Abstract
Primary induction failure (PIF) in acute myeloid leukemia (AML) patients is associated with poor outcome, with allogeneic hematopoietic stem cell transplantation (HCT) being the sole curative therapeutic option. Here, we retrospectively evaluated long-term outcomes of 220 AML patients undergoing allogeneic HCT after PIF who never achieved remission, and identified clinical and molecular risk factors associated with treatment response and ultimate prognosis. In this high-risk population, disease-free survival was 25.2% after 5 years and 18.7% after 10 years, while overall survival rates were 29.8% and 21.6% after 5 and 10 years of HCT, respectively. 10-year non-relapse mortality was 32.5%, and 48.8% of patients showed disease relapse within 10 years after allogeneic HCT. Adverse molecular risk features determined at initial diagnosis, poor performance status at the time of allogeneic HCT, and long diagnosis-to-HCT intervals were associated with unfavorable prognosis. Collectively, our data suggests that immediate allogeneic HCT after PIF offers long-term survival and cure in a substantial subset of cases and that high-risk AML patients who never achieved complete response during induction might benefit from early donor search.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic stem cell transplantation (HCT) is the standard consolidation treatment for most patients with adverse-risk acute myeloid leukemia (AML) [1, 2]. Outcomes of patients with AML undergoing allogeneic HCT have significantly improved over the last decades, mostly due to advances in stem cell harvesting modalities, supportive care, and infection management [3,4,5]. Furthermore, the introduction of reduced toxicity conditioning (RTC) regimens has made allogeneic HCT accessible for less fit and elderly patients [6,7,8,9,10]. Achieving complete remission (CR) before allogeneic HCT has been associated with improved clinical outcomes [11, 12]. Thus, AML patients with primary induction failure (PIF) who never achieve CR are often not considered for immediate allogeneic HCT and receive various other re-induction strategies, although allogeneic HCT represents the only curative option for these patients [2, 13,14,15,16]. Recently, Stelljes et al. reported that patients with relapsed/refractory AML had similar survival rates regardless of whether they proceeded directly to allogeneic HCT or underwent intensive remission induction prior to HCT within the ASAP trial [16]. Yet, median follow-up of this study was 37 months and the value of immediate allogeneic HCT in this clinical setting for induction of long-term response and durable remission is largely unclear.
In this single-center retrospective analysis, we explored long-term outcomes of 220 AML patients with PIF undergoing allogeneic HCT with active disease at our institution over a period of 30 years between 1989 and 2019, and investigated molecular and clinical features associated with clinical prognosis.
Subjects and methods
Patient selection and study design
We evaluated clinical and molecular data from 220 patients receiving allogeneic HCT after PIF at the University Medical Center Freiburg (Germany) between 1989 and 2019, with PIF being defined as never achieving CR during induction or re-induction, assessed either by cytomorphology or molecular measurable residual disease (MRD), as defined by the NCI (Version 23.06d, Code C70622) [2]. Patients provided written informed consent for the use of their data for clinical research after approval by the local Ethics Committee (22-1490-S1-retro). All analyses were performed in accordance with the Declaration of Helsinki.
Clinical and molecular examinations as well as laboratory analyses were performed as part of standard clinical care. Patients were treated at the discretion of the treating physician and according to institutional standards and national/international guidelines. Molecular risk at diagnosis and response was categorized according to the respective guidelines and criteria applicable at the time the patient was treated [17]. Throughout the manuscript, analyses were performed considering the entire cohort as well as distinct transplant periods: (i) 1989–2000, (ii) 2001–2010, and (iii) 2011–2019 (Table 1).
