Abstract
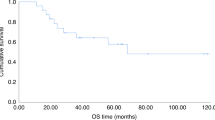
To improve disease control without increasing the toxicity of a reduced-intensity allogeneic hematopoietic cell transplantation (HCT) in multiple myeloma (MM), a phase I trial was performed using an antibody-radionuclide conjugate targeting CD45 (90Y-DOTA-BC8) as conditioning. 90Y-DOTA-BC8 was combined with fludarabine and low-dose TBI followed by allogeneic HCT in patients with MM and ≥1 adverse risk characteristic at diagnosis, relapse after autologous transplant, or plasma cell leukemia (PCL). The primary objective was to estimate the maximum tolerated radiation absorbed dose. Fourteen patients were treated (one with PCL, nine failed prior autologous HCT, and nine with ≥1 adverse cytogenetics). Absorbed doses up to 32 Gy to liver were delivered. No dose-limiting toxicities occurred. Non-hematologic toxicities were manageable and included primarily gastrointestinal (43%) and metabolic/electrolyte disturbances (36%). Treatment-related mortality at 100 days was 0%. At a median follow-up of 5 years, the overall survival was 71% (median not reached) and the progression-free survival was 41% (median 40.9 months). The incorporation of CD45-targeted radioimmunotherapy (RIT) into a reduced-intensity allogeneic HCT is well-tolerated and may induce long-term remissions among patients with poor-risk MM, supporting further development of RIT-augmented conditioning regimens for HCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33:2863–9. https://doi.org/10.1200/jco.2015.61.2267.
Roos-Weil D, Moreau P, Avet-Loiseau H, Golmard J-L, Kuentz M, Vigouroux S, et al. Impact of genetic abnormalities after allogeneic stem cell transplantation in multiple myeloma: a report of the Société Française de Greffe de Moelle et de Thérapie Cellulaire. Haematologica. 2011;96:1504–11. https://doi.org/10.3324/haematol.2011.042713.
Dhakal B, Vesole DH, Hari PN. Allogeneic stem cell transplantation for multiple myeloma: is there a future? Bone Marrow Transpl. 2016;51:492–500. https://doi.org/10.1038/bmt.2015.325.
Crawley C, Iacobelli S, Bjorkstrand B, Apperley JF, Niederwieser D, Gahrton G. Reduced-intensity conditioning for myeloma: lower nonrelapse mortality but higher relapse rates compared with myeloablative conditioning. Blood. 2007;109:3588–94. https://doi.org/10.1182/blood-2006-07-036848.
Pagel JM, Gooley TA, Rajendran J, Fisher DR, Wilson WA, Sandmaier BM, et al. Allogeneic hematopoietic cell transplantation after conditioning with 131 I–anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood. 2009;114:5444–53.
Cassaday RD, Press OW, Pagel JM, Rajendran JG, Gooley TA, Fisher DR et al. Phase I Study of a CD45-Targeted Antibody-Radionuclide Conjugate for High-Risk Lymphoma. Clin Cancer Res. 2019. https://doi.org/10.1158/1078-0432.Ccr-19-1567.
Cheson BD. Radioimmunotherapy of non-Hodgkin lymphomas. Blood. 2003;101:391–8. https://doi.org/10.1182/blood-2002-06-1793.
Gonsalves WI, Timm MM, Rajkumar SV, Morice WG, Dispenzieri A, Buadi FK, et al. The prognostic significance of CD45 expression by clonal bone marrow plasma cells in patients with newly diagnosed multiple myeloma. Leuk Res. 2016;44:32–9. https://doi.org/10.1016/j.leukres.2016.03.003.
Rajendran JG, Fisher DR, Gopal AK, Durack LD, Press OW, Eary JF. High-dose (131)I-tositumomab (anti-CD20) radioimmunotherapy for non-Hodgkin’s lymphoma: adjusting radiation absorbed dose to actual organ volumes. J Nucl Med Off Publ Soc Nucl Med. 2004;45:1059–64.
Loevinger RBT, Watson EE. MIRD primer for absorbed dose calculations, Rev. ed. New York, NY: Society of Nuclear Medicine, Incorporated; 1991.
Fisher DR. Internal dosimetry for systemic radiation therapy. Semin Radiat Oncol. 2000;10:123–32. https://doi.org/10.1053/srao.2000.0123.
