Abstract
Over half of chronic myeloid leukemia (CML) patients in deep molecular response do not lose the major molecular response (MMR) after stopping treatment with tyrosine kinase inhibitors (TKI). This strategy is safe in clinical trials, but its applicability in the real-life setting remains unsettled. We describe the outcomes after TKI discontinuation in a nationwide series of 236 CML patients. Median follow-up from treatment discontinuation was 21.5 months and 5 patients died from CML-unrelated causes. TKI therapy was reinitiated due to MMR loss (n = 52), increase ≥ 1 log in BCR-ABL transcript level without losing MMR (n = 12), patient preference (n = 2), and withdrawal syndrome (n = 1). Treatment-free remission rate at 4 years was 64% (95% confidence interval, CI: 55%–72%). Cumulative incidence of molecular recurrence at 3 years was 33% (95% CI: 26%–38%). TKI treatment for < 5 years and MR4.5 duration shorter than 4 years were both associated with higher incidence of molecular recurrence. No patient had disease progression. Response status at last control was: MR4.5 (n = 196), MR4 (n = 15), MMR (n = 14), complete cytogenetic response (n = 10), and other (n = 1). A significant increase in Hb and cholesterol levels was observed after imatinib withdrawal. Our results demonstrate that TKI treatment discontinuation is feasible in real-life clinical practice.
Similar content being viewed by others
Introduction
Life expectancy of patients with chronic myeloid leukemia (CML) is currently approaching that of the general population, as a result of the successful treatment with tyrosine kinase inhibitors (TKIs)1. During the first 1 or 2 years of treatment, most patients achieve a significant decrease in the leukemic burden to a level that protects them from disease transformation2. Later on, about 30–50% of patients achieve a state of undetectable or nearly undetectable molecular residual disease that is generally sustained if treatment compliance is good3,4,5. Despite such excellent efficacy, several studies have demonstrated the persistence of leukemic stem cells in CML patients with deep molecular response (DMR)6,7, with this supporting the need to maintain treatment indefinitely to prevent disease recurrence. However, mere persistence of leukemia stem cells at such low level may not predict disease progression or resistance. Of concern, long-term TKI therapy is not devoid of potential medical risks for the patients8 and poses a considerable economic burden to the healthcare systems9, although this has been eased with the arrival of generic imatinib.
The notion of the need of lifelong treatment in CML has been challenged in recent years by the results of numerous clinical trials demonstrating that over half of the patients in DMR can remain relapse-free after stopping TKI therapy10,11,12,13. Data on more than 2500 patients who discontinued treatment in sustained DMR have demonstrated that this approach is feasible in the setting of controlled clinical trials 14,15,16. Most relapses are observed during the first year after treatment cessation but late relapses can occasionally occur, and therefore close monitoring is of crucial importance for the safety of this strategy17.
It is of note that achieving a treatment-free remission (TFR) has quickly become a key goal of CML therapy18,19, even though no general consensus on its applicability in clinical practice has been established14,15,20. Moreover, information on the safety of TKI cessation outside of clinical trials is still limited21,22,23,24. Only recently, a number of publications have focused on the selection of optimal candidates for treatment cessation outside of clinical trials and on the minimum requirements for an adequate monitoring15,16,18,25,26,27. In this study, we describe the outcomes after TKI discontinuation in a large series of CML patients from Spain.
Methods
Patients
The present retrospective study comprised a series of 236 patients in chronic-phase CML, who discontinued TKI treatment outside of clinical trials between April 2009 and February 2018 in 33 Spanish institutions associated to the Grupo Español de Leucemia Mieloide Crónica (GELMC). After central review, all cases met the following inclusion criteria: (a) TKI treatment duration for at least 3 years; (b) sustained MR4.5 (BCR-ABL1 International Scale (IS) ≤ 0.0032% or undetectable BCR-ABL1 in samples with ≥ 32,000 ABL1 transcripts) in ≥ 4 consecutive determinations (one single point in MR4 was acceptable) during a minimum of 2 years before treatment discontinuation; (c) strict molecular monitoring in a reference laboratory expressing the results on the IS with sensitivity > 4-log. Patients who had undergone allogeneic hematopoietic stem cell transplantation were excluded, but other previous pharmacological therapies were permitted.