Statistical analysis
Data was collected prospectively and analyzed in a retrospective fashion. Primary endpoints were disease-free survival (DFS) and overall survival (OS). Other endpoints were time to relapse and time to non-relapse mortality (NRM). DFS was calculated from allogeneic HCT to AML relapse or death from any cause, OS was calculated from allogeneic HCT to death from any cause. NRM was calculated from allogeneic HCT to death without previous relapse. In case of a missing event, patients were censored at the last known follow-up time point. Time-to-event variables were visualized using the Kaplan–Meier method and log-rank tests were applied to evaluate survival differences. Curves for NRM and time to relapse were calculated using a cumulative-incidence model with the opposing event considered as competing. Univariate and multivariate risk analyses were conducted using Cox proportional hazards regression for OS and DFS.
All statistical analyses were performed using R-Studio 4.0.0 (2020-04-24) and Rx64 4.0.0 (2020-04-24). For log-rank and Cox regression analyses, the survival- package was used. For cumulative-incidence calculation, the cmprsk-package was used. P < 0.05 were considered statistically significant. Data analysis and graph generation was conducted using the dplyr and survminer-packages, packages of the R-tidyverse, and Prism Graph Pad 5.0.
Results
We enrolled 220 AML patients in our study, undergoing allogeneic HCT after PIF between 1989 and 2019 (1989–2000: n = 36; 2001–2010: n = 108; 2011–2019: n = 76). Detailed patient, disease, and transplant-related characteristics are summarized in Tables 1 and 2. Median age of patients was 55 (range: 20–75), increasing from 42 in the first decade (1989–2000) to 57 in the second (2001–2010) and third decade (2011–2019, Table 1). This correlated with the proportion of patients treated with RTC, from 13.9% between 1989 and 2000 to 61.8% between 2011 and 2019 (Table 2). Molecular risk was assessed in 63.2% of patients, while molecular information was missing in 36.8% of AML cases. The proportion of patients with information on the molecular risk group increased over time with the availability of the respective technologies (Table 1) [17]. Most patients were diagnosed with de novo AML (61.4%), while 29.5% had secondary AML (sAML) emerging from myelodysplastic syndrome (MDS) or myeloproliferative neoplasm (MPN), and 9.1% of patients suffered from treatment-related AML (tAML). Patients received in median two lines of treatment before allogeneic HCT (range: 1–6), median time from diagnosis to allogeneic HCT was 3.48 months (range: 0.48–14.52 months) (Table 1). Unmodified myeloablative conditioning regiments (MAC) were used in 92 (41.8%) patients, while 125 (56.8%) patients were treated with RTC, and 3 (1.4%) patients received RIC regimens (Table 2). GvHD prophylaxis regimens consisted of either cyclosporin A (CsA) in combination with Alemtuzumab (35.9%) or anti-T lymphocyte globulin (ATG (Grafalon), 33.2%), or different strategies including mycophenolate-Mofetil (MMF) or methotrexate (30.9%) (Table 2). In 35% of cases, patients received stem cells from matched related donors (MRD), in 41.1% from matched unrelated donors (Table 2).
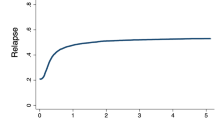
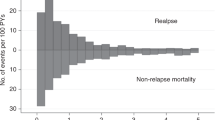
The median follow-up of our study was 8.5 years (range: 0.06–25.4 years). Disease-free survival at 1 year was reached in 39.8% of AML patients, in 25.2% after 5 and 18.7% after 10 years of allogeneic HCT (Fig. 1a and Table 3). Median OS was 1.05 years, while 1-year and 5-year OS were 50.4% and 29.8%, respectively, and 21.6% of patients were alive 10 years after allogeneic HCT (Fig. 1a and Table 3). Survival rates were largely stable over time. For example, 5-year OS in patients treated between 1998-2000 was 28.7%, in those receiving HCT between 2001 and 2010 28.7%, and 30.3% in patients treated between 2011 and 2019 (Table 3). Non-relapse mortality (NRM) for the whole cohort after 5 years was 29.3% and 32.5% after 10 years (Fig. 1b and Table 3). Acute graft versus host disease (aGvHD) was observed in 55.5% of patients, while 30% of patients suffered from chronic graft versus host disease (cGvHD) (Table 2). Relapse rates of the entire cohort after 5 and 10 years were 44.9% and 48.8%, respectively (Fig. 1b and Table 3). Over time, relapse rates varied slightly, with a 5-year relapse rate of 45.7% between 1998 and 2000, 50.9% in 2001–2010, and 33.3% between 2011 and 2019 (Table 3), reflecting small variations of risk factors within the patient populations.
