Abstract
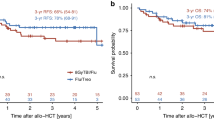
Clofarabine (Clo) is an immunosuppressive purine analog that may have better anti-leukemic activity than fludarabine (Flu). The addition of total body irradiation (TBI) to conditioning regimens has been widely investigated. However, the use of single agent Clo in combination with intermediate doses of TBI ranging from 4 to 8 Gy has not been studied yet. This study is a double center, observational, retrospective study of patients with high-risk hematological malignancies diagnosed from 2012 to 2021, treated at the American University of Beirut Medical Center in Beirut (AUBMC), Lebanon, and Saint-Antoine Hospital (SAH) in Paris, France. It aims to identify the outcome of patients with high-risk hematological malignancies who underwent allogeneic stem cell transplant (allo-SCT) and received Clo and TBI (4–8 Gy) before transplant. Data regarding patient baseline characteristics, disease-related factors, and transplant outcomes including graft-versus-host disease (GVHD), Non-relapse mortality (NRM), progression-free survival (PFS), and overall survival (OS), were collected. We identified 24 high-risk patients diagnosed with a hematological malignancy. The median age at transplant was 37 years (range 22–78). At the time of the transplant, only 15 patients (63%) were in complete remission (CR). All patients received Clo/TBI (4–8 Gy). After a median follow-up of 40 months, the cumulative incidences of grade II-III acute GVHD, grade IV acute GVHD, and chronic GVHD were 50%, 4%, and 8%, respectively. NRM at 100 days, and 1 year after transplant was 4% and 25%, respectively. 17% of the patients had a relapse or progression of the disease by the end of the study. The 2-year PFS and OS were 50% and 56%, respectively. The median PFS and OS were 66 and 68 months respectively. As a conclusion, Clo/TBI (4–8 Gy) as a conditioning regimen for allo-SCT in high-risk patients confers disease control with an acceptable toxicity profile.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets obtained, organized, and analyzed during the current study are available from the corresponding author on reasonable request.
References
Agura E, Cooper B, Holmes H, Vance E, Berryman RB, Maisel C, et al. Report of a phase II study of clofarabine and cytarabine in de novo and relapsed and refractory AML patients and in selected elderly patients at high risk for anthracycline toxicity. Oncologist. 2011;16:197–206. https://doi.org/10.1634/theoncologist.2010-0220.
Diaconescu R, Flowers CR, Storer B, Sorror ML, Maris MB, Maloney DG, et al. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood. 2004;104:1550–8. https://doi.org/10.1182/blood-2004-03-0804.
McClune BL, Weisdorf DJ. Reduced-intensity conditioning allogeneic stem cell transplantation for older adults: is it the standard of care? Curr Opin Hematol. 2010;17:133–8. https://doi.org/10.1097/MOH.0b013e3283366ba4.
Pulsipher MA, Boucher KM, Wall D, Frangoul H, Duval M, Goyal RK, et al. Reduced-intensity allogeneic transplantation in pediatric patients ineligible for myeloablative therapy: results of the Pediatric Blood and Marrow Transplant Consortium Study ONC0313. Blood. 2009;114:1429–36. https://doi.org/10.1182/blood-2009-01-196303.
Saito T, Kanda Y, Kami M, Kato K, Shoji N, Kanai S, et al. Therapeutic potential of a reduced-intensity preparative regimen for allogeneic transplantation with cladribine, busulfan, and anti-thymocyte globulin against advanced/refractory acute leukemia/lymphoma. Clin Cancer Res. 2002;8:1014–20.
Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and post grafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–9. https://doi.org/10.1182/blood-2002-05-1340.
Kornblit B, Maloney DG, Storb R, Storek J, Hari P, Vucinic V, et al. Fludarabine and 2-Gy TBI are superior to 2 Gy TBI as conditioning for HLA-matched related hematopoietic cell transplantation: a phase III randomized trial. Biol Blood Marrow Transplant. 2013;19:1340–7. https://doi.org/10.1016/j.bbmt.2013.06.002.
Ghanem H, Kantarjian H, Ohanian M, Jabbour E. The role of clofarabine in acute myeloid leukemia. Leuk Lymphoma. 2013;54:688–98. https://doi.org/10.3109/10428194.2012.726722.
Kantarjian H, Gandhi V, Cortes J, Verstovsek S, Du M, Garcia-Manero G. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–86. https://doi.org/10.1182/blood-2003-03-0925.