Molecular relapse was defined as consecutively detectable peripheral blood BCR-ABL1 transcripts showing a ≥ 1 log increase or loss of major molecular response (MMR) in any single sample. During the study period, both situations could trigger TKI resumption at the discretion of the attending physician based on the prevailing recommendations at that time.
The study was approved by the Ethics Committee of the Hospital Clínico Universitario of Valencia and registered by the AEMPS (Agencia Española de Medicamentos y Productos Sanitarios), under the reference INC-IMA-2017–01. Informed consent for the inclusion in the study was obtained in accordance with the requirements by local ethics committees.
Statistical analysis
Quantitative data are expressed as median and interquartile range (IQR) and qualitative data as percentages. The non-parametric Skillings–Mack test was used for comparing the laboratory values at baseline and at different time points after treatment discontinuation28. Differences in the distribution of categorical and continuous variables were evaluated by the χ2-test and the Mann–Whitney U-test, respectively.
TFR was estimated by the method of Kaplan–Meier and defined as the time from TKI discontinuation to the date of restarting therapy for any reason or the date of last contact if treatment was not restarted. Incidence of molecular relapse was calculated using the cumulative incidence function with resumption of TKI treatment in the absence of molecular relapse and death in MMR as competing events. For the remaining patients, follow-up was censored at the date of the last molecular monitoring. The following patients’ clinical characteristics were evaluated for their potential relationship with the incidence of molecular relapse: sex, age at discontinuation, Sokal risk score, type of BCR-ABL1 transcript (e13a2 vs. e14a2), history of TKI resistance, number of TKI lines before discontinuation (1 vs. more than one), type of TKI at the time of discontinuation (imatinib vs. others), prior exposure to interferon, aggregated time on TKI treatment, and duration of MR4.5 before discontinuation. Optimal prognostic cutoffs for time on TKI treatment and duration of MR4.5 before discontinuation were selected by receiver-operating characteristic curve analysis of molecular relapse at 1 year. Univariate analyses of factors predicting molecular relapse were done within the framework of competing risks by the method of Fine and Gray29.
All thee performed with IBM SPSS 22.0 (SPSS, Chicago, IL, USA) and Stata 11 (https://www.stata.com).
Results
Patients’ characteristics and clinical outcome
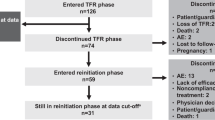
Demographics and treatment history of the whole series is summarized in Table 1. Median age at time of stopping TKI treatment was 61 years (IQR: 52–72) and 123 (52%) patients were females. Sokal risk score at diagnosis was high in only 17 (8%) patients. BCR-ABL transcript was typical in 232 cases (e13a2 = 70, e14a2 = 104, both = 6, p210 but undetermined isoform = 52) and atypical in 4 cases (e14a3 = 1, e13a3 = 1, e1a2 = 2). Frontline TKI treatment consisted of imatinib (n = 217), nilotinib (n = 15), dasatinib (n = 2), or bosutinib (n = 2). Second-line TKI treatment consisted of imatinib (n = 3), nilotinib (n = 22), or dasatinib (n = 27). Third-line TKI treatment was imatinib (n = 3), nilotinib (n = 11), bosutinib (n = 4), or ponatinib (n = 2). At the time of stopping treatment, most patients (74%) were receiving imatinib. All patients were in DMR, but the main reasons for TKI cessation were the presence of side effects or a concomitant disease (n = 66), an attempt to achieve a TFR in the absence of clinically relevant TKI toxicity (n = 166) and pregnancy or planning pregnancy (n = 4).