a Kaplan–Meier analysis of overall survival (continuous line) and disease-free survival (dotted line) of the entire patient cohort. b cumulative-incidence model of relapse rates (continuous line) and non-relapse mortality (dotted line) of the entire patient cohort. OS overall survival, DFS disease-free survival, NRM non-relapse mortality, HCT hematopoietic cell transplantation.
At 1 month after HCT, 206 patients (93.6%) were alive and 59 patients (26.8%) were alive at the time of the final study analysis. The main cause of death after HCT was treatment failure and AML progression (57.8%), while infections (19.3%), GvHD (11.2%), and treatment-related toxicity (8.4%) were leading causes of NRM (Table 4). Secondary malignancies led to death in 1.9% of patients (Table 4).
In Cox regression analyses incorporating known molecular and clinical risk factors, poor performance status (ECOG > 1; DFS: p = 0.001, HR: 2.3, 95% CI: 1.4–3.7; OS: p = 8*10−5, HR: 2.8, 95% CI: 1.7–4.6), adverse molecular risk at AML diagnosis (vs. intermediate/favorable risk; DFS: p = 0.03, HR: 1.6, 95% CI: 1.1–2.4; OS: p = 0.01, HR: 1.7, 95% CI: 1.1–2.6), and long diagnosis-to-HCT interval (DFS: 0.01, HR: 1.1, 95% CI: 1.0–1.2; OS: p = 0.005, HR: 1.1, 95% CI: 1.0–1.2) were strongly and independently associated with unfavorable DFS and OS (Fig. 2a, b). This was confirmed by log rank analyses, revealing that DFS and OS was shorter in patients with adverse molecular risk factors (vs. intermediate/favorable risk; DFS: p = 0.01, HR: 1.64, 95% CI: 1.12–2.41; OS: p = 0.003, HR: 1.81, 95% CI: 1.22–2.67, Fig. 3a, b), ECOG > 1 (DFS: p = 1*10−4, HR: 2.44, 95% CI: 1.24–4.79; OS: p = 9*10−7, HR: 3.06, 95% CI: 1.45–6.46, Fig. 3c, d), and a time from diagnosis to HCT greater than 180 days (DFS: p = 0.001, HR: 1.88, 95% CI: 1.16–3.04; OS: p = 2*10−4, HR: 2.05, 95% CI: 1.25–3.37, Fig. 3e, f). Importantly, the association between these clinical features and outcomes was maintained when restricting the analyses to patients proceeding to allogeneic HCT after PIF during first line therapy (Supplementary Table 1 and Supplementary Fig. 1). Notably, age, donor type (MRD vs. MMUD/MUD/MMRD), GvHD-prophylaxis, conditioning regimen (RTC vs. MAC), and AML subtypes did not influence clinical outcomes (Fig. 2). The presence of circulating blasts before conditioning has previously been shown to be associated with unfavorable outcomes [18]. Our data support this finding, we also found shorter DFS and OS in patients with circulating blasts compared to patients with no measurable blasts in peripheral blood (Log-rank analyses: DFS: p = 2*10−5, HR: 2.00, CI: 1.44–2.7; OS: p = 0.001, HR: 1.72, CI: 1.25–2.37, Supplementary Fig. 2).
