Abstract
Our previous study showed that H3 receptor antagonists reduced neuronal apoptosis and cerebral infarction in the acute stage after cerebral ischemia, but through an action independent of activation of histaminergic neurons. Because enhanced angiogenesis facilitates neurogenesis and neurological recovery after ischemic stroke, we herein investigated whether antagonism of H3R promoted angiogenesis after brain ischemia. Photothrombotic stroke was induced in mice. We showed that administration of H3R antagonist thioperamide (THIO, 10 mg·kg−1·d−1, i.p., from D1 after cerebral ischemia) significantly improved angiogenesis assessed on D14, and attenuated neurological defects on D28 after cerebral ischemia. Compared with wild-type mice, Hrh3−/− mice displayed more blood vessels in the ischemic boundary zone on D14, and THIO administration did not promote angiogenesis in these knockout mice. THIO-promoted angiogenesis in mice was reversed by i.c.v. injection of H3R agonist immepip, but not by H1 and H2 receptor antagonists, histidine decarboxylase inhibitor α-fluoromethylhistidine, or histidine decarboxylase gene knockout (HDC−/−), suggesting that THIO-promoted angiogenesis was independent of activation of histaminergic neurons. In vascular endothelial cells (bEnd.3), THIO (10−9−10−7 M) dose-dependently facilitated cell migration and tube formation after oxygen glucose deprivation (OGD), and H3R knockdown caused similar effects. We further revealed that H3R antagonism reduced the interaction between H3R and Annexin A2, while knockdown of Annexin A2 abrogated THIO-promoted angiogenesis in bEnd.3 cells after OGD. Annexin A2-overexpressing mice displayed more blood vessels in the ischemic boundary zone, which was reversed by i.c.v. injection of immepip. In conclusion, this study demonstrates that H3R antagonism promotes angiogenesis after cerebral ischemia, which is independent of activation of histaminergic neurons, but related to the H3R on vascular endothelial cells and its interaction with Annexin A2. Thus, H3R antagonists might be promising drug candidates to improve angiogenesis and neurological recovery after ischemic stroke.
Similar content being viewed by others
Introduction
Stroke is the second leading cause of death and a major cause of severe disability for adults worldwide [1]. According to the different pathological mechanisms, stroke is divided into ischemic stroke and hemorrhagic stroke, of which ischemic stroke is the main type, accounting for 60%–80% [2]. Ischemic stroke mainly results from stenosis or obstruction of blood vessels. Currently, the only FDA-approved treatment is the thrombolytic therapy by tissue plasminogen activator (tPA) at the early stage of cerebral ischemia. However, this treatment has many shortcomings, such as limited therapeutic window (4.5–6 h) and intracranial hemorrhage [3]. Therefore, it is particularly urgent to find new therapeutic targets or drugs for ischemic stroke.
The pathophysiological process of ischemic stroke is complicated. The early stage involves neuronal excitotoxic reaction, inflammatory reaction and apoptosis, while the later stage mainly refers glial scar formation, angiogenesis and neurogenesis [4, 5]. In recent decades, lots of studies focused on neuroprotective drugs, but these drugs are mainly aimed at the early stage of cerebral ischemia and almost failed in clinical trials [6]. Therefore, promoting neurological repair at the later stage of ischemia may be a new approach to treat ischemic stroke. It has been found that the density of microvessels in the peripheral ischemic area is correlated to the survival of patients suffering from stroke [7]. The regeneration of blood vessels restores the supply of oxygen and nutrients to the insulted brain tissue. It can also guide newly generated neurons to ischemic boundary zone, provide nutritional support and clear the injured tissue, thereby promoting the reconstruction of neurovascular units [5]. It has been reported that vascular endothelial growth factor (VEGF), erythropoietin (EPO) or statins can promote angiogenesis after ischemia, however, these approaches have limited efficacy, and growth factors cannot easily pass the blood-brain barrier or have side effects [8]. New targets or drugs that are capable of effectively promoting angiogenesis will be beneficial to neurological repair after cerebral ischemia.
