Abstract
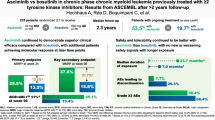
Asciminib is approved for patients with Philadelphia chromosome–positive chronic-phase chronic myeloid leukemia (CML-CP) who received ≥2 prior tyrosine kinase inhibitors or have the T315I mutation. We report updated results of a phase 1, open-label, nonrandomized trial (NCT02081378) assessing the safety, tolerability, and antileukemic activity of asciminib monotherapy 10–200 mg once or twice daily in 115 patients with CML-CP without T315I (data cutoff: January 6, 2021). After ≈4-year median exposure, 69.6% of patients remained on asciminib. The most common grade ≥3 adverse events (AEs) included increased pancreatic enzymes (22.6%), thrombocytopenia (13.9%), hypertension (13.0%), and neutropenia (12.2%); all-grade AEs (mostly grade 1/2) included musculoskeletal pain (59.1%), upper respiratory tract infection (41.7%), and fatigue (40.9%). Clinical pancreatitis and arterial occlusive events (AOEs) occurred in 7.0% and 8.7%, respectively. Most AEs occurred during year 1; the subsequent likelihood of new events, including AOEs, was low. By data cutoff, among patients without the indicated response at baseline, 61.3% achieved BCR::ABL1 ≤ 1%, 61.6% achieved ≤0.1% (major molecular response [MMR]), and 33.7% achieved ≤0.01% on the International Scale. MMR was maintained in 48/53 patients who achieved it and 19/20 who were in MMR at screening, supporting the long-term safety and efficacy of asciminib in this population.

Similar content being viewed by others
Introduction
Tyrosine kinase inhibitors (TKIs) targeting the ABL and BCR::ABL1 adenosine triphosphate (ATP) binding sites have significantly extended the lives of patients with chronic myeloid leukemia (CML) [1,2,3,4,5]. However, intolerance and resistance to ATP-competitive TKIs, resulting in decreased quality of life and increased risk of progressive disease (PD), remain challenges [3,4,5,6]. ATP-competitive TKIs may have off-target effects from lack of specificity for BCR::ABL1 that can be associated with long-term safety risks and treatment discontinuation [1, 7,8,9,10,11,12,13]. Treatment resistance may result from emergent BCR::ABL1 mutations that are sensitive to only specific TKIs, including the T315I mutation, which confers resistance to almost all approved TKIs [2,3,4, 14].
As patients without satisfactory treatment outcomes advance through successive lines of TKIs, treatment failure rates increase, survival rates decrease [13, 15, 16], and many do not achieve optimal responses [1, 2, 10, 17,18,19,20,21,22,23,24]. New treatment options with improved antileukemic activity and long-term tolerability are needed for patients with resistance or intolerance to multiple TKIs.
Asciminib is the first BCR::ABL1 inhibitor that specifically targets the ABL myristoyl pocket (STAMP), allosterically restoring inhibition of the ABL1 kinase [25,26,27,28]. Unlike ATP-competitive TKIs, asciminib maintains activity against most ATP-binding site mutations, including T315I [25,26,27]. Asciminib’s target selectivity and specificity were predicted to minimize off-target effects, potentially reducing AEs in patients requiring long-term therapy [25,26,27, 29]. Its high potency may also drive rapid, sustained, and deeper molecular responses [25,26,27]; in the ASCEMBL trial, deeper, faster responses were achieved with asciminib versus bosutinib [28, 30]. Asciminib was approved in the US in 2021, with subsequent approvals worldwide, and was added to National Comprehensive Cancer Network guidelines as a new option for adults with Philadelphia chromosome–positive (Ph+) chronic-phase CML (CML-CP) previously treated with ≥2 TKIs or who have the T315I mutation [2, 31]. This approval was supported by results from the randomized phase 3 ASCEMBL trial (NCT03106779) and cohorts of patients in the current phase 1 trial (NCT02081378) receiving asciminib monotherapy, including heavily pretreated patients with Ph+ CML-CP/accelerated phase (AP) with or without T315I [28, 29, 31].
Prior analysis from this phase 1 trial after a median follow-up of 14 months first provided safety and efficacy data for asciminib in patients with CML-CP/AP with or without T315I [29]. Among patients with CML-CP without T315I, major molecular response (MMR; BCR::ABL1 ≤ 0.1% on the International Scale [IS]) was achieved or maintained by 12 months in 44 of 91 evaluable patients (48%) (median response duration, >61 weeks), whereas 4 patients lost MMR (1 with MMR at baseline). Of 51 patients, 14 (27%) with BCR::ABL1IS > 1% at baseline achieved MMR by 12 months. Updated results from a cohort of patients with T315I-mutated CML-CP will be reported separately. Here we report updated safety and efficacy results from this trial in patients with CML-CP without T315I who were treated with asciminib monotherapy over a median duration of exposure of ≈4 years.
Methods
Study oversight
The study was designed collaboratively by the sponsor (Novartis Pharmaceuticals) and study investigators. The sponsor collected and analyzed data in conjunction with the authors. All authors contributed to the development and writing of the manuscript and vouch for the accuracy and completeness of the data and the study’s fidelity to the protocol.
Study design
The methods of this open-label, nonrandomized, first-in-human study of asciminib (Supplementary Fig. S1) have been described in detail elsewhere [29]. This analysis focused on the cohort of patients (n = 115) with Ph+ CML-CP without T315I at screening who received asciminib monotherapy at varying doses twice daily (10–200 mg) or once daily (80–200 mg) in the dose-escalation or -expansion parts of this study. Patients were ≥18 years of age; had hematologic, cytogenetic, or molecular evidence of disease that was relapsed or refractory to ≥2 prior TKIs; or were intolerant of ≥2 prior TKIs (per European LeukemiaNet 2009 recommendations [32]).
The primary objective was to determine the maximum tolerated dose and/or recommended dose for the expansion of asciminib monotherapy. Secondary objectives included assessing safety, tolerability, preliminary antileukemic activity, and pharmacokinetic profile in plasma of asciminib. Additional details are in the Supplementary Methods.
