Abstract
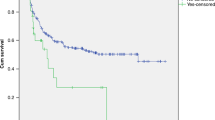
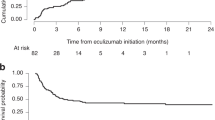
Transplant-associated thrombotic microangiopathy (TA-TMA) is an increasingly recognized complication of hematopoietic cell transplant that can result in multi-organ failure (MOF). Patients undergoing high-dose chemotherapy with autologous stem cell transplant (aHCT) for neuroblastoma require good organ function to receive post-transplant radiation and immunotherapy. We examined TA-TMA incidence and transplant outcomes in patients with neuroblastoma receiving different transplant preparative regimens. Sixty patients underwent aHCT using high-dose chemotherapy: 41 patients received carboplatin/etoposide/melphalan (CEM), 13 patients busulfan/melphalan (Bu/Mel) and six patients received tandem transplant (cyclophosphamide/thiotepa and CEM). TA-TMA with MOF was diagnosed in 13 patients (21.7%) at a median of 18 days after aHCT. TA-TMA occurred in 12 patients receiving CEM and in 1 after cyclophosphamide/thiotepa. There were no incidences of TA-TMA after Bu/Mel regimen. Six of 13 patients with TA-TMA and MOF received terminal complement blocker eculizumab for therapy. They all recovered organ function and received planned post-transplant therapy. Out of seven patients who did not get eculizumab, two died from TA-TMA complications and four progressed to ESRD. We conclude that the CEM regimen is associated with a high incidence of clinically significant TA-TMA after aHCT and eculizumab can be safe and effective treatment option to remediate TA-TMA associated MOF.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK. et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–73.
Kreissman SG, Seeger RC, Matthay KK, London WB, Sposto R, Grupp SA. et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol. 2013;14:999–1008. https://doi.org/10.1016/S1470-2045(13)70309-7.
Grupp SA, Stern JW, Bunin N, Nancarrow C, Ross AA, Mogul M. et al. Tandem high-dose therapy in rapid sequence for children with high-risk neuroblastoma. J Clin Oncol. 2000;18:2567–75.
Desai AV, Heneghan MB, Li Y, Bunin NJ, Grupp SA, Bagatell R. et al. Toxicities of busulfan/melphalan versus carboplatin/etoposide/melphalan for high-dose chemotherapy with stem cell rescue for high-risk neuroblastoma. Bone Marrow Transplant. 2016;51:1204–10. https://doi.org/10.1038/bmt.2016.84.
Uderzo C, Bonanomi S, Busca A, Renoldi M, Ferrari P, Iacobelli M. et al. Risk factors and severe outcome in thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Transplantation. 2006;82:638–44. https://doi.org/10.1097/01.tp.0000230373.82376.46.
Laskin BL, Goebel J, Davies SM, Khoury JC, Bleesing JJ, Mehta PA. et al. Early clinical indicators of transplant-associated thrombotic microangiopathy in pediatric neuroblastoma patients undergoing auto-SCT. Bone Marrow Transplant. 2011;46:682–9.
Jodele S, Laskin BL, Dandoy CE, Myers KC, El-Bietar J, Davies SM. et al. A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29:191–204. https://doi.org/10.1016/j.blre.2014.11.001.
Jodele S, Fukuda T, Vinks A, Mizuno K, Laskin BL, Goebel J. et al. Eculizumab Therapy in Children with Severe Hematopoietic Stem Cell Transplantation-Associated Thrombotic Microangiopathy. Biol Blood Marrow Transplant. 2013;20:518–25. https://doi.org/10.1016/j.bbmt.2013.12.565.
Ricklin D, Cines DB. TMA: beware of complements. Blood. 2013;122:1997–9. https://doi.org/10.1182/blood-2013-07-512707.
Jodele S, Fukuda T, Mizuno K, Vinks AA, Laskin BL, Goebel J. et al. Variable Eculizumab Clearance Requires Pharmacodynamic Monitoring to Optimize Therapy for Thrombotic Microangiopathy after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2016;22:307–15. https://doi.org/10.1016/j.bbmt.2015.10.002.
Jodele S, Davies SM, Lane A, Khoury J, Dandoy C, Goebel J. et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124:645–53. https://doi.org/10.1182/blood-2014-03-564997.
Laskin BL, Nehus E, Goebel J, Furth S, Davies SM, Jodele S. Estimated versus measured glomerular filtration rate in children before hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:2056–61. https://doi.org/10.1016/j.bbmt.2014.07.008.
Laskin BL, Nehus E, Goebel J, Khoury JC, Davies SM, Jodele S. Cystatin C-estimated glomerular filtration rate in pediatric autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1745–52. https://doi.org/10.1016/j.bbmt.2012.06.006.
Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW. et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–47.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. https://doi.org/10.1186/cc2872cc2872 [pii].
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76.
Au WY, Ma ES, Lee TL, Ha SY, Fung AT, Lie AK. et al. Successful treatment of thrombotic microangiopathy after haematopoietic stem cell transplantation with rituximab. Br J Haematol. 2007;137:475–8.
Jodele S, Laskin BL, Goebel J, Khoury JC, Pinkard SL, Carey PM. et al. Does early initiation of therapeutic plasma exchange improve outcome in pediatric stem cell transplant-associated thrombotic microangiopathy?. Transfusion. 2013;53:661–7.doi: 1111/j.1537-2995.2012.03776.x.
Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C. et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–81. https://doi.org/10.1056/NEJMoa1208981.
Greenbaum LA, Fila M, Ardissino G, Al-Akash SI, Evans J, Henning P. et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016;89:701–11. https://doi.org/10.1016/j.kint.2015.11.026.
Jodele S, Dandoy CE, Danziger-Isakov L, Myers KC, El-Bietar J, Nelson A. et al. Terminal Complement Blockade after Hematopoietic Stem Cell Transplantation Is Safe without Meningococcal Vaccination. Biol Blood Marrow Transplant. 2016;22:1337–40. https://doi.org/10.1016/j.bbmt.2016.03.032.
Mahler MB, Taur Y, Jean R, Kernan NA, Prockop SE, Small TN. Safety and immunogenicity of the tetravalent protein-conjugated meningococcal vaccine (MCV4) in recipients of related and unrelated allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:145–9. https://doi.org/10.1016/j.bbmt.2011.07.027.
Myers KC, Lawrence J, Marsh RA, Davies SM, Jodele S. High-dose methylprednisolone for veno-occlusive disease of the liver in pediatric hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2013;19:500–3. https://doi.org/10.1016/j.bbmt.2012.11.011.
Myers KC, Dandoy C, El-Bietar J, Davies SM, Jodele S. Veno-occlusive disease of the liver in the absence of elevation in bilirubin in pediatric patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:379–81. https://doi.org/10.1016/j.bbmt.2014.09.026.
Richardson PG, Riches ML, Kernan NA, Brochstein JA, Mineishi S, Termuhlen AM. et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. 2016;127:1656–65. https://doi.org/10.1182/blood-2015-10-676924.
de Fontbrune FS, Galambrun C, Sirvent A, Huynh A, Faguer S, Nguyen S. et al. Use of Eculizumab in Patients With Allogeneic Stem Cell Transplant-Associated Thrombotic Microangiopathy: A Study From the SFGM-TC. Transplantation. 2015;99:1953–9. https://doi.org/10.1097/TP.0000000000000601.
Kim SS, Patel M, Yum K, Keyzner A. Hematopoietic stem cell transplant-associated thrombotic microangiopathy: review of pharmacologic treatment options. Transfusion. 2015;55:452–8. https://doi.org/10.1111/trf.12859.
Jodele S, Dandoy CE, Myers KC, El-Bietar J, Nelson A, Wallace G. et al. New approaches in the diagnosis, pathophysiology, and treatment of pediatric hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transfus Apher Sci. 2016;54:181–90. https://doi.org/10.1016/j.transci.2016.04.007.
Dandoy C, Davies SM, Hirsch R, Chima RS, Paff Z, Cash M. et al. Abnormal echocardiography seven days after stem cell transplant may be an early indicator of thrombotic microangiopathy. Biol Blood Marrow Transplant. 2015;21:113–8. https://doi.org/10.1016/j.bbmt.2014.09.028.
Lerner D, Dandoy C, Hirsch R, Laskin B, Davies SM, Jodele S. Pericardial effusion in pediatric SCT recipients with thrombotic microangiopathy. Bone Marrow Transplant. 2014;49:862–3. https://doi.org/10.1038/bmt.2014.40.
Skerka C, Zipfel PF, Muller D, Micklisch S, Riedl M, Zimmerhackl LB. et al. The autoimmune disease DEAP-hemolytic uremic syndrome. Semin Thromb Hemost. 2010;36:625–32. https://doi.org/10.1055/s-0030-1262884.
Jodele S, Licht C, Goebel J, Dixon BP, Zhang K, Sivakumaran TA. et al. Abnormalities in the alternative pathway of complement in children with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Blood. 2013;122:2003–7. https://doi.org/10.1182/blood-2013-05-501445.
Acknowledgements
We thank Dr. Tsuyoshi.Fukuda and Dr. Kana Mizuno from Division of Pharmacology at CCHMC for eculizumab PK/PD studies, Dr. Bradley.Dixon and Ms.Thelma Kathman and the staff of the Nephrology Clinical Laboratory at CCHMC for their assistance with eculizumab serum concentration and CH50 testing, Dr. Ralph Gruppo and Ms. Mary Block and the staff of Hematology Clinical laboratory at CCHMC for their assistance with sC5b-9 testing, Ms. Sue Pinkard and the Hoxworth Blood Center team at the University of Cincinnati for their assistance with therapeutic plasma exchange procedures, Mark Mueller, RN and Suzanne Berger, RN for coordinating neuroblastoma patient care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SJ and SD have a US Provisional patent application for methods and compositions related to transplant-associated thrombotic microangiopathy. The remaining authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jodele, S., Dandoy, C.E., Myers, K. et al. High-dose Carboplatin/Etoposide/Melphalan increases risk of thrombotic microangiopathy and organ injury after autologous stem cell transplantation in patients with neuroblastoma. Bone Marrow Transplant 53, 1311–1318 (2018). https://doi.org/10.1038/s41409-018-0159-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0159-8
This article is cited by
-
Advancing therapy for neuroblastoma
Nature Reviews Clinical Oncology (2022)
-
Antineoplastics
Reactions Weekly (2018)