Histamine, a neurotransmitter and modulator in the brain, is involved in learning and memory, sleep and wakefulness, feeding, etc. Our previous studies have shown that histamine exerts a multi-target neuroprotective effect in different periods after cerebral ischemia [9, 10]. At the early stage of cerebral ischemia, histamine can promote the metabolism of glutamate to alleviate the excitatory injury of neurons [11], while at the late stage, histamine can inhibit the formation of glial scars and promote neurogenesis [12]. In addition, studies have found that histamine promoted the proliferation and migration of endothelial cells in vitro and induced angiogenesis in subcutaneous tissue [13]. However, histamine has low ability of crossing the blood-brain barrier and is closely related to peripheral inflammation. Histamine H1 receptor (H1R) and H2 receptor (H2R) are located in postsynapses, while H3 receptor (H3R) is mainly located on presynapses to negatively regulate histamine synthesis and release [14]. It has been reported that H3R antagonists alleviate narcolepsy, inhibit epilepsy and improve cognitive ability [15, 16]. Our previous study also found that H3 receptor antagonists reduced neuronal apoptosis and cerebral infarction in the acute stage after cerebral ischemia, but through an action independent of histaminergic neurons [17]. Since H3R is mainly distributed in the brain, its ligands may have few peripheral side effects. So, H3R may be viewed as promising target for brain disorders, but the role of H3R and its antagonist in angiogenesis after focal cerebral ischemia is still unclear.
The present study revealed that antagonism or knockout of H3R promoted angiogenesis after focal cerebral ischemia, which is independent of histaminergic neurons, but related to the H3R on vascular endothelial cells involving a regulation of Annexin A2 (ANXA2). Histamine H3R can serve as a new target for the treatment of ischemic stroke.
Materials and methods
Animals
Male mice from the C57BL/6 strain, 8–12-week-old, including wild type (WT), HDC−/−, Hrh3−/− and ANXA2+/+(Anxa2 knock-in mice) genotypes were used. The WT mice were purchased from Vital River Laboratories (Beijing, China). The Hrh3−/− mice were supplied by Johnson and Johnson Pharmaceutical Research and Development, LLC (La Jolla, CA, USA), bred and maintained by the Jackson Laboratory. The HDC−/− mice were kindly provided by Prof. Hiroshi Ohtsu, Department of Engineering, School of Medicine, Tohoku University [18]. ANXA2+/+ were commercially generated by standard homologous recombination at Cyagen (Suzhou, China). The HDC−/−, Hrh3−/− and ANXA2+/+ mice were identified by reverse transcriptase–PCR. The PCR primers for the Hdc gene were sense 5’-ACCCCATCTACCTCCGACAT-3’, antisense 5’-ACCGAATCACAAACCACAGC-3’. The PCR primers for the Hrh3 gene were sense 5’-CACACCCTTCCTCAGCGTTA-3’, antisense 5’-CCCTTTTGAGTGAGCGTGG-3’. The PCR primers for the Anxa2 gene were sense 5’-CCTCTGCTAACCATGTTCATGCC-3’, antisense 5’-TAGTTCCGAAGCATCATACTGGGC-3’. All experiments and protocols were approved by the Zhejiang University Animal Experimentation Committee and were in complete compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Photothrombotic stroke model
The model of focal cerebral ischemia in mice was established by irradiating photosensitive reagent Rose Bengal solution (Sigma-Aldrich, MO, USA) with cold light source [19]. Photochemical reaction of Rose Bengal produced singlet oxygen, leading to vascular endothelium damage and platelets aggregate and resulting in focal cerebral ischemia. Mice were fixed on the stereotaxic apparatus, and the skull was exposed through a midline incision and the connective tissue was removed. A cold light source with a luminous round spot with a diameter of 2 mm and a light intensity of 16000 lux was pasted vertically above the skull, and its center was shifted 1.5 mm to the right from Bregma. Five minutes after the administration of Rose Bengal solution (100 mg/kg, i.p.), the skull was illuminated for 15 min. After irradiation, the cold light source was removed, the scalp was sutured and disinfected, and the mice were placed in an incubator until they woke up. During the whole operation, the animal temperature was maintained at 37 ± 0.5 °C by a temperature control lamp.