Study assessments
AEs were coded using the Medical Dictionary for Regulatory Activities version 23.1 and graded according to the Common Terminology Criteria for Adverse Events version 4.03. Molecular response was assessed using real-time, quantitative, reverse-transcriptase polymerase chain reaction. Results were reported as the ratio of BCR::ABL1 to ABL1 on the IS [33]. BCR::ABL1 mutational analyses were performed using Sanger sequencing. Molecular and mutational assessments were performed centrally by ICON (Portland, OR, USA).
Statistical analyses
The data cutoff date was January 6, 2021, and all patients who received ≥1 study drug dose were included. The MMR rate by each time point was defined as the proportion of MMR-evaluable patients (i.e., those not in MMR and without atypical BCR::ABL1 transcripts at screening) who had achieved MMR by that time point. Rates of BCR::ABL1IS ≤ 1% or deep molecular response (DMR; MR4 [BCR::ABL1IS ≤ 0.01%] and MR4.5 [BCR::ABL1IS ≤ 0.0032%]) were calculated similarly. Event-free survival (EFS) was estimated using the Kaplan–Meier method, with events defined as treatment discontinuation due to AEs, on-treatment progression to AP/blast crisis (BC), and on-treatment death for any reason. An EFS analysis that included BCR::ABL1IS > 10% at 6 months and >1% at ≥12 months as events is reported in the Supplementary Material. Progression-free survival (PFS) and overall survival (OS) were not analyzed because patients were not followed after treatment discontinuation.
Results
Patients
This analysis included 115 patients with CML-CP without T315I who were enrolled in the study (May 2014–October 2019) and received asciminib monotherapy (Fig. 1A, Supplementary Table S1 and Supplementary Fig. S2). At data cutoff, most patients (n = 80 [69.6%]) remained on study treatment; 35 patients (30.4%) had discontinued, most frequently due to AEs (n = 13) or physician decision (n = 8; mostly due to lack of efficacy). The overall median duration of exposure was 4.2 years (range, 0.04–6.55 years), and ranged from 3.8 to 4.8 years depending on the treatment line (Supplementary Fig. S3); 99 patients (86.1%) were exposed for ≥48 weeks and 88 (76.5%) for ≥96 weeks. Two patients (1.7%) died on treatment (defined as on treatment or within 30 days of the last study drug dose), and deaths were unrelated to asciminib per investigator assessment (Fig. 1A and Supplementary Material): 1 patient died due to cardiac arrest and had a history of suspected contributory comorbidities (e.g., systemic scleroderma and ischemic heart disease), and another patient with a history of bladder cancer and urostomy died due to worsening of general physical condition.
A Disposition of patients with CML-CP without BCR::ABL1 T315I mutations who received asciminib monotherapy. B History of prior TKIs. AE adverse event, CML chronic myeloid leukemia, CP chronic phase, IS International Scale, MMR major molecular response (BCR::ABL1 ≤ 0.1% on the IS), MR4 BCR::ABL1IS ≤ 0.01%, MR4.5 BCR::ABL1IS ≤ 0.0032%, TKI tyrosine kinase inhibitor.
Patients were heavily pretreated; the majority (71.3%) received ≥3 prior TKIs (Table 1). Three patients (2.6%) who were positive for T315I on enrollment had received one prior TKI, per eligibility criteria; mutation was not confirmed by the central laboratory. Imatinib was the first-line TKI for 56% of patients (Fig. 1B); dasatinib (38%) and nilotinib (34%) were the most frequent second-line therapies. TKI use patterns were more complex in the third line and beyond. Additional baseline demographics are in Supplementary Table S2. At screening, BCR::ABL1 mutations were detected in 12 patients (10.4%), with 2 (1.7%) having multiple mutations; 29 patients (25.2%) were not evaluable (n = 10) or had low levels of BCR::ABL1 leading to lack of amplification (n = 19) (Table 1 and Supplementary Table S3).
Safety
Treatment-emergent all-grade AEs regardless of relationship to the study drug were reported in all patients (Table 2); those reported in ≥20% were musculoskeletal pain, upper respiratory tract infection, fatigue, increased pancreatic enzymes (mostly asymptomatic, as reported in detail below), abdominal pain, arthralgia, headache, diarrhea, nausea, hypertension, rash, vomiting, thrombocytopenia, pruritus, increased hepatic enzymes, and dizziness. Grade ≥3 AEs reported in ≥10% of patients were increased pancreatic enzymes, thrombocytopenia, hypertension, and neutropenia.
A side-by-side comparison of the first-ever occurrence of any-grade AEs (incidence) versus the persistence, recurrence, and late onset of any-grade AEs (first ever, recurring, and ongoing [prevalence]) by year shows that most AEs occurred early after treatment initiation; the likelihood of newly occurring events after the first year of treatment was low (Fig. 2). All instances of newly occurring thrombocytopenia and nearly all instances of newly occurring neutropenia, anemia, increased lipase, increased amylase, and dyslipidemia were reported in the first year. Upper respiratory tract infections and constitutional events such as musculoskeletal pain and fatigue were the only events to occur at later timepoints, were mostly grade 1/2, and did not lead to treatment discontinuation.
Percentages are calculated based on the number of patients at risk of an event (left column: patients with ongoing treatment who were event free at the start of the interval; right column: patients with ongoing treatment at the start of the interval). In the left column (incidence), the number of patients at risk of an event differs from year to year, and percentages in each year should thus not be summed. A patient with multiple occurrences of an event within the same time interval was counted only once in that time interval. AE adverse event, URTI upper respiratory tract infection.
AEs led to treatment discontinuation in 13 patients (11.3%) (increased lipase, n = 4; increased amylase, pancreatitis, and thrombocytopenia, n = 2 each; all other AEs, n = 1 each) (Supplementary Table S4). In 69 patients (60.0%), AEs could be managed by dose interruption or adjustment per protocol.
