Abstract
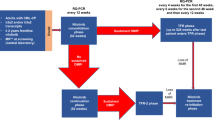
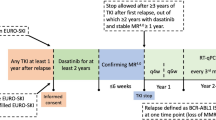
The ENESTop study evaluated treatment-free remission (TFR) in patients with chronic myeloid leukemia (CML) in chronic phase who had received ≥3 years of tyrosine kinase inhibitor therapy and achieved sustained deep molecular response only after switching from imatinib to nilotinib. After 1-year nilotinib consolidation, 126 patients attempted TFR. At 48 weeks (primary analysis), 57.9% (73/126) were in TFR. In the present analysis at 5 years, 42.9% (54/126) were in TFR. Since the 48-week analysis, among patients who left the TFR phase, 58% (11/19) did not have a loss of molecular response and discontinued for other reasons. Of the 59 patients who reinitiated nilotinib upon loss of major molecular response (MMR) or confirmed loss of MR4, 98.3% regained MMR, 94.9% regained MR4, and 93.2% regained MR4.5. Overall adverse event rates decreased over the 5 years of TFR. In patients reinitiating nilotinib, there was a cumulative increase in cardiovascular events with longer nilotinib exposure. No disease progression or CML-related deaths were reported. Overall, these results confirm the durability and safety of TFR for patients receiving second-line nilotinib. Cardiovascular risk should be carefully managed, particularly when reinitiating treatment after TFR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sasaki K, Strom SS, O’Brien S, Jabbour E, Ravandi F, Konopleva M, et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015;2:e186–93.
Bower H, Bjorkholm M, Dickman PW, Hoglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34:2851–7.
Jabbour E. Chronic myeloid leukemia: first-line drug of choice. Am J Hematol. 2016;91:59–66.
Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84.
Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17–23.
Hochhaus A, Saussele S, Rosti G, Mahon FX, Janssen JJWM, Hjorth-Hansen H, et al. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv41–51.
Mahon FX. Treatment-free remission in CML: who, how, and why? Hematol Am Soc Hematol Educ Program. 2017;2017:102–9.
Cortes J, Rea D, Lipton JH. Treatment-free remission with first- and second-generation tyrosine kinase inhibitors. Am J Hematol. 2019;94:346–57.
National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology—Chronic Myeloid Leukemia (Version 2.2020). https://www.nccn.org/professionals/physician_gls/pdf/cml_blocks.pdf. Accessed 4 Dec 2020.
Annunziata M, Bonifacio M, Breccia M, Castagnetti F, Gozzini A, Iurlo A, et al. Current strategies and future directions to achieve deep molecular response and treatment-free remission in chronic myeloid leukemia. Front Oncol. 2020;10:883.
Jiang Q, Liu ZC, Zhang SX, Gale RP. Young age and high cost are associated with future preference for stopping tyrosine kinase inhibitor therapy in Chinese with chronic myeloid leukemia. J Cancer Res Clin Oncol. 2016;142:1539–47.
Villemagne Sanchez LA, O’Callaghan C, Gough K, Hall K, Kashima Y, Seymour JF, et al. Patient perceptions of treatment-free remission in chronic myeloid leukemia. Leuk Lymphoma. 2018;59:406–15.
Mahon F-X, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–35.
Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122:515–22.
Rousselot P, Charbonnier A, Cony-Makhoul P, Agape P, Nicolini FE, Varet B, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32:424–30.
Imagawa J, Tanaka H, Okada M, Nakamae H, Hino M, Murai K, et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol. 2015;2:e528–35.
Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A, et al. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35:298–305.
Rea D, Nicolini FE, Tulliez M, Guilhot F, Guilhot J, Guerci-Bresler A, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017;129:846–54.
Okada M, Imagawa J, Tanaka H, Nakamae H, Hino M, Murai K, et al. Final 3-year results of the dasatinib discontinuation trial in patients with chronic myeloid leukemia who received dasatinib as a second-line treatment. Clin Lymphoma Myeloma Leuk. 2018;18:353–60.e1.
Chen KK, Du TF, Xiong PS, Fan GH, Yang W. Discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia with losing major molecular response as a definition for molecular relapse: a systematic review and meta-analysis. Front Oncol. 2019;9:372.
Hughes TP, Leber B, Cervantes F, Spector N, Pasquini R, Clementino NCD, et al. Sustained deep molecular responses in patients switched to nilotinib due to persistent BCR-ABL1 on imatinib: final ENESTcmr randomized trial results. Leukemia. 2017;31:2529–31.
Mahon FX, Boquimpani C, Kim DW, Benyamini N, Clementino NCD, Shuvaev V, et al. Treatment-free remission after second-line nilotinib treatment in patients with chronic myeloid leukemia in chronic phase: results from a single-group, phase 2, open-label study. Ann Intern Med. 2018;168:461–70.
Shah NP, Garcia-Gutierrez V, Jimenez-Velasco A, Larson S, Saussele S, Rea D, et al. Dasatinib discontinuation in patients with chronic-phase chronic myeloid leukemia and stable deep molecular response: the DASFREE study. Leuk Lymphoma. 2020;61:650–9.
Ross DM, Masszi T, Gomez Casares MT, Hellmann A, Stentoft J, Conneally E, et al. Durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96-week update of the ENESTfreedom study. J Cancer Res Clin Oncol. 2018;144:945–54.
Radich JP, Hochhaus A, Masszi T, Hellmann A, Stentoft J, Casares MTG, et al. Treatment-free remission following frontline nilotinib in patients with chronic phase chronic myeloid leukemia: 5-year update of the ENESTfreedom trial. Leukemia. 2021. https://doi.org/10.1038/s41375-021-01205-5.