Forest plots showing hazard ratios for DFS (a) and OS (b) estimated by univariate and multivariate Cox proportional hazards regression outcome analyses, incorporating patient-, disease-, and treatment-specific characteristics. P-values were calculated using the Walden test. ECOG Eastern Cooperative Oncology Group Score, HCT hematopoietic cell transplantation, CMV cytomegalovirus, AML acute myeloid leukemia, sAML secondary acute myeloid leukemia, IM intermediate, fav favorable, n.a. not assessed, M(M)UD (mis)matched unrelated donor, MMRD mismatched related donor, MRD matched related donor, PB peripheral blood, BM bone marrow, Dg diagnosis, RTC reduced toxicity conditioning, MAC myeloablative conditioning, ATG anti-T lymphocyte globulin, Campath, Alemtuzumab.
Kaplan–Meier analyses of DFS (a) and OS (b) in patients with adverse molecular risk (n = 77) vs. intermediate/favorable molecular risk (n = 62). Patients with unknown molecular risk are also shown (n = 81). Kaplan–Meier analyses of DFS (c) and OS (d) in patients with ECOG 0–1 (n = 173) vs. 2–4 (n = 22). Kaplan–Meier analyses of DFS (e) and OS (f) in patients with Diagnosis-to-HCT interval of less than 6 months (n = 185) vs. at least 6 months (n = 35). OS overall survival, DFS disease-free survival, HCT hematopoietic cell transplantation, ECOG Eastern Cooperation Oncology Group score, IM intermediate, fav favorable, n.a. not assessed.
Discussion
Primary induction failure is associated with poor prognosis in patients with AML [17, 19,20,21,22]. In this situation, re-induction with cytarabine- or venetoclax-based therapies followed by allogeneic HCT in CR is considered the standard procedure to induce long-term remissions [23]. However, Stelljes et al. recently demonstrated in a large randomized phase III trial (ASAP trial), which enrolled 281 patients with relapsed/refractory AML, that re-induction with high-dose cytarabine and mitoxantrone before allogeneic HCT did not result in higher early overall response rates or a survival advantage compared to immediate conditioning and allogeneic HCT without prior remission induction [16]. While the median follow-up of this study was relatively short with 37 months, we here add evidence that allogeneic HCT with active disease at time of transplantation can also lead to long-term remission and cure in a substantial subset of cases. In our retrospective single-center study, we analyzed 220 AML patients with PIF undergoing allogeneic HCT while not being in CR over a period of 30 years. Median OS for the whole group was 1.05 years, with a 5-year OS of 29.8%. These results are largely concordant with previous studies. For example, Craddock et al. demonstrated a 5-year OS of 22% in refractory AML patients who underwent unrelated donor transplantation between 1994 and 2006 [8]. In a retrospective study led by Nagler et al. that included more than 3400 patients with relapsed/refractory AML treated with allogeneic HCT from unrelated donors, 2-year DFS and OS were 29.8% and 36.5% after a median follow-up of 4 years [24]. Importantly, our results were also comparable to studies investigating survival in high-risk AML patients undergoing HCT in CR/CRi [25] and might be superior to those investigating treatment with hypomethylating agents and venetoclax without allogeneic HCT in relapsed/refractory AML patients (Median OS: 5 months, 60-day mortality rate: 33.3%) [26]. Collectively, our data indicates that immediate allogeneic HCT in refractory AML patients with active disease might be a reasonable alternative to repeated remission induction strategies before proceeding to HCT, and represents a feasible strategy to induce long-term survival and durable remissions.
The other major finding of our study is the association of outcomes with adverse molecular risk factors at AML diagnosis, performance status, and the diagnose-to-HCT interval, while age and donor type did not correlate with prognosis in our cohort. The role of most of these risk factors for outcome prediction after allogeneic HCT is discussed controversially in previous studies. For example, the prognostic value of donor types has been addressed by several register trials over the last years, showing either no significant difference between siblings and unrelated donors [27, 28], more favorable outcomes in patients undergoing HCT with unrelated donors [29], or worse prognosis with haploidentical donors [29, 30]. In our analysis, we found no significant association of donor types with patient outcomes, likely due to advances in treatment and transplant modalities over decades that might contribute to omitting the influence of donor source. Similarly, in opposite to other studies in AML and MDS, patient’s age was not prognostic in our analysis [31, 32]. On the other hand, the lack of an association between conditioning protocols (RTC vs. MAC) and outcomes after HCT, the predictive value of molecular risk factors and the performance status have been demonstrated before and are in line with our results [9, 14, 33,34,35]. Importantly, we found that patients with a long diagnosis-to-HCT interval show shorter DFS and OS, indicating that a delay of allogeneic HCT, repetitive re-induction, and prolonged presence of the disease might contribute to impaired patient conditions before HCT [8, 27]. Separately, higher disease activity before conditioning, measured by the presence of circulating blasts, led to unfavorable outcomes, which is in line with the findings reported by Duval et al. [18].