Clinically important safety information (Supplementary Methods), grouped into categories, is reported in Table 3 and Supplementary Materials. The two most frequently reported categories were gastrointestinal (GI) toxicity (72.2%) and hypersensitivity (44.3%). The most frequent GI events (≥20% of patients) were abdominal pain (37.4%), diarrhea (30.4%), nausea (28.7%), and vomiting (26.1%). Grade 3 GI events were reported in 5 patients (4.3%) and did not require treatment discontinuation; dose adjustments and interruptions occurred in 7 (6.1%) and 9 (7.8%) patients, respectively. Most hypersensitivity events were low-grade skin conditions such as rash (27.8%) and urticaria (5.2%); grade 3 events occurred in 4 patients (3.5%) only. Treatment discontinuation was required in 1 patient (0.9%) (bronchospasm and rash) and dose adjustments and interruptions in 1 (0.9%) and 3 (2.6%) patients, respectively. No grade 4 events were reported.
Pancreatic enzyme elevations were reported in 46 patients (40.0%) (grade 3, n = 23 [20.0%]; grade 4, n = 3 [2.6%]). The majority were asymptomatic and included increased lipase (n = 43 [37.4%]; grade 3, n = 21 [18.3%]; grade 4, n = 2 [1.7%]) and increased amylase (n = 22 [19.1%]; grade 3, n = 4 [3.5%]; grade 4, n = 1 [0.9%]). Pancreatic enzyme elevations led to treatment discontinuation in 4 patients (3.5%) (increased lipase, n = 4; increased amylase, n = 2) and were managed by dose adjustment in 13 (11.3%) and treatment interruption in 14 (12.2%).
Both early- and late-onset clinical pancreatitis events were reported in 8 patients (7.0%) (grade 3, n = 4 [3.5%]; grade 4, n = 0) (Supplementary Table S5 and Supplementary Fig. S4) and led to treatment discontinuation in 2 patients (1.7%) (grade 2, n = 1; grade 3, n = 1); 1 had a prior history of pancreatic steatosis and pancreatic enzyme elevations and the other had late-onset (day 505) pancreatitis. Pancreatic enzyme levels were not available for 2 patients at the time of pancreatitis events, based on investigator reporting (Supplementary Fig. S4D, E). Pancreatitis was managed by dose adjustment (n = 3 [2.6%]) and/or interruption (n = 4 [3.5%]) and resolved in all patients except one (persistent grade 2 with only radiologic findings) who had a history of acute pancreatitis and had not experienced improvement by the time of treatment discontinuation due to PD.
The most frequently reported myelosuppression events (≥10% of patients) were thrombocytopenia, anemia, and neutropenia. Thrombocytopenia was reported in 29 patients (25.2%) (grade 3, n = 2 [1.7%]; grade 4, n = 14 [12.2%]) and was associated with bleeding events in 7, including mild contusion, melena, petechiae, and grade 3 epistaxis and hematemesis (n = 1 each). Anemia was reported in 20 patients (17.4%), with half being grade 1 (n = 4 [3.5%]) or 2 (n = 6 [5.2%]) and the other half being grade 3. Neutropenia was reported in 19 patients (16.5%) (grades 3 and 4, n = 7 [6.1%] each) and was associated with infection in 2 patients (pneumonia and conjunctivitis, n = 1 each).
Treatment discontinuation for myelosuppression events occurred in 2 patients (1.7%); both had a previous history of and discontinued due to thrombocytopenia. These events were otherwise managed by dose adjustment (thrombocytopenia, n = 5 [4.3%]; anemia, n = 0; neutropenia, n = 3 [2.6%]) or treatment interruption (thrombocytopenia, n = 8 [7.0%]; anemia, n = 1 [0.9%]; neutropenia, n = 4 [3.5%]). Myelosuppression-related events tended to occur on treatment initiation and rarely persisted or occurred at later timepoints (Fig. 2). Of note, no dose-related trends were observed in myelosuppression or pancreatic events.
Ten patients (8.7%) experienced arterial occlusive events (AOEs) (Supplementary Table S6). Grade 1/2 angina pectoris was reported in 4 patients (3.5%) and resolved in all (1 requiring dose interruption and 2 requiring concomitant medication). Grade 3 events were reported in 5 patients (myocardial infarction, n = 2; myocardial ischemia, n = 1; coronary artery disease, n = 1; peripheral arterial occlusive disease and arterial bypass occlusion, n = 1). No grade 4 AOEs occurred. Of the 10 patients with AOEs, 8 had prior exposure to dasatinib, 6 to nilotinib, 5 to bosutinib, and 1 to ponatinib. Most had ≥1 baseline cardiovascular (CV) risk factor, including history of hyperlipidemia (n = 8), hypertension (n = 6), obesity (n = 4), and prior AOEs (n = 4); smoking status was not collected. No treatment discontinuations occurred due to AOEs; dose adjustments or interruptions occurred in 3 (2.6%) and 4 (3.5%) patients, respectively. One patient who had a myocardial ischemia event died due to cardiac arrest (unrelated to asciminib; see “Patients” and Supplementary Materials).
Seven patients (6.1%) had cardiac failure–related events (grade 3, n = 4 [3.5%]; grade 4, n = 1 [0.9%]), including 2 who also experienced AOEs (Supplementary Table S7); no treatment discontinuations occurred due to these events, and treatment interruption occurred in 3 patients (2.6%). Of the 7 patients, 5 had received 4 prior TKIs (previous ponatinib, n = 1); most had ≥1 baseline CV risk factor, including hyperlipidemia (n = 5), hypertension (n = 5), obesity (n = 3), and prior cardiac conditions (n = 4).