At the study closing date in 15 May 2018, median follow-up from treatment discontinuation was 21.5 months (IQR: 10–39) and 5 patients had died while in MMR due to CML-unrelated causes. In 67 patients (28%), TKI therapy was reinitiated due to molecular relapse (loss of MMR: n = 52, increase ≥ 1 log in BCR-ABL transcript level at two consecutive assessments without losing MMR: n = 12), patient preference (n = 2), and severe withdrawal syndrome (n = 1). One additional patient lost MMR after 20 months from treatment cessation but decided not to be retreated, with spontaneous recovery of MMR in subsequent determinations. The cumulative incidence of molecular recurrence was 20% (95% confidence interval (CI): 16%–25%) at 6 months, 26% (95% CI: 21%–30%) at 1 year, and 33% (95% CI: 26%–38%) at 3 years (Fig. 1). Forty-nine relapses (75% of total) occurred in the first 6 months, eight between months 7 and 12, and eight later than 12 months. The latest loss of MMR was detected 30 months after TKI discontinuation. One patient restarted treatment 44 months after TKI discontinuation due to ≥ 1 log increase in BCR-ABL1 transcripts in two consecutive positive samples without losing MMR. The probability of TFR at 1 and 4 years was 72.5% (95% CI: 66%–78%) and 64% (95% CI: 55%–72%), respectively (Fig. 2). Among the 164 patients who remained in MMR without treatment, 10 lost MR4.5 (without loss of MR4) and 19 lost MR4 on at least one occasion during the follow-up period. The aggregated time off treatment for the whole series was 4559 months.
Safety and laboratory data after TKI discontinuation
All patients remained in complete hematologic response and none progressed to the advanced phases of CML. At the time of restarting treatment, the median BCR-ABL1 IS was 0.3% (IQR: 0.1–1.17), with this value being > 5% in only seven instances. Most patients (81%) received the same TKI that they were taking before treatment cessation. In 44 of the 52 patients (85%) who lost MMR, treatment was initiated within the first month after detection of the molecular relapse. Median follow-up after treatment resumption was 20 months (IQR: 9–37). Among the 64 patients who restarted treatment due to molecular recurrence, 46 of 52 regained MMR after a median time of 3 months (IQR: 1–5), 50 of 64 regained MR4 after a median time of 3.5 months (IQR: 2–6), and 47 of 64 regained MR4.5 after a median time of 5 months (IQR: 2–8). Six patients did not regain MMR, but the follow-up of five of them was still short (< 3 months). The remaining one was a heart transplantation recipient with chronic renal failure, who was unable to maintain TKI therapy continuously, due to several infectious episodes and pleural effusion. At last control, the response status was as follows: MR4.5 (n = 196), MR4 (n = 15), MMR (n = 14), complete cytogenetic response (n = 10), and other (n = 1).
A total of 51 patients (22%) developed musculoskeletal or joint pain after treatment cessation. In one patient, imatinib had to be reinitiated at low doses due to severe pain and joint deformities in both hands that did not respond to anti-inflammatory drugs.
In 66 patients in DMR, the main reason for TKI cessation was the presence of side effects or a concomitant disease. Among the 62 cases in whom evolutive data were available, a complete resolution of such toxicity was noted in 33 (53%) patients, a partial improvement was seen in 17 (28%) patients, whereas no significant change was observed in the remainder.
We evaluated several hematological and biochemical parameters at baseline and at 3 and 6 months following TKI cessation. At the time of treatment discontinuation, patients on imatinib had on average lower values of Hb, leukocytes, and cholesterol, and higher creatinine levels than patients on nilotinib, although mostly within normal ranges (Table 2). In patients stopping imatinib, a significant increase in the Hb levels, leukocyte counts, total lymphocyte counts, and platelet counts was observed (Fig. 3). At 6 months, increases in Hb levels ≥ 1 g/dL and ≥ 2 g/dL were observed in 51% and 25% of patients, respectively. Among the anemic patients, the median Hb increase was 1.8 mg/dL (IQR: 0.9–2.5), with 72% and 47% of cases experiencing increases in Hb levels ≥ 1 g/dL and ≥ 2 g/dL, respectively. The serum creatinine decreased slightly, whereas cholesterol levels showed a significant increase (Fig. 3). Median increase in total cholesterol level at 6 months was of 29.5 mg/dL (IQR: 12.2–41.7), with 53% of patients with a baseline cholesterol < 200 mg/dL reaching a cholesterol level above such figure. By contrast, discontinuation of nilotinib was not followed by any relevant change in laboratory values, except for a mild decrease in the leukocyte counts (data not shown). Finally, the number of patients who discontinued other TKI was too small to draw any conclusions on this subject.