Shanmuganathan N, Braley JA, Yong ASM, Hiwase DK, Yeung DT, Ross DM, et al. Modeling the safe minimum frequency of molecular monitoring for CML patients attempting treatment-free remission. Blood. 2019;134:85–9.
Rousselot P, Loiseau C, Delord M, Cayuela JM, Spentchian M. Late molecular recurrences in patients with chronic myeloid leukemia experiencing treatment-free remission. Blood Adv. 2020;4:3034–40.
Richter J, Söderlund S, Lübking A, Dreimane A, Lotfi K, Markevärn B, et al. Musculoskeletal pain in patients with chronic myeloid leukemia after discontinuation of imatinib: a tyrosine kinase inhibitor withdrawal syndrome? J Clin Oncol. 2014;32:2821–3.
Saussele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30:1638–47.
Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–54.
Kantarjian HM, Hughes TP, Larson RA, Kim DW, Issaragrisil S, le Coutre P, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. 2021;35:440–53.
Leong D, Aghel N, Hillis C, Siegal D, Karampatos S, Rangarajan S, et al. Tyrosine kinase inhibitors in chronic myeloid leukaemia and emergent cardiovascular disease. Heart. 2021;107:667–73.
Aghel N, Delgado DH, Lipton JH. Cardiovascular toxicities of BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia: preventive strategies and cardiovascular surveillance. Vasc Health Risk Manag. 2017;13:293–303.
Ross DM, Arthur C, Burbury K, Ko BS, Mills AK, Shortt J, et al. Chronic myeloid leukaemia and tyrosine kinase inhibitor therapy: assessment and management of cardiovascular risk factors. Intern Med J. 2018;48:5–13.
Saussele S, Richter J, Guilhot J, Gruber FX, Hjorth-Hansen H, Almeida A, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19:747–57.
Radich JP, Hochhaus A, Giles FJ, Ross DM, Saglio G, Hughes TP, et al. Analyses of predictors of durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline or second-line nilotinib. Blood. 2019;134 Supplement_1:2932.
Ritchie EK, Catchatourian R, Klisovic RB, Pinilla-Ibarz J, Deininger MW, Erba HP, et al. Results from enestgoal: a phase 2 study of treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) who switched from imatinib to nilotinib. Blood. 2017;130 Supplement 1:2875.
Rea D, Kyrcz-krzemien S, Pungolino E, Falzetti F, Bogdanovic A, Medina Almeida A, et al. Molecular response by 24 months of nilotinib 300 mg bid treatment in patients with chronic myeloid leukemia (CML) who failed to achieve dmr with imatinib: Results from the enestpath study. HemaSphere. 2020;4:328–9.
Acknowledgements
The authors would like to thank all study sites and participating countries, the study investigators, and all study participants and their families. The authors also thank Tim Harries of Novartis Pharmaceuticals UK Ltd, London, UK, for providing medical editorial assistance. This study was sponsored and funded by Novartis Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
TPH has received honoraria and research funding from and is a member of a board of directors or advisory committee at Bristol-Myers Squibb and Novartis. NDC has received financial support for congresses from EMS. MF has received honoraria from and is a member of a speakers bureau for BMS, Novartis and Pfizer. JHL has received research funding from and provided consulting to Ariad, Bristol-Myers Squibb, Novartis and Pfizer. AGT has received honoraria from Bristol-Myers Squibb, Novartis, and Pfizer. BM is a member of a speakers bureau for Bristol-Myers Squibb and Novartis. FEN has received honoraria and research funding from Incyte Biosciences and Novartis, and provided consultancy to Analysis Group Inc. and Sun Pharma. NT has received honoraria from Bristol-Myers Squibb, Novartis Pharma KK and Pfizer Japan Inc., and research funding from Novartis Pharma KK and Pfizer Japan Inc. TS has provided consultancy to and received honoraria and research funding from Bristol-Myers Squibb, Incyte, Novartis and Pfizer, and provided consultancy to and received honoraria from Adamed. DWK has received honoraria and research funding from Bristol-Myers Squibb, ILYANG, Novartis and Pfizer, is a member of a speakers bureau for Bristol-Myers Squibb, Novartis and Pfizer, is a member of a board of directors or advisory committee at Bristol-Myers Squibb and Pfizer, and has provided consultancy for ILYANG and Novartis. RFC is employed by, is an equity holder in and has received research funding from Novartis. RT and CB are employed by Novartis. FXM h0as received honoraria from ARIAD, Bristol-Myers Squibb, Novartis and Pfizer, and has received research funding from Novartis. This study was sponsored and funded by Novartis Pharmaceuticals.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hughes, T.P., Clementino, N.C.D., Fominykh, M. et al. Long-term treatment-free remission in patients with chronic myeloid leukemia after second-line nilotinib: ENESTop 5-year update. Leukemia 35, 1631–1642 (2021). https://doi.org/10.1038/s41375-021-01260-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01260-y
This article is cited by
-
Overcoming BCR::ABL1 dependent and independent survival mechanisms in chronic myeloid leukaemia using a multi-kinase targeting approach
Cell Communication and Signaling (2023)
-
Guidelines for the treatment of chronic myeloid leukemia from the NCCN and ELN: differences and similarities
International Journal of Hematology (2023)
-
Treatment-Free Remission: the New Goal in CML Therapy
Current Hematologic Malignancy Reports (2021)