Efficacy
Of 115 patients, 9 had atypical or unknown transcripts (BCR::ABL1IS could not be determined) and were excluded from all molecular response analyses. After excluding 20 additional patients who were in MMR at screening, 86 who were evaluable remained (BCR::ABL1IS > 0.1% at screening) for analysis of cumulative MMR. Of these 86, 53 (61.6%) achieved MMR by data cutoff (Table 4 and Supplementary Fig. S5). Most responses were achieved by week 48; however, the cumulative MMR rate continued to increase over time, with the first MMR being attained by up to week 228 of treatment (median time to MMR based on time-to-event analysis, 132 weeks; 95% CI, 96–206 weeks). Cumulative MMR rates ranged from 52.5% to 75.0% in patients receiving asciminib in the third, fourth, or later lines (Fig. 3A). There was no obvious correlation between MMR rate and treatment line; similar trends of increasing response over time were observed. MMR rates were similar between patients with BCR::ABL1IS > 0.1% to 1% (87.5%) and BCR::ABL1IS > 1% to 10% at screening (81.0%) (Fig. 3B); half of the patients with BCR::ABL1IS > 0.1% to 10% at screening achieved MMR by week 72, with additional responses reported at later timepoints. Of 41 patients with BCR::ABL1IS > 10% at screening, 15 (36.6%) achieved MMR by data cutoff; approximately half (n = 8) achieved MMR by week 72.
Cumulative rate of A MMR by number of lines of prior TKI therapy, B MMR by BCR::ABL1IS level at screening, and C BCR::ABL1IS ≤ 1% by BCR::ABL1IS level at screening. Treatment discontinuations for any reason were treated as competing events. BCR::ABL1IS BCR::ABL1 on the International Scale, L line of asciminib treatment (e.g., 3L indicates patients who were treated with asciminib in the third line), MMR major molecular response (BCR::ABL1IS ≤ 0.1%), TKI tyrosine kinase inhibitor.
Of 53 patients who achieved MMR, 5 lost MMR by data cutoff, while 48 maintained MMR or achieved deeper responses. Of 20 patients who were in MMR at screening, the majority maintained or achieved deeper responses, and only 1 lost MMR by data cutoff. The Kaplan–Meier estimated rate of durable MMR at 96 weeks was 94% (95% CI, 86.4%–100.0%) among patients not in MMR at screening who achieved MMR at any time and 94.4% (95% CI, 84.4%–100%) among patients in MMR at screening. All, except 1 of the 6 patients who lost MMR, received asciminib in the fourth line or later (Supplementary Fig. S3). By data cutoff, 2 of these 6 patients had discontinued treatment after loss of MMR (1 due to AE and 1 due to lack of efficacy).
Sixty-two patients were evaluable (BCR::ABL1IS > 1% at screening) in the analysis of cumulative BCR::ABL1IS ≤ 1%. Thirty-eight (61.3%) achieved BCR::ABL1IS ≤ 1% by data cutoff, with most achieving this response by week 24 (Table 4 and Supplementary Fig. S5). Of 21 patients with BCR::ABL1IS > 1% to 10% at screening, nearly all (n = 19 [90.5%]) achieved BCR::ABL1IS ≤ 1% by week 24 (Fig. 3C). Of 41 patients with BCR::ABL1IS > 10% at screening, 19 (46.3%) achieved BCR::ABL1IS ≤ 1% by data cutoff.
There were 101 and 105 patients who had BCR::ABL1IS > 0.01% and >0.0032% at screening, respectively, and were thus evaluable for analyses of cumulative MR4 and MR4.5, respectively. Nearly one-third of these patients achieved DMR (MR4, n = 34 [33.7%]; MR4.5, n = 32 [30.5%]) by data cutoff, with most responses achieved by week 36 (Table 4 and Supplementary Fig. S5). The cumulative MR4.5 rate showed similar trends regardless of treatment line (Supplementary Fig. S3). Of 41 patients who had BCR::ABL1IS > 10% at screening, 3 achieved MR4.5 by data cutoff (1 received third-line asciminib; 2 received fourth-line asciminib).
The Kaplan–Meier estimated EFS rate at 96 weeks was 87% (95% CI, 80%–93%), and median time to EFS was not reached (Fig. 4). Of 12 patients with BCR::ABL1 mutations detected at screening, 6 achieved MMR by data cutoff (Supplementary Table S3). Four of these 12 (2 of whom achieved MMR) had additional treatment-emergent mutations (M244V, V289I, and myristoyl-pocket mutations G463S, V468F, and I502L) detected post screening; 3 of the 4 discontinued treatment (AE, n = 1; PD, n = 2). One patient with no mutations at screening had a newly emerged myristoyl-pocket mutation (G463D) detected post screening, did not achieve MMR, and discontinued treatment (physician decision/lack of efficacy). Details of these cases are reported in the Supplementary Materials.
A EFS for patients overall. B EFS by BCR::ABL1IS at screening. EFS was estimated using the Kaplan–Meier method, with treatment discontinuation due to AEs, on-treatment progression to AP/BC, and on-treatment death for any reason considered as events. Survival data were not collected after patients discontinued the study. AE adverse event, AP accelerated phase, BC blast crisis, EFS event-free survival.
Discussion
Updated analysis of this phase 1 trial in heavily pretreated patients with CML-CP without T315I demonstrates the continued safety, tolerability, and substantial, durable efficacy of asciminib; with a median exposure of ≈4 years, most patients (69.6%) remained on asciminib. No new safety signals arose in this patient population. A significant proportion of patients achieved MMR or DMR, and very few lost MMR. The cumulative rate of MMR and DMR continued to increase with additional patients achieving responses even 3 years after initiation of treatment. Discontinuation due to PD (6.1%) and AEs (11.3%) occurred infrequently; 2 patients died on treatment, unrelated to asciminib. In studies of other TKIs (including nilotinib, dasatinib, bosutinib, and ponatinib) in heavily pretreated patients, 24%–62% remained on treatment [9, 10, 17, 19, 21, 22, 24, 34,35,36,37], including 24% to ≈56% in bosutinib studies [9, 24] and 33%–53% in ponatinib studies [10, 37].