Predictors of molecular relapse
Table 3 summarizes the analysis of factors associated with increased risk of molecular relapse. As can be seen, shorter periods of both TKI treatment and RM4.5 before TKI discontinuation were significantly associated with increased risk of molecular recurrence. Indeed, the cumulative incidence of relapse at 3 years in patients who had received TKI treatment for more than 5 years was 30% (95% CI: 24%–38%), as compared with 59% (95% CI: 38%–80%) for those treated for a shorter period (Supplemental Figure S1). Patients in sustained MR4.5 for more than 4 years before TKI cessation had a 25% (95% CI: 18%–35%) cumulative incidence of molecular relapse at 3 years, as compared with 46% (95% CI: 34%–59%) for those with a shorter period in MR4.5 (Supplemental Figure S2). Type of TKI at the time of discontinuation (imatinib vs. others) had no effect on the subsequent risk of molecular relapse (subhazard ratio (SHR): 0.77; 95% CI: 0.44–1.33; P = 0.35).
According to Hughes and Ross15, optimal candidates for treatment discontinuation in clinical practice would be those fulfilling all the following criteria: (a) patients in chronic-phase CML, who have a low or intermediate Sokal risk score and a typical BCR-ABL transcript (e13a2 or e14a2); (b) at least 8 years on TKI treatment without history of resistance; and (c) sustained MR4.5 lasting ≥ 2 years. In the present series, 58% of patients fulfilled these criteria as optimal candidates for TKI discontinuation. However, the cumulative incidence of molecular relapse for the optimal candidates did not significantly differ from the “less than optimal” group (SHR: 0.85, 95% CI: 0.47–1.52, P = 0.58; Supplemental Figure S3).
Discussion
In the present study, the safety of TKI discontinuation in clinical practice was evaluated in a nationwide series of 236 CML patients from 33 Spanish centers. Overall, the treatment-free survival was of 64% at 4 years. Most patients who fail to maintain TFR regained a DMR after 3–5 months of restarting treatment. No case of disease progression was documented. These data confirm the applicability of this treatment strategy outside of clinical trials in Spain.
In fact, the results from the current series compare favorably with the ~50% TFR rate reported in most clinical trials on TKI discontinuation10,11,12,13. Moreover, in 18% of our patients the reason for treatment resumption was an increase in BCR-ABL1 transcript levels without losing MMR. It is currently known that a proportion of such cases, particularly those presenting an increase in BCR-ABL1 transcript levels after the first 6 months of stopping treatment (i.e., “the late molecular relapses”)12,17 could have spontaneously regained DMR without therapy. However, this information was not available at the time of TKI cessation in our earlier cases, whereas in other instances the decision of resuming treatment before MMR loss might reflect the cautious approach of the attending physicians to prevent any complication related to stopping TKI therapy outside of a clinical trial. Nevertheless, the favorable TFR of the current series likely derives from the prolonged exposure to TKI treatment (median ~10 years) and the sustained duration in DMR (median ~5 years) before TKI discontinuation in our patients, which largely exceeded the minimal thresholds of clinical trials. Actually, these two variables may be the most important factors affecting the probability of molecular relapse-free survival27. In the recently published EURO-SKI trial13, the probability of maintaining MMR at 6 months was of 61% in imatinib-treated patients with DMR duration of more than 3.1 years, whereas it was of 44% only in those with a shorter period in DMR.