Our study harbors several limitations, which include its retrospective nature, heterogeneity of the patient cohort, in particular a younger median age in patients treated between 1989 and 2000, and the lack of various clinical and molecular parameters in a substantial subset of patients. For example, information on molecular risk or the HCT-CI score is largely missing in patients treated within the first decade of the study. Further limitations include heterogenic therapeutic approaches such as an increased use of RTC in the last decade, the unavailability of CsA/Alemtuzumab between 1989–2000, and variable graft sources over time (41.7% bone marrow graft between 1989–2000 and only 1.3% between 2011–2019). However, to our knowledge, this study offers the longest follow up of AML patients with PIF undergoing allogeneic HCT while not being in CR.
Collectively, we demonstrated that immediate allogeneic HCT in AML patients with active disease represents a valid alternative to intensive remission induction and provides long-term survival and cure in a significant proportion of patients, highlighting the importance of allogeneic HCT as the most effective treatment option in this high-risk group. Our data further suggests starting donor search at AML diagnosis and to immediately proceed with conditioning and allogeneic HCT in refractory patients whenever a donor is available.
Code availability
R-code can be obtained from the authors upon request.
References
for the European Society for Blood and Marrow Transplantation (EBMT), Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40,000 transplants annually. Bone Marrow Transpl. 2016;51:786–92.
Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77.
Balsat M, Renneville A, Thomas X, De Botton S, Caillot D, Marceau A, et al. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: a study by the Acute Leukemia French Association Group. JCO. 2017;35:185–93.
Getta BM, Devlin SM, Levine RL, Arcila ME, Mohanty AS, Zehir A, et al. Multicolor flow cytometry and multigene next-generation sequencing are complementary and highly predictive for relapse in acute myeloid leukemia after allogeneic transplantation. Biol Blood Marrow Transplant. 2017;23:1064–71.
Freeman SD, Hills RK, Virgo P, Khan N, Couzens S, Dillon R, et al. Measurable residual disease at induction redefines partial response in acute myeloid leukemia and stratifies outcomes in patients at standard risk without NPM1 mutations. JCO. 2018;36:1486–97.
Eapen M, Brazauskas R, Hemmer M, Perez WS, Steinert P, Horowitz MM, et al. Hematopoietic cell transplant for acute myeloid leukemia and myelodysplastic syndrome: conditioning regimen intensity. Blood Adv. 2018;2:2095–103.
Wall SA, Devine S, Vasu S. The who, how and why: allogeneic transplant for acute myeloid leukemia in patients older than 60 years. Blood Rev. 2017;31:362–9.
Craddock C, Labopin M, Pillai S, Finke J, Bunjes D, Greinix H, et al. Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia. 2011;25:808–13.
Bonifazi F, Pavoni C, Peccatori J, Giglio F, Arpinati M, Busca A, et al. Myeloablative conditioning with thiotepa-busulfan-fludarabine does not improve the outcome of patients transplanted with active leukemia: final results of the GITMO prospective trial GANDALF-01. Bone Marrow Transpl. 2022;57:949–58.
Niederwieser D, Hasenclever D, Berdel WE, Biemond BJ, Al-Ali HK, Chalandon Y, et al. Increased LFS Following Hematopoietic Cell Transplantation As Compared to Conventional Consolidation therapy in patients >60 years with AML in first complete remission and a matched donor: results of a randomized phase III study. Blood 2022;140:2126–9.