The most frequently reported AEs grouped by common pathophysiological features were GI toxicity (72.2%), musculoskeletal pain (59.1%), hypersensitivity (mainly dermatologic events [44.3%]), and upper respiratory tract infection (41.7%); these events were generally mild and manageable. AEs (including hematologic) generally occurred early after treatment initiation, a pattern also observed in the phase 3 ASCEMBL trial, where most first-ever AEs (including hematologic) occurred in the first 6 months [30]. Myelosuppression overall was reported in one-third of patients (34.8%); these events mainly occurred early, particularly thrombocytopenia (25.2%), which was reported only in the first year. The short latency period suggests potent suppression of the leukemic clone with incomplete recovery and/or both disease- and therapy-related inhibition of nonleukemic hematopoiesis [38].
Pancreatic toxicity, including pancreatic enzyme elevations, is a broad safety concern for patients with CML receiving TKIs [2, 11, 39]. The pancreas was identified as a target organ of asciminib toxicity in dogs (but not rats and monkeys) [40]. However, the risk factors and mechanism of pancreatic toxicity with TKIs targeting ABL, including asciminib, are unknown. In this long-term clinical setting, events were mainly asymptomatic pancreatic enzyme elevations (40%) and generally manageable with dose adjustments, leading to discontinuation in only 3.5% of patients. Clinical pancreatitis was reported in 8 patients (7.0%) and led to discontinuation in 2 (1.7%). Of note, no cases of pancreatitis were reported in ASCEMBL, and grade ≥3 increased lipase occurred in only 3.8% of patients receiving asciminib [28]. Regular monitoring of pancreatic function is recommended during asciminib treatment, with dose interruption or reduction as needed.
CV toxicity is observed with all ABL kinase inhibitors, including ponatinib, and warrants a personalized approach for optimal CV risk management in patients with CML [41, 42]. Due to multiple confounding factors, including heavy pretreatment with TKIs that potentially exacerbated or led to the development of baseline CV risk factors in patients experiencing AOEs (8.7%) or cardiac failure events (6.1%) in this analysis, the causal or contributory role of asciminib remains uncertain. Importantly, data on long-term exposure from this analysis are reassuring; no increase in the frequency or severity of AOEs with longer asciminib exposure was observed. With longer follow-up, the safety profile of asciminib remained unchanged compared with previously published data [29], raising no new concerns.
MMR is a well-established treatment goal [2] and is associated with improved outcomes, including OS and PFS [43,44,45,46]. However, achieving MMR in third or later lines of therapy may be difficult; major treatment goals for these patients include preventing PD and achieving and maintaining other protective response thresholds, including BCR::ABL1IS ≤ 1% [1, 2]. Many patients in this trial (61.3%) achieved BCR::ABL1IS ≤ 1% by data cutoff, including 53.2% by 48 weeks (≈12 months). Although other trials have reported similar response in patients with CML-CP without T315I after ≥2 prior TKIs, responses appear to be achieved earlier with asciminib; in ASCEMBL, 50.8% of patients receiving asciminib had this response by 48 weeks (≈12 months) [47], whereas, in the OPTIC trial, 46.2% of patients receiving ponatinib achieved this response by 36 months [48]. In the PACE trial, 49% of patients with CML without T315I after ≥1 prior TKI achieved a correlate of BCR::ABL1IS ≤ 1% by 57 months [2, 10].
In this updated analysis, the cumulative MMR rate continued to increase with longer asciminib treatment duration regardless of line of therapy or baseline disease characteristics, with two-thirds of patients (61.6%) achieving MMR by data cutoff; rates ranged from 52.5% to 75.0% by treatment line, confirming the benefit of asciminib in this heavily pretreated population for whom options are very limited. These data compare favorably with those in other later-line TKI studies [10, 13, 19, 20, 22, 34, 35, 49]. Acknowledging potentially variable response levels prior to change in therapy among these studies, MMR rates were 15% in patients with or without T315I receiving third- and fourth-line bosutinib (median follow-up, 28.5 months) [49] and 35% in patients without T315I in the PACE 5-year analysis of third-line or later ponatinib [10].
Compared with lesser depth of response, DMR is associated with improved outcomes, including better OS, EFS, and failure-free survival and reduced risk of progression to AP/BC [50,51,52] and is a required level of response for treatment-free remission eligibility [1, 2]. Approximately one-third (33.7%) of evaluable patients in this analysis achieved MR4 with asciminib; this appears favorable compared with 26% in a 5-year analysis of patients without T315I treated with ponatinib, with the caveat that response levels prior to treatment may have differed from those in the current study [10]. Of 41 patients receiving asciminib with BCR::ABL1IS > 10% at screening (a highly refractory population), 46.3% achieved BCR::ABL1IS ≤ 1%, 36.6% achieved MMR, and 7.3% (n = 3) achieved MR4.5.
This longer-term follow-up demonstrates the durability of response achieved with asciminib; only 5 of 53 patients who achieved MMR lost this response (2 of 5 remain in BCR::ABL1IS ≤ 1%). This durability, with median time to MMR of 132 weeks with asciminib, appears favorable compared with that of ponatinib in the PACE 5-year analysis, in which ≈41% of patients without T315I who achieved MMR lost this response and median time to MMR was not reached [10].
Newly emerged BCR::ABL1 mutations were detected in 5 patients (M244V, V289I, and myristoyl-pocket mutations G463D, G463S, V468F, and I502L). Of 12 patients with mutations detected at screening (E255K, F317L, G250E, L248V, V299L, M244V, Y253H), 6 achieved MMR; however, at this time, data are too limited to draw conclusions on the impact of mutations on the efficacy of asciminib.
In conclusion, asciminib, with its novel mechanism specifically targeting the ABL myristoyl pocket, is safe, well tolerated, and provides durable longer-term responses, including DMR in heavily pretreated patients with CML-CP without T315I. With a median treatment duration of ≈4 years, more than two-thirds of patients remained on therapy. These extended safety and response data from the phase 1 study complement results from ASCEMBL, which demonstrated superior efficacy and favorable safety with asciminib compared with bosutinib [28, 30]. Expanded clinical investigation of asciminib is warranted, including in patients with newly diagnosed CML-CP. A phase 3 trial (NCT04971226) is enrolling patients with CML-CP to evaluate asciminib versus all other approved first-line TKIs (imatinib, nilotinib, dasatinib, and bosutinib). This trial, along with other planned and ongoing studies and routine pharmacovigilance activities, will further characterize the efficacy and safety profile of asciminib, collect additional mutation data, and define optimal use for patients with CML.