Soni S, Abdel-Azim H, McManus M, Nemecek E, Sposto R, Woolfrey A, et al. Phase I study of clofarabine and 2-Gy total body irradiation as a nonmyeloablative preparative regimen for hematopoietic stem cell transplantation in pediatric patients with hematologic malignancies: a therapeutic advances in childhood leukemia Consortium study. Biol Blood Marrow Transplant. 2017;23:1134–41. https://doi.org/10.1016/j.bbmt.2017.03.037.
Becker PS, Kantarjian HM, Appelbaum FR, Storer B, Pierce S, Shan J, et al. Retrospective comparison of clofarabine versus fludarabine in combination with high-dose cytarabine with or without granulocyte colony-stimulating factor as salvage therapies for acute myeloid leukemia. Haematologica. 2013;98:114–8. https://doi.org/10.3324/haematol.2012.063438.
Chevallier P, Labopin M, de La Tour RP, Lioure B, Bulabois CE, Huynh A, et al. SFGM-TC. Clofarabine versus fludarabine-based reduced-intensity conditioning regimen before allogeneic transplantation in adults with AML/MDS. Cancer Med. 2016;5:3068–76. https://doi.org/10.1002/cam4.880.
Chevallier P, Peterlin P, Garnier A, Le Bourgeois A, Mahé B, Dubruille V, et al. Clofarabine-based reduced intensity conditioning regimen with peripheral blood stem cell graft and post-transplant cyclophosphamide in adults with myeloid malignancies. Oncotarget. 2018;9:33528–35. https://doi.org/10.18632/oncotarget.26083.
Krakow EF, Gyurkocza B, Storer BE, Chauncey TR, McCune JS, Radich, et al. Phase I/II multisite trial of optimally dosed clofarabine and low-dose TBI for hematopoietic cell transplantation in acute myeloid leukemia. Am J Hematol. 2020;95:48–56. https://doi.org/10.1002/ajh.25665.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1. https://doi.org/10.1016/j.bbmt.2014.12.001.
Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016;51:906–12. https://doi.org/10.1038/bmt.2016.130.
Picod A, Bonnin A, Battipaglia G, Giannotti F, Ruggeri A, Brissot E, et al. Defibrotide for sinusoidal obstruction syndrome/veno-occlusive disease prophylaxis in high-risk adult patients: a single-center experience study. Biol Blood Marrow Transplant. 2018;24:1471–5. https://doi.org/10.1016/j.bbmt.2018.02.015.
Atoui A, Omeirat N, Fakhreddine O, El Alam R, Kanafani Z, Abou Dalle I, et al. The use of voriconazole as primary prophylaxis for invasive fungal infections in patients undergoing allogeneic stem cell transplantation: a single center’s experience. J Fungi. 2021;7:925. https://doi.org/10.3390/jof7110925.
Storb R, Sandmaier BM. Nonmyeloablative allogeneic hematopoietic cell transplantation. Haematologica. 2016;101:521–30. https://doi.org/10.3324/haematol.2015.132860.
Michallet M, Le QH, Mohty M, Prebet T, Nicolini F, Boiron JM, et al. Predictive factors for outcomes after reduced-intensity conditioning hematopoietic stem cell transplantation for hematological malignancies: a 10-year retrospective analysis from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Exp Hematol. 2008;36:535–44.
Ho VT, Kim HT, Aldridge J, Liney D, Kao G, Armand P, et al. Use of matched unrelated donors compared with matched related donors is associated with lower relapse and superior progression-free survival after reduced-intensity conditioning hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1196–204.
Luger SM, Ringdén O, Zhang M-J, Perez WS, Bishop MR, Bornhauser M, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47:203–11.
Acknowledgements
The authors thank the nursing staff and all the physicians at our two centers for providing excellent patient care.
Author information
Authors and Affiliations
Contributions
JLC, the corresponding and first author, came up with the idea given the gap in the literature. JLC also supervised the study wrote and reviewed the manuscript extensively. GB, the second author, synthesized the manuscript writing, contributed in data collection, and reviewed the paper extensively. AA, KT and LS contributed extensively in the data collection and cleaning. AZ contributed to patient and case database organization and management. NM, IAD, AB, MM, RD reviewed the manuscript and were in charge of patients and all authors reviewed its final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study gained international review board approval and ethics committee approval in the AUMBC and SAH. All patients gave informed consent for the collection of their data in this database. Data were collected from chart reviews, and test results were cross-checked using various methods of verification, including matching of several sources of data, on-site verification, and computerized searches for discrepancy errors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El Cheikh, J., Bidaoui, G., Atoui, A. et al. Clofarabine and total body irradiation (TBI) as conditioning regimen for allogeneic stem cell transplantation in high-risk acute leukemia patients: a two-center retrospective cohort study. Bone Marrow Transplant 58, 667–672 (2023). https://doi.org/10.1038/s41409-023-01947-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-01947-z