Strict molecular monitoring and a swift resumption of TKI treatment upon detection of molecular relapse are essential for the safety of the discontinuation strategy in clinical practice26. To this respect, Spain has an extensive network of certified laboratories for BCR-ABL1 molecular monitoring in the public health system, where the great majority of CML patients are managed. In our series, most patients resumed TKI treatment within the first month after detection of MMR loss. Occasionally, treatment initiation was delayed due to an ongoing pregnancy. Of note, the BCR-ABL1 transcript levels at the time of restarting treatment were above the threshold of 5% IS in only 7 cases, with this suggesting that an adequate monitoring was performed in the setting of common clinical practice. Indeed, disease resistance was not an issue in patients who required TKI therapy due to molecular relapse, in line with published data from clinical trials14.
Several findings on the evolution of the laboratory parameters after TKI discontinuation deserve to be mentioned. At the time of treatment cessation, the Hb levels and the leukocyte counts were significantly lower in patients receiving imatinib than in those on nilotinib treatment, with this suggesting a stronger myelosuppressive effect of the former. In fact, a dose-dependent inhibitory effect of imatinib on normal progenitor cells has experimentally been demonstrated in vitro30 and in vivo31. In a previous study including CML patients who were in DMR with imatinib 400 mg daily, a dose reduction to 300 mg daily resulted in a median Hb increase of 1 g/dL among the anemic patients32. Our present data confirmed that the myelosuppressive effect of long-term treatment with imatinib is reversible, as patients stopping this drug experienced a significant increase in all hematologic values33. In particular, the increase in Hb levels observed in anemic patients at 6 months from imatinib discontinuation (≥ 1 g/dL and ≥ 2 g/dL in 72% and 47% of patients, respectively) might be of clinical significance, as chronic fatigue has been identified as the most important factor limiting health-related quality of life in patients on long-term therapy with imatinib34. By contrast, nilotinib withdrawal did not appear to have any major impact on the hematologic values. With regard to the biochemical parameters, patients who stopped imatinib had higher serum creatinine levels at baseline, but experienced a significant reduction in creatinine levels afterwards, in line with previous reports suggesting that imatinib can decrease glomerular filtration35,36. On the other hand, it is well recognized the early onset hypercholesterolemia induced by nilotinib37, with such effect being potentially involved in the pathogenesis of the occlusive arterial events associated with this drug38. Current guidelines recommend a proactive approach towards controlling the hyperlipidemia induced by nilotinib with lifestyle interventions and lipid-lowering therapies8. Despite such recommendations, total cholesterol levels at the time of TKI discontinuation were significantly higher in nilotinib-treated patients than in those receiving imatinib (median difference ~20 mg/dL). Such situation changed completely after TKI treatment discontinuation, as cholesterol levels significantly increased following imatinib withdrawal. As a result of this tendency, at 6 months after treatment discontinuation patients who stopped imatinib ended up with total cholesterol levels that were significantly higher than those of patients stopping nilotinib. In this sense, plasma lipid levels have been reported to normalize within one month of imatinib treatment in small case series39. Imatinib has also been shown to delay the development of atherosclerosis in experimental models40 and to induce the regression of type 2 diabetes mellitus41. Consequently, the potential influence of cessation of imatinib treatment in the long-term risk of cardiovascular events needs to be evaluated in future studies.
Several experts’ recommendations14,15,16,25,26 and formal guidelines18,27 have recently been published focusing on the selection of candidates for TKI discontinuation in clinical practice. In general, there is consensus on the need to define a minimum duration of TKI exposure and DMR before treatment discontinuation is to be attempted. However, significant differences in the thresholds for the minimal duration of TKI exposure have been proposed, ranging from 3 to 8 years. In our series, we observed that shorter periods of both TKI treatment and MR4.5 before TKI discontinuation were significantly associated with increased risk of molecular recurrence, with the best cutoffs for discrimination being at 5 years and 4 years, respectively. Despite that, the predictive value of such clinical factors to accurately identify non-relapsing patients at the individual level was poor. Moreover, when we compared the incidence of molecular relapse in optimal candidates for TKI treatment discontinuation according to the criteria from Hughes and Ross15 (58% of patients in our series) with that of the remaining patients, no difference was observed. In line with other authors16, we think that the emphasis on defining minimal criteria for discontinuation TKI therapy in clinical practice should not overlook what rate of TFR would be acceptable for each individual patient, depending on the specific TKI-related risks and patient preferences.