Data availability
Novartis is committed to sharing access to patient-level data and supporting clinical documents from eligible studies with qualified external researchers. These requests will be reviewed and approved by an independent review panel based on scientific merit. All data provided will be anonymized to respect the privacy of patients who have participated in the trial, consistent with applicable laws and regulations. This trial data availability follows the criteria and process described at https://www.clinicalstudydatarequest.com/.
References
Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Chronic Myeloid Leukemia. 2022;V1.2023.
Jabbour E, Parikh SA, Kantarjian H, Cortes J. Chronic myeloid leukemia: mechanisms of resistance and treatment. Hematol Oncol Clin North Am. 2011;25:981–95.
Patel AB, O’Hare T, Deininger MW. Mechanisms of resistance to ABL kinase inhibition in chronic myeloid leukemia and the development of next generation ABL kinase inhibitors. Hematol Oncol Clin North Am. 2017;31:589–612.
Hirji I, Gupta S, Goren A, Chirovsky DR, Moadel AB, Olavarria E, et al. Chronic myeloid leukemia (CML): association of treatment satisfaction, negative medication experience and treatment restrictions with health outcomes, from the patient’s perspective. Health Qual Life Outcomes. 2013;11:167.
Phillips KM, Pinilla-Ibarz J, Sotomayor E, Lee MR, Jim HS, Small BJ, et al. Quality of life outcomes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a controlled comparison. Support Care Cancer. 2013;21:1097–103.
Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–54.
Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boque C, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naive chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333–40.
Cortes JE, Khoury HJ, Kantarjian HM, Lipton JH, Kim DW, Schafhausen P, et al. Long-term bosutinib for chronic phase chronic myeloid leukemia after failure of imatinib plus dasatinib and/or nilotinib. Am J Hematol. 2016;91:1206–14.
Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393–404.
Steegmann JL, Baccarani M, Breccia M, Casado LF, Garcia-Gutierrez V, Hochhaus A, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648–71.
Hantschel O, Rix U, Superti-Furga G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leuk Lymphoma. 2008;49:615–9.
Cortes J, Lang F. Third-line therapy for chronic myeloid leukemia: current status and future directions. J Hematol Oncol. 2021;14:44.
Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–80.
Akard LP, Albitar M, Hill CE, Pinilla-Ibarz J. The “hit hard and hit early” approach to the treatment of chronic myeloid leukemia: implications of the updated National Comprehensive Cancer Network clinical practice guidelines for routine practice. Clin Adv Hematol Oncol. 2013;11:421–32.
Bosi GR, Fogliatto LM, Costa TEV, Grokoski KC, Pereira MP, Bugs N, et al. What happens to intolerant, relapsed or refractory chronic myeloid leukemia patients without access to clinical trials? Hematol Transfus Cell Ther. 2019;41:222–8.
Lomaia E, Zaritskey A, Shuvaev V, Martynkevich I, Fominykh M, Ovsyannikova E, et al. Efficacy of tyrosine kinase inhibitors in third line therapy in chronic phase chronic myeloid leukemia. Blood. 2015;126:abstract 4051.
Hochhaus A, Breccia M, Saglio G, Garcia-Gutierrez V, Rea D, Janssen J, et al. Expert opinion-management of chronic myeloid leukemia after resistance to second-generation tyrosine kinase inhibitors. Leukemia. 2020;34:1495–502.
Garg RJ, Kantarjian H, O’Brien S, Quintas-Cardama A, Faderl S, Estrov Z, et al. The use of nilotinib or dasatinib after failure to 2 prior tyrosine kinase inhibitors: long-term follow-up. Blood. 2009;114:4361–8.
Ribeiro BF, Miranda EC, Albuquerque DM, Delamain MT, Oliveira-Duarte G, Almeida MH, et al. Treatment with dasatinib or nilotinib in chronic myeloid leukemia patients who failed to respond to two previously administered tyrosine kinase inhibitors - a single center experience. Clinics (Sao Paulo, Brazil). 2015;70:550–5.
Giles FJ, Abruzzese E, Rosti G, Kim DW, Bhatia R, Bosly A, et al. Nilotinib is active in chronic and accelerated phase chronic myeloid leukemia following failure of imatinib and dasatinib therapy. Leukemia. 2010;24:1299–301.
Ibrahim AR, Paliompeis C, Bua M, Milojkovic D, Szydlo R, Khorashad JS, et al. Efficacy of tyrosine kinase inhibitors (TKIs) as third-line therapy in patients with chronic myeloid leukemia in chronic phase who have failed 2 prior lines of TKI therapy. Blood. 2010;116:5497–500.
Cortes J, Quintas-Cardama A, Jabbour E, O’Brien S, Verstovsek S, Borthakur G, et al. The clinical significance of achieving different levels of cytogenetic response in patients with chronic phase chronic myeloid leukemia after failure to front-line therapy: is complete cytogenetic response the only desirable endpoint? Clin Lymphoma Myeloma Leuk. 2011;11:421–6.
Hochhaus A, Gambacorti-Passerini C, Abboud C, Gjertsen BT, Brummendorf TH, Smith BD, et al. Bosutinib for pretreated patients with chronic phase chronic myeloid leukemia: primary results of the phase 4 BYOND study. Leukemia. 2020;34:2125–37.
Wylie AA, Schoepfer J, Jahnke W, Cowan-Jacob SW, Loo A, Furet P, et al. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature. 2017;543:733–7.
Schoepfer J, Jahnke W, Berellini G, Buonamici S, Cotesta S, Cowan-Jacob SW, et al. Discovery of asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1. J Med Chem. 2018;61:8120–35.
Manley PW, Barys L, Cowan-Jacob SW. The specificity of asciminib, a potential treatment for chronic myeloid leukemia, as a myristate-pocket binding ABL inhibitor and analysis of its interactions with mutant forms of BCR-ABL1 kinase. Leuk Res. 2020;98:106458.