Laser speckle contrast imaging and analysis
The cerebral blood flow (CBF) of mice was measured by RLFSI III laser speckle imaging system (RWD Life Science, CA, USA). Based on the blur of laser speckle pattern caused by blood cell movement, LSCI can detect the change of blood flow with time through a simple instrument [20, 21]. The mice were fixed on the stereotactic apparatus after anesthesia (1% Pelltobarbitalum Natricum, 6 mL/kg, i.p.). Exposed skull was irradiated with 784 nm, 80 mW laser, and the blood flow was detected by CCD camera. The image was acquired by customized software (RWD Life Science, CA, USA) with 5-ms exposure time and 10-frames/s acquisition speed for 5 s. During imaging, normal saline could be used to wipe the skull to keep moist. The scalp was sutured and the mice was put into the incubator until it waked up. The CBF of infarcted region of interest (ROI) (2 mm diameter circle) was analyzed. The relative cerebral blood flow (rCBF) was obtained by the ratio of the CBF on the 3rd and 14th days after operation to the CBF before the operation.
Behavioral experiments
The motor function of mice after cerebral ischemia was evaluated by grid-walking task and cylinder task 1 d before the surgery, and at 1, 3, 7, 14, 21 and 28 d after surgery [19, 22]. The grid-walking task was performed according to previous reports [19]. The specific parameters of the elevated wire grid were as follows: 32 cm × 20 cm × 50 cm (length × width × height); 1.2 cm × 1.2 cm (the size of square wire meshes). Mouse was placed individually on the elevated wire grid to walk freely for 5 min. A camera is placed under the grid to capture the whole walking process of the mouse and record the number of stepping errors (foot faults) and total steps of each foot. The percentage of foot faults of each foot was calculated according to the following formulas: foot faults ratio = foot faults number/total steps number × 100%; total steps number = foot faults number + no foot faults number. During walking, if the animal rest with the wrist on the edge of the grid or its limbs fell into the grid without support, it would be considered as a foot fault.
In the cylinder task, mice were placed in an uncovered transparent Plexiglas cylinder. When standing in the cylinder, the mouse would support their body with one or both forelimbs against the wall. The parameters of the cylinder are: 10 cm × 15 cm (diameter × height). A camera was placed 20 cm above the cylinder to capture the video until the mouse freely explore for 5 min. The time that each animal spent on both forelimbs, either the right forelimb, or the left forelimb propping to the wall was counted. Asymmetry index was calculated by the following formula: asymmetry index = (affected side time − control side time)/(affected side time + control side time + bilateral time) × 100%.
Drug administration
Animals were intraperitoneally injected with the H3R antagonist thioperamide (THIO, 3 or 10 mg·kg−1·d−1, Sigma-Aldrich, MO, USA) one day after focal ischemia operation, and then once per day. H1R antagonist pyrilamine (PYRI, 10 mg·kg−1·d−1, Sigma-Aldrich, MO, USA) or H2R antagonist cimetidine (CIM, 10 mg·kg−1·d−1, Sigma-Aldrich, MO, USA) were intraperitoneally injected 15 min before treatment with thioperamide. α-Fluoromethylhistidine (α-FMH, 5 μg/d in saline, a kind gift from Dr Kamei Chiaki, i.c.v.) or H3R agonist immepip (IMME, 1 μg/d in saline, Sigma-Aldrich, MO, USA, i.c.v.) was given at 15 min before treatment with thioperamide.
Assessment of lesion volume
To quantify the lesion volume, serial coronal brain sections were cut at 30 μm on a cryostat (Leica, Germany) and collected every 300 μm. The slices were stained with toluidine blue (1%, 20 min), dehydrated with ethanol and cleared using xylene. The lesion volume was observed under light microscopy, and then traced and quantified by ImageJ software.