In conclusion, the present results contribute to reassure the safety of TKI treatment discontinuation in real-life clinical practice, under close molecular monitoring. Resolution of TKI-related toxicity might translate into a potential health improvement for the patients. Special attention should be paid to the increase in cholesterol levels observed after imatinib discontinuation. Finally, further studies addressing the biological factors underlying the mechanisms of disease control after TKI treatment cessation are warranted.
References
Bower, H. et al. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J. Clin. Oncol. 34, 2851–2857 (2016).
Hochhaus, A. et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N. Engl. J. Med. 376, 917–927 (2017).
Branford, S. et al. Early molecular response and female sex strongly predict stable undetectable BCR-ABL1, the criteria for imatinib discontinuation in patients with CML. Blood 121, 3818–3824 (2013).
Hochhaus, A. et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 30, 1044–1054 (2016).
Cortes, J. E. et al. Final 5-year study results of DASISION: the Dasatinib versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients Trial. J. Clin. Oncol. 34, 2333–2340 (2016).
Chu, S. et al. Persistence of leukemia stem cells in chronic myelogenous leukemia patients in prolonged remission with imatinib treatment. Blood 118, 5565–5572 (2011).
Chomel, J. C. et al. Leukemic stem cell persistence in chronic myeloid leukemia patients with sustained undetectable molecular residual disease. Blood 118, 3657–3660 (2011).
Steegmann, J. L. et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia 30, 1648–1671 (2016).
The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood 121, 4439–4442 (2013).
Etienne, G. et al. Long-term follow-up of the French stop imatinib (STIM1) study in patients with chronic myeloid leukemia. J. Clin. Oncol. 35, 298–305 (2017).
Ross, D. M. et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood 122, 515–522 (2013).
Rousselot, P. et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J. Clin. Oncol. 32, 424–430 (2014).
Saussele, S. et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 19, 747–757 (2018).
Saussele, S., Richter, J., Hochhaus, A. & Mahon, F. X. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia 30, 1638–1647 (2016).
Hughes, T. P. & Ross, D. M. Moving treatment-free remission into mainstream clinical practice in CML. Blood 128, 17–23 (2016).
Laneuville, P. When to stop tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. Curr. Treat. Options Oncol. 19, 15 (2018).
Rea, D. & Mahon, F. X. How I manage relapse of chronic myeloid leukaemia after stopping tyrosine kinase inhibitor therapy. Br. J. Haematol. 180, 24–32 (2018).
Hochhaus, A. et al. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 28, iv41–iv51 (2017).
Mahon, F. X. & Etienne, G. Deep molecular response in chronic myeloid leukemia: the new goal of therapy? Clin. Cancer Res. 20, 310–322 (2014).
Deininger, M. W. Molecular monitoring in CML and the prospects for treatment-free remissions. Hematol. Am. Soc. Hematol. Educ. Program. 2015, 257–263 (2015).
Benjamini, O. et al. Patient-driven discontinuation of tyrosine kinase inhibitors: single institution experience. Leuk. Lymphoma 55, 2879–2886 (2014).
Kong, J. H. et al. Does the frequency of molecular monitoring after tyrosine kinase inhibitor discontinuation affect outcomes of patients with chronic myeloid leukemia? Cancer 123, 2482–2488 (2017).
Takahashi, N. et al. Discontinuation of imatinib in Japanese patients with chronic myeloid leukemia. Haematologica 97, 903–906 (2012).
Ferrero, D. et al. How many patients can proceed from chronic myeloid leukaemia diagnosis to deep molecular response and long-lasting imatinib discontinuation? A real life experience. Br. J. Haematol. 176, 669–671 (2017).
Shah, N. P. Front-line treatment options for chronic-phase chronic myeloid leukemia. J. Clin. Oncol. 36, 220–224 (2018).