Rea D, Mauro MJ, Boquimpani C, Minami Y, Lomaia E, Voloshin S, et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood. 2021;138:2031–41.
Hughes TP, Mauro MJ, Cortes JE, Minami H, Rea D, DeAngelo DJ, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N Engl J Med. 2019;381:2315–26.
Novartis Pharmaceuticals Corporation. Scemblix (asciminib) [package insert]. Novartis Pharmaceuticals Corporation: East Hanover, NJ; 2021.
Rea D, Mauro MJ, Boquimpani C, Minami Y, Lomaia E, Voloshin S, et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood. 2021;138:2031–41.
Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–51.
Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37.
Ongoren S, Eskazan AE, Suzan V, Savci S, Erdogan Ozunal I, Berk S, et al. Third-line treatment with second-generation tyrosine kinase inhibitors (dasatinib or nilotinib) in patients with chronic myeloid leukemia after two prior TKIs: real-life data on a single center experience along with the review of the literature. Hematology. 2018;23:212–20.
Russo Rossi A, Breccia M, Abruzzese E, Castagnetti F, Luciano L, Gozzini A, et al. Outcome of 82 chronic myeloid leukemia patients treated with nilotinib or dasatinib after failure of two prior tyrosine kinase inhibitors. Haematologica. 2013;98:399–403.
Quintas-Cardama A, Kantarjian H, Jones D, Nicaise C, O’Brien S, Giles F, et al. Dasatinib (BMS-354825) is active in Philadelphia chromosome-positive chronic myelogenous leukemia after imatinib and nilotinib (AMN107) therapy failure. Blood. 2007;109:497–9.
Cortes JE, Apperley J, Lomaia E, Moiraghi B, Sutton MU, Pavlovsky C, et al. OPTIC primary analysis: a dose-optimization study of 3 starting doses of ponatinib. Presented at: EHA 2021 Virtual Meeting. Abstract S153.
Sneed TB, Kantarjian HM, Talpaz M, O’Brien S, Rios MB, Bekele BN, et al. The significance of myelosuppression during therapy with imatinib mesylate in patients with chronic myelogenous leukemia in chronic phase. Cancer. 2004;100:116–21.
Rea D. Management of adverse events associated with tyrosine kinase inhibitors in chronic myeloid leukemia. Ann Hematol. 2015;94:S149–58.
Novartis Pharmaceuticals Corporation. NDA/BLA multi-disciplinary review and evaluation (40) SCEMBLIX (asciminib). 2021.
Cirmi S, El Abd A, Letinier L, Navarra M, Salvo F. Cardiovascular toxicity of tyrosine kinase inhibitors used in chronic myeloid leukemia: an analysis of the FDA adverse event reporting system database (FAERS). Cancers (Basel). 2020;12:826.
Santoro M, Mancuso S, Accurso V, Di Lisi D, Novo G, Siragusa S. Cardiovascular issues in tyrosine kinase inhibitors treatments for chronic myeloid leukemia: a review. Front Physiol. 2021;12:675811.
Hehlmann R, Lauseker M, Saussele S, Pfirrmann M, Krause S, Kolb HJ, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31:2398–406.
Castagnetti F, Gugliotta G, Breccia M, Stagno F, Iurlo A, Albano F, et al. Long-term outcome of chronic myeloid leukemia patients treated frontline with imatinib. Leukemia. 2015;29:1823–31.
Jabbour E, Kantarjian HM, Saglio G, Steegmann JL, Shah NP, Boque C, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2014;123:494–500.
Hehlmann R, Lauseker M, Jung-Munkwitz S, Leitner A, Muller MC, Pletsch N, et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-alpha in newly diagnosed chronic myeloid leukemia. J Clin Oncol. 2011;29:1634–42.
Mauro MJ, Minami Y, Rea D, Hochhaus A, Lomaia E, Voloshin S, et al. Efficacy and safety results from ASCEMBL, a multicenter, open-label, phase 3 study of asciminib, a first-in-class STAMP inhibitor, vs bosutinib in patients with chronic myeloid leukemia in chronic phase after ≥2 prior tyrosine kinase inhibitors: update after 48 weeks. Presented at: ASH 63rd Annual Meeting and Exposition; Atlanta, GA; December 11–14, 2021. Abstract 310.
Deininger MW, Apperley JF, Arthur CK, Chuah C, Hochhaus A, De Lavallade H, et al. Post hoc analysis of responses to ponatinib in patients with chronic-phase chronic myeloid leukemia (CP-CML) by baseline BCR-ABL1 level and baseline mutation status in the OPTIC trial. Presented at: ASH 63rd Annual Meeting and Exposition; Atlanta, GA; December 11–14, 2021. Abstract 307.
Khoury HJ, Cortes JE, Kantarjian HM, Gambacorti-Passerini C, Baccarani M, Kim DW, et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood. 2012;119:3403–12.
Falchi L, Kantarjian HM, Wang X, Verma D, Quintas-Cardama A, O’Brien S, et al. Significance of deeper molecular responses in patients with chronic myeloid leukemia in early chronic phase treated with tyrosine kinase inhibitors. Am J Hematol. 2013;88:1024–9.
Hehlmann R, Muller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32:415–23.
Etienne G, Dulucq S, Nicolini FE, Morrisset S, Fort MP, Schmitt A, et al. Achieving deeper molecular response is associated with a better clinical outcome in chronic myeloid leukemia patients on imatinib front-line therapy. Haematologica. 2014;99:458–64.
Acknowledgements
The study and work presented here were sponsored and funded by Novartis Pharmaceuticals Corporation. Financial support for medical editorial assistance was provided by Novartis. We thank Christopher Edwards, PhD, CMPP, and Chris Hofmann, PhD, of Nucleus Global for medical editorial assistance with this manuscript. MCH received partial salary support from the following sources: a research grant from the Jonathan David Foundation, a VA Merit Review Grant (I01BX005358), and an NCI R21 grant (R21CA263400).