Walter RB, Kantarjian HM, Huang X, Pierce SA, Sun Z, Gundacker HM, et al. Effect of complete remission and responses less than complete remission on survival in acute myeloid leukemia: a combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M. D. Anderson Cancer Center Study. JCO. 2010;28:1766–71.
Weisdorf DJ, Millard HR, Horowitz MM, Hyare PS, Champlin R, Ho V, et al. Allogeneic transplantation for advanced acute myeloid leukemia: the value of complete remission. Cancer. 2017;123:2025–34.
Burnett AK, Wheatley K, Goldstone AH, Stevens RF, Hann IM, Rees JHK, et al. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UK MRC AML 10 trial. Br J Haematol. 2002;118:385–400.
Ustun C, Le-Rademacher J, Wang HL, Othus M, Sun Z, Major B, et al. Allogeneic hematopoietic cell transplantation compared to chemotherapy consolidation in older acute myeloid leukemia (AML) patients 60–75 years in first complete remission (CR1): an alliance (A151509), SWOG, ECOG-ACRIN and CIBMTR Study. Leukemia. 2019;33:2599–609.
Jabbour E, Daver N, Champlin R, Mathisen M, Oran B, Ciurea S, et al. Allogeneic stem cell transplantation as initial salvage for patients with acute myeloid leukemia refractory to high-dose cytarabine-based induction chemotherapy. Am J Hematol. 2014;89:395–8.
Stelljes M, Middeke JM, Bug G, Wagner EM, Mueller LP, Christoph S, et al. In patients with relapsed/refractory AML sequential conditioning and immediate allogeneic stem cell transplantation (allo-HCT) results in similar overall and leukemia-free survival compared to intensive remission induction chemotherapy followed by Allo-HCT: results from the randomized phase III ASAP trial. Blood. 2022;140:9–11.
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–8.
Buckley SA, Wood BL, Othus M, Hourigan CS, Ustun C, Linden MA, et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: a meta-analysis. Haematologica. 2017;102:865–73.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP–EBMT analysis on patients with AML in remission. Bone Marrow Transpl. 2016;51:610–1.
Andersen PK, Klein JP, Zhang M. Testing for centre effects in multi‐centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18:1489–500.
Hougaard P. Frailty models for survival data. Lifetime Data Anal. 1995;1:255–73.
Stubbins RJ, Francis A, Kuchenbauer F, Sanford D. Management of acute myeloid leukemia: a review for general practitioners in oncology. Curr Oncol. 2022;29:6245–59.
Nagler A, Ngoya M, Galimard JE, Labopin M, Bornhäuser M, Stelljes M, et al. Longitudinal outcome over two decades of unrelated allogeneic stem cell transplantation for relapsed/refractory acute myeloid leukemia: an ALWP/EBMT analysis. Clin Cancer Res. 2022;28:4258–66.
Begna, Ali KH, Naseema Gangat W, Elliott MA, Al-Kali A, Litzow MR, et al. Mayo Clinic experience with 1123 adults with acute myeloid leukemia. Blood Cancer J. 2021;11:46.
Morsia E, McCullough K, Joshi M, Cook J, Alkhateeb HB, Al‐Kali A, et al. Venetoclax and hypomethylating agents in acute myeloid leukemia: Mayo Clinic series on 86 patients. Am J Hematol. 2020;95:1511–21.
Brissot E, Labopin M, Stelljes M, Ehninger G, Schwerdtfeger R, Finke J, et al. Comparison of matched sibling donors versus unrelated donors in allogeneic stem cell transplantation for primary refractory acute myeloid leukemia: a study on behalf of the Acute Leukemia Working Party of the EBMT. J Hematol Oncol. 2017;10:130.