Mahon, F. X. Treatment-free remission in CML: who, how, and why? Hematol. Am. Soc. Hematol. Educ. Program. 2017, 102–109 (2017).
Rea, D. et al. Discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia: recommendations for clinical practice from the French Chronic Myeloid Leukemia Study Group. Cancer 124, 2956–2963 (2018).
Skillings, J. H. & Mack, G. A. On the use of a Friedman-type statistic in balanced and unbalanced block designs. Technometrics 23, 171–177 (1981).
Fine, J. P. & Gray, R. J. A proportional hazards model for the subdistribution of competing risks. J. Am. Stat. Assoc. 94, 496–509 (1999).
Bartolovic, K. et al. Inhibitory effect of imatinib on normal progenitor cells in vitro. Blood 103, 523–529 (2004).
Agis, H. et al. In vivo effects of imatinib mesylate on human haematopoietic progenitor cells. Eur. J. Clin. Invest. 36, 402–408 (2006).
Cervantes, F. et al. Imatinib dose reduction in patients with chronic myeloid leukemia in sustained deep molecular response. Ann. Hematol. 96, 81–85 (2017).
Park J. S., et al. Change of health-related profiles after Imatinib cessation in chronic phase chronic myeloid leukemia patients. Leuk. Lymphoma 57, 341–357 (2016).
Efficace, F. et al. Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia 27, 1511–1519 (2013).
Marcolino, M. S. et al. Imatinib treatment duration is related to decreased estimated glomerular filtration rate in chronic myeloid leukemia patients. Ann. Oncol. 22, 2073–2079 (2011).
Yilmaz, M. et al. Estimated glomerular filtration rate changes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. Cancer 121, 3894–3904 (2015).
Rea, D. et al. Early onset hypercholesterolemia induced by the 2nd-generation tyrosine kinase inhibitor nilotinib in patients with chronic phase-chronic myeloid leukemia. Haematologica 99, 1197–1203 (2014).
Valent, P. et al. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood 125, 901–906 (2015).
Gottardi, M., Manzato, E. & Gherlinzoni, F. Imatinib and hyperlipidemia. N. Engl. J. Med. 353, 2722–2723 (2005).
Lassila, M. et al. Imatinib attenuates diabetes-associated atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 24, 935–942 (2004).
Veneri, D., Franchini, M. & Bonora, E. Imatinib and regression of type 2 diabetes. N. Engl. J. Med. 352, 1049–1050 (2005).
Acknowledgements
We are indebted to all members of GELMC for participating in the present study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
41408_2018_125_MOESM4_ESM.tif
Figure S3 Cumulative incidence of molecular relapse in optimal candidates for TKI discontinuation in clinical practice (as defined by Hughes & Ross) compared to “less than optimal” candidates
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hernández-Boluda, J.C., Pereira, A., Pastor-Galán, I. et al. Feasibility of treatment discontinuation in chronic myeloid leukemia in clinical practice: results from a nationwide series of 236 patients. Blood Cancer Journal 8, 91 (2018). https://doi.org/10.1038/s41408-018-0125-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-018-0125-0
This article is cited by
-
Late relapse of chronic myeloid leukemia after allogeneic bone marrow transplantation points to KANSARL (KANSL1::ARL17A) alteration: a case report with insights on the molecular landscape
Annals of Hematology (2024)
-
Real-world therapeutic response and tyrosine kinase inhibitor discontinuation in chronic phase-chronic myeloid leukemia: data from the French observatory
Annals of Hematology (2022)
-
RNA-binding protein IGF2BP1 maintains leukemia stem cell properties by regulating HOXB4, MYB, and ALDH1A1
Leukemia (2020)
-
Treatment-free remission in chronic myeloid leukemia: the patient perspective and areas of unmet needs
Leukemia (2020)
-
Tyrosine Kinase Inhibitor Therapy Discontinuation for Patients with Chronic Myeloid Leukaemia in Clinical Practice
Current Hematologic Malignancy Reports (2019)