Pagel JM, Appelbaum FR, Eary JF, Rajendran J, Fisher DR, Gooley T, et al. 131I–anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood. 2006;107:2184–91.
Chiesa C, Botta F, Coliva A, Maccauro M, Devizzi L, Guidetti A, et al. Absorbed dose and biologically effective dose in patients with high-risk non-Hodgkin’s lymphoma treated with high-activity myeloablative 90Y-ibritumomab tiuxetan (Zevalin). Eur J Nucl Med Mol Imaging. 2009;36:1745–57. https://doi.org/10.1007/s00259-009-1141-x.
Matthews DC, Appelbaum FR, Eary JF, Fisher DR, Durack LD, Bush SA, et al. Development of a marrow transplant regimen for acute leukemia using targeted hematopoietic irradiation delivered by 131I-labeled anti-CD45 antibody, combined with cyclophosphamide and total body irradiation. Blood. 1995;85:1122–31.
Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988;6:1562–8. https://doi.org/10.1200/jco.1988.6.10.1562.
Storer BE. Small-sample confidence sets for the MTD in a phase I clinical trial. Biometrics. 1993;49:1117–25.
Manier S, Salem KZ, Park J, Landau DA, Getz G, Ghobrial IM. Genomic complexity of multiple myeloma and its clinical implications. Nat Rev Clin Oncol. 2017;14:100–13. https://doi.org/10.1038/nrclinonc.2016.122.
Maloney DG, Molina AJ, Sahebi F, Stockerl-Goldstein KE, Sandmaier BM, Bensinger W, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102:3447–54. https://doi.org/10.1182/blood-2002-09-2955.
Rotta M, Storer BE, Sahebi F, Shizuru JA, Bruno B, Lange T, et al. Long-term outcome of patients with multiple myeloma after autologous hematopoietic cell transplantation and nonmyeloablative allografting. Blood. 2009;113:3383–91. https://doi.org/10.1182/blood-2008-07-170746.
Crawley C, Lalancette M, Szydlo R, Gilleece M, Peggs K, Mackinnon S, et al. Outcomes for reduced-intensity allogeneic transplantation for multiple myeloma: an analysis of prognostic factors from the Chronic Leukaemia Working Party of the EBMT. Blood. 2005;105:4532–9. https://doi.org/10.1182/blood-2004-06-2387.
Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E 3rd, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12:1195–203. https://doi.org/10.1016/s1470-2045(11)70243-1.
Maffini E, Storer BE, Sandmaier BM, Bruno B, Sahebi F, Shizuru JA, et al. Long-term follow up of tandem autologous-allogeneic hematopoietic cell transplantation for multiple myeloma. Haematologica. 2019;104:380–91. https://doi.org/10.3324/haematol.2018.200253.
Bensinger WI, Green DJ, Burwick N, Becker PS. A prospective study of lenalidomide monotherapy for relapse after Allo-SCT for multiple myeloma. Bone Marrow Transplant. 2014;49:492–5. https://doi.org/10.1038/bmt.2013.219.
Green DJ, Maloney DG, Storer BE, Sandmaier BM, Holmberg LA, Becker PS, et al. Tandem autologous/allogeneic hematopoietic cell transplantation with bortezomib maintenance therapy for high-risk myeloma. Blood Adv. 2017;1:2247–56. https://doi.org/10.1182/bloodadvances.2017010686.
Giaccone L, Evangelista A, Patriarca F, Sorasio R, Pini M, Carnevale-Schianca F, et al. Impact of new drugs on the long-term follow-up of upfront tandem autograft-allograft in multiple myeloma. Biol Blood Marrow Transplant. 2018;24:189–93. https://doi.org/10.1016/j.bbmt.2017.09.017.
O’Steen S, Comstock ML, Orozco JJ, Hamlin DK, Wilbur DSS, Jones JC, et al. The alpha emitter astatine-211 targeted to CD38 can eradicate multiple myeloma in a disseminated disease model. Blood. 2019. https://doi.org/10.1182/blood.2019001250.
Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380:1726–37. https://doi.org/10.1056/NEJMoa1817226.
Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36:2267–80. https://doi.org/10.1200/jco.2018.77.8084.