Brissot E, Labopin M, Ehninger G, Stelljes M, Brecht A, Ganser A, et al. Haploidentical versus unrelated allogeneic stem cell transplantation for relapsed/refractory acute myeloid leukemia: a report on 1578 patients from the Acute Leukemia Working Party of the EBMT. Haematologica. 2019;104:524–32.
Barbullushi K, Labopin M, Kröger N, Finke J, Stelljes M, Ganser A, et al. Outcomes of allogeneic hematopoietic stem cell transplantation from different donor types in primary refractory acute myeloid leukemia: a report from the ALWP of the EBMT. Blood. 2022;140:10613–5.
Battipaglia G, Boumendil A, Labopin M, Ciceri F, Tischer J, Stelljes M, et al. Unmanipulated haploidentical versus HLA-matched sibling allogeneic hematopoietic stem cell transplantation in relapsed/refractory acute myeloid leukemia: a retrospective study on behalf of the ALWP of the EBMT. Bone Marrow Transpl. 2019;54:1499–510.
Carré M, Porcher R, Finke J, Ehninger G, Koster L, Beelen D, et al. Role of age and hematopoietic cell transplantation-specific comorbidity index in myelodysplastic patients undergoing an allotransplant: a retrospective study from the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2020;26:451–7.
Michallet M, Thomas X, Vernant J, Kuentz M, Socié G, Espérou-Bourdeau H, et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Société Française de Greffe de Moelle (SFGM). Bone Marrow Transpl. 2000;26:1157–63.
Modi D, Deol A, Kim S, Ayash L, Alavi A, Ventimiglia M, et al. Age does not adversely influence outcomes among patients older than 60 years who undergo allogeneic hematopoietic stem cell transplant for AML and myelodysplastic syndrome. Bone Marrow Transpl. 2017;52:1530–6.
Bertz H, Potthoff K, Finke J. Allogeneic stem-cell transplantation from related and unrelated donors in older patients with myeloid leukemia. JCO. 2003;21:1480–4.
Armand P, Kim HT, DeAngelo DJ, Ho VT, Cutler CS, Stone RM, et al. Impact of cytogenetics on outcome of De novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transpl. 2007;13:655–64.
Acknowledgements
This study was performed at the Freiburg University Medical Center, data was provided by the Department of Hematology, Oncology and Stem Cell Transplantation. Documentation and organization of patients’ primary data and follow-up data was performed by Irmgard Matt of the Department of Hematology, Oncology and Stem Cell Transplantation.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conception and design: MMJ, JF, and FS. Administrative support: JD-A, RW, HB, CW, JD, RZ, JF, and FS. Provision of study materials or patients: JD-A, RW, HB, CW, JD, RZ, JF, and FS. Collection and assembly of data: MMJ, HB, CW, RZ, JF, and FS. Data analysis and interpretation: MMJ, GI, JF, and FS. Manuscript writing: All authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Corresponding author
Ethics declarations
Competing interests
MMJ declares no competing interests. FS receives research funding from Roche Sequencing Solutions, Gilead, and Takeda, and receives honoraria from AstraZeneca. JF declares no competing interests. GI declares no competing interests. JD-A received speakers honoraria from Roche, Amgen, Riemser, SOBI, IPSEN, NovoNordisk and AstraZeneca and travel support from Lilly, Roche, Gilead and SOBI. JD declares no competing interests. CW received honoraria from Takeda. HB declares no competing interests. RW received honoraria from Abbvie, Amgen, BMS/Celgene, Janssen, Kite/Gilead, Novartis, Pfizer and Sanofi. RZ received honoraria from Incyte, Novartis, Sanofi and Mallinckrodt all outside of the work reported here.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mozaffari Jovein, M., Ihorst, G., Duque-Afonso, J. et al. Long-term follow-up of patients with acute myeloid leukemia undergoing allogeneic hematopoietic stem cell transplantation after primary induction failure. Blood Cancer J. 13, 179 (2023). https://doi.org/10.1038/s41408-023-00953-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-023-00953-0