Immunohistochemistry staining
Mice were transcardially perfused with a solution containing 0.9% NaCl at 4 °C, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PBS, pH 7.4). Coronal sections of brain tissue were obtained using a cryostat (Leica, Germany) and were mounted onto coated glass slides. Slices were incubated with Lectin (1:200, Vector Laboratories, CA, USA) or anti-CD31 (1:200, Millipore, MO, USA) at 4 °C overnight and goat IgG-Alexa 594 (1:400, Invitrogen, MA, USA) and at room temperature. Sections were mounted in mounting medium with 4’,6-diamidino-2-phenylindole (DAPI) (1:1000, Sigma-Aldrich, MO, USA). All sections are observed under a confocal microscope (FV1000, Olympus, Japan) or fluorescent microscope (BX51, Olympus, Japan). All quantifications were carried out at a similar coronal position and analyzed in a blind fashion.
Cell culture and oxygen-glucose deprivation (OGD) treatment
Mouse brain microvascular endothelial cells (bEnd.3) were purchased from Zeye Biology (Shanghai, China) and incubated with DMEM media (Gibco, New Zealand) supplemented with 10% fetal bovine serum (FBS, Gibco, New Zealand). Cells were cultured in a humidified atmosphere of 95% O2 and 5% CO2 at 37 °C.
To mimic ischemia in vitro, the medium of bEnd.3 cells was replaced with glucose-free DMEM and cells were transferred to a sealed chamber (Billups-Rothenburg, CA, USA) loaded with mixed gas containing 5% CO2 and 95% N2 at 37 °C for 2 h. Cells were returned to normoxic culture conditions and different concentrations of thioperamide (10−9, 10−8 and 10−7 M) were added during reperfusion. Additionally, some bEnd.3 cells treated identically but not subjected to OGD were used as control. Alternatively, bEnd.3 cells were transfected with H3R or ANXA2 knockdown plasmid (sh-Hrh3 or sh-Anxa2) or H3R overexpression plasmid (Hrh3-mCherry) at 24 h before OGD exposure. At least three independent experiments were performed for tube formation, transwell and wound healing assays, Western blot and co-immunoprecipitation.
Tube formation assay
The bEnd.3 cells were plated into u-slides (Ibidi, Germany) coated with matrigel (BD Biosciences, CA, USA) at a density of 1 × 104 cells per well and incubated in DMEM at 37 °C for 4 h. The formation of tubes was observed under a microscope and photographed. Three to five independent fields were assessed for each well and the number of meshes, total tube length, and the number of branching points were quantified using the ImageJ software.
Transwell assay
The bEnd.3 cells suspended in the complete medium were seeded in the upper chamber at a density of 1.2 × 105 cells per well. Four hours later, the medium in the upper chamber was changed to 0.1% FBS in DMEM, and the lower chamber was filled with 500 μL DMEM with 5% FBS. After incubation for 24 h at 37 °C, nonmigrating cells were removed with a cotton swab and the migrated cells through the membrane were fixed in 4% PFA and stained with 0.1% crystal violet solution (Beyotime, China). Three to five independent fields were assessed for each well and the number of migrated cells were quantified using the ImageJ software.
Wound healing assay
The bEnd.3 cell monolayer was scraped in a straight line with a 200 μL pipette tip and the cell debris was removed by PBS. The wounded monolayer cells were replaced with serum-free DMEM medium and cultured in a humidified atmosphere of 95% O2 and 5% CO2 at 37 °C for 24 h. Images were observed with a microscope at 0 h and 24 h after injury. The injury area between the sides of the scratch was measured using the ImageJ software.
Western blot and co-immunoprecipitation
For Western blotting, the total protein concentrations (40 μg) from bEnd.3 cells were separated using SDS-PAGE gels and transferred onto nitrocellulose membranes. The membranes were incubated with rabbit anti-H3R (1:1000, Abcam, UK), mouse anti-ANXA2 (1:1000, Abcam, UK) or mouse anti-GAPDH (1:5000, Kangchen, China) primary antibodies. Secondary antibodies conjugated with HRP against rabbit or mouse IgG (1:3000, Lianke, China) were applied. Images were acquired with the Tanon Chemiluminescent Imaging System (Tanon, China) and analyzed by ImageJ software. The results were expressed as the target protein/GAPDH ratio and then normalized to the values measured in the control groups.
