing regimens. Ann Oncol. 2009;20:309–18.
Treon SP, Branagan AR, Hunter Z, Santos D, Tournhilac O, Anderson KC. Paradoxical increases in serum IgM and viscosity levels following rituximab in Waldenstrom’s macroglobulinemia. Ann Oncol. 2004;15:1481–3.
Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–9.
Kochenderfer JN, Somerville RPT, Lu T, Yang JC, Sherry RM, Feldman SA, et al. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther. 2017;25:2245–53.
Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC, Hosing CM, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25:285–95.
Zhu Y, Tan Y, Ou R, Zhong Q, Zheng L, Du Y, et al. Anti-CD19 chimeric antigen receptor-modified T cells for B-cell malignancies: a systematic review of efficacy and safety in clinical trials. Eur J Haematol. 2016;96:389–96.
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56.
Jain T, Bar M, Kansagra AJ, Chong EA, Hashmi SK, Neelapu SS, et al. Use of chimeric antigen receptor T cell therapy in clinical practice for relapsed/refractory aggressive B cell non-Hodgkin lymphoma: an expert panel opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2019;25:2305–21.
Walz A, Peveri P, Aschauer H, Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987;149:755–61.
Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–91.
Sanmamed MF, Carranza-Rua O, Alfaro C, Onate C, Martin-Algarra S, Perez G, et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin Cancer Res. 2014;20:5697–707.
Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol. 2017;28:1988–95.
Jacoby E, Bielorai B, Avigdor A, Itzhaki O, Hutt D, Nussboim V, et al. Locally produced CD19 CAR T cells leading to clinical remissions in medullary and extramedullary relapsed acute lymphoblastic leukemia. Am J Hematol. 2018;93:1485–92.
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–38.
Marin-Acevedo JA, Chirila RM, Dronca RS. Immune checkpoint inhibitor toxicities. Mayo Clin Proc. 2019;94:1321–9.
Wang J, Hu Y, Yang S, Wei G, Zhao X, Wu W, et al. Role of fluorodeoxyglucose positron emission tomography/computed tomography in predicting the adverse effects of chimeric antigen receptor T cell therapy in patients with non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2019;25:1092–8.
Kong BY, Menzies AM, Saunders CA, Liniker E, Ramanujam S, Guminski A, et al. Residual FDG-PET metabolic activity in metastatic melanoma patients with prolonged response to anti-PD-1 therapy. Pigment Cell Melanoma Res. 2016;29:572–7.
Acknowledgements
We acknowledge the kind support of all physicians and laboratory team of the Division of Hematology and Bone Marrow Transplantation, Sheba Medical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Danylesko, I., Shouval, R., Shem-Tov, N. et al. Immune imitation of tumor progression after anti-CD19 chimeric antigen receptor T cells treatment in aggressive B-cell lymphoma. Bone Marrow Transplant 56, 1134–1143 (2021). https://doi.org/10.1038/s41409-020-01156-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01156-y
This article is cited by
-
Intrathecal bivalent CAR T cells targeting EGFR and IL13Rα2 in recurrent glioblastoma: phase 1 trial interim results
Nature Medicine (2024)
-
Real-life experiences with CAR T-cell therapy with idecabtagene vicleucel (ide-cel) for triple-class exposed relapsed/refractory multiple myeloma patients
BMC Cancer (2023)
-
Th17.1 cell driven sarcoidosis-like inflammation after anti-BCMA CAR T cells in multiple myeloma
Leukemia (2023)
-
Lymphoma pseudoprogression observed on [18F]FDG PET-CT scan 15 days after CAR-T infusion
European Journal of Nuclear Medicine and Molecular Imaging (2022)
-
Diagnostic Accuracy of 2-[18F]FDG-PET and whole-body DW-MRI for the detection of bone marrow metastases in children and young adults
European Radiology (2022)