Watanabe K, Terakura S, Martens AC, van Meerten T, Uchiyama S, Imai M, et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 zeta chimeric antigen receptor-modified effector CD8+ T cells. J Immunol. 2015;194:911–20. https://doi.org/10.4049/jimmunol.1402346.
Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25:214–21. https://doi.org/10.1016/j.coi.2012.12.003.
McLellan AD, Ali Hosseini, Rad SM. Chimeric antigen receptor T cell persistence and memory cell formation. Immunol Cell Biol. 2019;97:664–74. https://doi.org/10.1111/imcb.12254.
Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24:563–71. https://doi.org/10.1038/s41591-018-0010-1.
Acknowledgements
This work was supported by grants R21 CA155911, T32 CA009515, R01 CA205248, and P30 CA015704 from the National Institutes of Health, National Cancer Institute and the Conquer Cancer Foundation. The authors thank the patients and family members who enrolled in NCI protocol NCT01503242. We acknowledge the nurses at the University of Washington Medical Center and the Seattle Cancer Care Alliance for clinical care contributions. We also acknowledge Nicholas Brown, Elizabeth Morrigan, and Monina Almeda for assistance with data collection, Kelsey Baker for statistical support, and Nancy Press for assistance with the figures.
Funding
NIH/NCI R21 CA155911, NIH/NCI T32 CA009515, NIH/NCI R01 CA205248, NIH/NCI P30 CA015704, Conquer Cancer Foundation.
Author information
Authors and Affiliations
Contributions
S.A.T. collected, analyzed and interpreted data and wrote the manuscript. D.J.G. collected, analyzed and interpreted data and co-wrote the manuscript. W.I.B. collected, analyzed and interpreted data and wrote the clinical trial. O.W.P., J.M.P., T.A.G., J.G.R. and D.R.F. contributed to the conception and design of the study. T.A.G. and D.G.C. analyzed the data. All authors contributed to data interpretation, manuscript editing and, aside from O.W.P.†, approval of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
BMS reports consulting for Kiadis Pharma, Actinium Pharmaceuticals, Frazier Healthcare Ventures, and Bristol-Meyers Squibb, equity ownership in AnaptysBio, Oncoresponse, Inipharm, EpiThany, and Blaze Bioscience, employment and equity ownership in Mavupharma, Inc., and research funding from Bellicum. LAH reports research funding from Seattle Genetics, Merck, Millennium Pharmaceuticals, Celgene, Sanofi-Aventis, royalties from UpToDate, and consulting for Jazz Pharmaceuticals and the National Comprehensive Cancer Network. JJO receives research funding from Actinium Pharmaceuticals. AKG reports grants and nonfinancial research support from Teva, Bristol-Myers Squibb, Merck, Takeda, Seattle Genetics, Pfizer, Janssen, Takeda, and Effector, and personal fees and nonfinancial support from Seattle Genetics, Pfizer, Janssen, Gilead Sciences, Sanofi-Aventis, Spectrum, Amgen, Aptevo, BRIM bio, Acerta, I-Mab-pharma, Compliment, Asana Bio, and Incyte. BGT holds patents and receives royalties and research funding from Mustang Bio. JMP reports consulting for Actinium Pharmaceuticals, Astrazeneca Pharmaceuticals, Bayer Healthcare Pharmaceuticals, F. Hoffman-La Roche, Pharmacyclics LLC, Teva Pharmaceuticals, consulting and personal fees from Celgene, Gilead Sciences, Janssen, personal fees from Millennium Pharmaceuticals, and honoraria from Seattle Genetics and Spectrum Pharmaceuticals. WIB reports consulting and personal fees from Amgen, Celgene, Janssen and personal fees from Millennium Pharmaceuticals. DJG receives research funding from Juno Therapeutics, Celgene, Merck, Seattle Genetics, Sanofi-Aventis, Cellectar Bio and consults for Cellectar Bio, Celgene, Seattle Genetics, and GSK. The remaining authors have disclosed no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tuazon, S.A., Sandmaier, B.M., Gooley, T.A. et al. 90Y-labeled anti-CD45 antibody allogeneic hematopoietic cell transplantation for high-risk multiple myeloma. Bone Marrow Transplant 56, 202–209 (2021). https://doi.org/10.1038/s41409-020-01000-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01000-3