Funding
This study was sponsored and funded by Novartis Pharmaceuticals.
Author information
Authors and Affiliations
Contributions
MJM, TPH, D-WK, DR, JEC, AH, KS, MB, MT, OO, HM, YTG, DJD, MCH, VGGdS, PlC, F-XM, JJWMJ, MD, NS, MBG, SC, FP, NA, MH and FL contributed to data acquisition and interpretation, writing and reviewing the manuscript, and reviewing and approving the final manuscript.
Corresponding author
Ethics declarations
Competing interests
MJM: Bristol Myers Squibb, Takeda, and Pfizer: personal fees. TPH: Novartis, Bristol Myers Squibb, and Enliven: consultancy, research funding. D-WK: Novartis, Bristol Myers Squibb, Pfizer, IL-YANG, and Takeda: grants. DR: Novartis, Pfizer, and Incyte: personal fees. JEC: Novartis, Pfizer, and Bristol Myers Squibb: grants, consulting fees. AH: Bristol Myers Squibb, Pfizer: institutional research support; Novartis and Incyte: institutional research support, personal fees. KS: Novartis: research funding, honoraria. MB: Bristol Myers Squibb, Celgene, Pfizer, Incyte, and Novartis: consultancy and honoraria; AbbVie: consultancy. MT: IMAGO: consultancy; Novartis and Takeda: research funding; Constellation Pharmaceuticals and Bristol Myers Squibb: membership on board of directors or advisory committees. OO: Amgen, Incyte, Celgene, Roche, Fusion Pharma, Novartis: honoraria. Amgen, Incyte, and Celgene: research funding. HM: Bayer, Bristol Myers Squibb Japan, Celgene, Chugai Pharma, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eisai, Kyowa Hakko Kirin, Lilly Japan, Novartis, Ono Pharmaceutical, Otsuka, Pfizer, Taiho Pharmaceutical, Takeda, MSD, and AbbVie: honoraria; Ono Pharmaceutical: consulting; Astellas Pharma, Chugai Pharma, Dainippon Sumitomo Pharma, Eisai, Kyowa Hakko Kirin, Lilly Japan, Ono Pharmaceutical, Taiho Pharmaceutical, Nippon Shinyaku, MSD, Boehringer Ingelheim, Daiichi Sankyo, Takeda, Nihonkayaku, Shionogi, Sanofi, Bayer Schering Pharma, Mitsubishi Tanabe Pharma, and Teijin Pharma: research funding. YTG: Pfizer, Johnson & Johnson, Amgen, MSD Pharma, Novartis, EUSA Pharma, Roche, Bristol Myers Squibb, and AbbVie: honoraria. DJD: AbbVie, Novartis, Blueprint, and GlycoMimetics: grants; AbbVie, Novartis, Blueprint and GlycoMimetics: research funding; AbbVie, Amgen, Autolus, Blueprint, Forty-Seven, GlycoMimetics, Incyte, Jazz, Kite, Novartis, Pfizer, Servier, and Takeda; consulting. AbbvVie, Amgen, Autolus, Blueprint, Forty-Seven, GlycoMimetics, Incyte, Jazz, Kite, Novartis, Pfizer, Servier, and Takeda: personal fees. MCH: Novartis, Deciphera, Theseus and Blueprint Medicines: consultancy; Deciphera: speaker’s bureau; Jonathan David Foundation, VA Merit Review Grant (I01BX005358), and NCI R21 grant (R21CA263400): partial salary support. Prior to 2019, MCH held an equity interest in MolecularMD. MCH holds multiple patents on the diagnosis and/or treatment of gastrointestinal stromal tumors; one patent on treatment has been licensed by Oregon Health & Science University to Novartis. VGGdS: Novartis, Pfizer, Bristol Myers Squibb, and Incyte: grants, nonfinancial support, and honoraria. PC: Pfizer, Novartis, and Incyte: honoraria. F-XM: Novartis: grants, honoraria, consultancy, and research funding; Bristol Myers Squibb and Pfizer: honoraria. JJWMJ: Novartis and Bristol Myers Squibb: research funding; Incyte: speaker’s fee; AbbVie, Novartis, Pfizer, and Incyte: honoraria; AbbVie, Alexion, Amgen, Astellas, Bristol Myers Squibb, Daiichi Sankyo, Janssen-Cilag, Olympus, Incyte, Sanofi Genzyme, Servier, Jazz, and Takeda: support for Apps for Care and Science nonprofit foundation of which JJWMJ is president. MD: Fusion Pharma, Blueprint, Pfizer, Novartis, Medscape, and Sangoma: consultancy; Pfizer: research funding; Takeda, Sangomo, and Blueprint: membership on board of directors or advisory committees. NS: Novartis: honoraria. Amgen: support for attending meetings and/or travel. MBG: National Cancer Institute, Amgen, Sanofi, Actinium Pharmaceuticals, Inc.: grants; Allogene: consulting. Sanofi and American Society of Hematology: honoraria. SC, FP, NA, and MH are employees of Novartis. FL: Bristol Myers Squibb, Incyte, and Celgene: consultancy, honoraria; Novartis: consultancy, honoraria, and research funding.
Ethics approval
The protocol was approved by the sites’ institutional review boards or ethics committees and conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonization. All patients provided written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mauro, M.J., Hughes, T.P., Kim, DW. et al. Asciminib monotherapy in patients with CML-CP without BCR::ABL1 T315I mutations treated with at least two prior TKIs: 4-year phase 1 safety and efficacy results. Leukemia 37, 1048–1059 (2023). https://doi.org/10.1038/s41375-023-01860-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-023-01860-w
This article is cited by
-
Cancer-associated thrombosis in hematologic malignancies
International Journal of Hematology (2024)
-
European LeukemiaNet laboratory recommendations for the diagnosis and management of chronic myeloid leukemia
Leukemia (2023)
-
Cardiovascular Adverse Events of Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia: Clinical Relevance, Impact on Outcome, Preventive Measures and Treatment Strategies
Current Treatment Options in Oncology (2023)