For co-immunoprecipitation, bEnd.3 cells transfected with Hrh3-mCherry and ANXA2-Flag plasmids were exposed to 2 h OGD and 24 h reperfusion with normal culture medium containing thioperamide. Cells were then harvested and lysed in a cell lysis buffer. The co-immunoprecipitation of H3R and ANXA2 was performed based on a previously described method [23]. The extracts of 500 μg protein were incubated with anti-Flag M2 Affinity Gel (25 μL, Sigma-Aldrich, MO, USA) overnight at 4 °C and washed three times with TBS. The precipitates were incubated with Flag peptide (4 μL, Sigma-Aldrich, MO, USA) for 30 min at 4 °C. Proteins were separated on 12% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were incubated with rabbit anti-mCherry (1:1000, Abcam, UK), mouse anti-Flag (1:1000, Abcam, UK) or mouse anti-GAPDH (1:5000, Kangchen, China) primary antibodies. Secondary antibodies conjugated with HRP against rabbit or mouse IgG (1:3000, Lianke, China) were applied. Images were acquired with the Tanon Chemiluminescent Imaging System (Tanon, China). The lanes marked “input” were loaded with 10% of the starting material used for immunoprecipitation.
Statistical analysis
Data are presented as mean ± SEM. Multiple comparisons were analyzed by One-way ANOVA followed by Tukey test, while two-tailed Student’s t test was applied for other comparisons between two groups. The data for rCBF on the 3rd and 14th days after operation and behavioral tests from 0 to 28 d were analyzed by Two-way ANOVA followed by Tukey test. For all analyses, the tests were two-sided and a P < 0.05 was considered statistically significant.
Results
Histamine H3R antagonism promotes angiogenesis and neurological function recovery at the late stage of focal cerebral ischemia
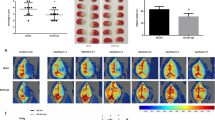
In order to evaluate the effect of antagonism of H3R on angiogenesis, we administered H3R antagonist thioperamide from 1 d after cerebral ischemia in photothrombotic stroke model, and observed the blood vessels in the ischemic boundary zone. Blood vessels marked with lectin was significantly reduced on the d 14, while thioperamide increased its density in a dose-dependent manner (Fig. 1a, b), but had no apparent effect at 3 d after ischemia (Fig. 1c, d). CD31 is thought to be a specific marker for blood vessels and the CD31 immunostaining results also showed that thioperamide increased the number of blood vessels at 14 d after ischemia (Fig. 1e, f). Meanwhile, the treatment of thioperamide enhanced CBF at 14 d, but not at 3 d after ischemia detected through laser speckle contrast imaging (Fig. 1g, h). Furthermore, IMME, an agonist of H3R, was administered with thioperamide to investigate whether the effect of thioperamide was exerted by blocking H3R. We found that the effect of thioperamide on angiogenesis can be reversed by IMME, while IMME alone had no obvious effect (Fig. 1i, j). Compared with wild-type mice, Hrh3−/− mice displayed more blood vessels at the ischemic boundary zone at 14 d after cerebral ischemia, and thioperamide did not further promote angiogenesis in these knockout mice (Fig. 1k, l). The above results validated that blocking H3R boosts angiogenesis after focal cerebral ischemia.
WT or Hrh3−/− mice were treated with thioperamide or together with immepip (IMME) after ischemia. a–d Representative microphotographs and quantification of lectin labeled blood vessels in the ischemic boundary zone on D14 (a) and D3 (c) after ischemia. n = 6–8. e, f Representative microphotographs and quantification of CD31 labeled blood vessels on D14 after ischemia. g Representative CBF pseudo-color microphotographs at D3 and D14. Black arrows indicate ischemic area. h Changes of rCBF. Day 0 stands for the day before operation. n = 5. i–l Representative microphotographs and quantification of lectin labeled blood vessels on d 14 after ischemia. n = 4-6. ***P < 0.001, **P < 0.01, *P < 0.05. Scale bar, 100 μm. The lesion area was outlined by a dashed line.
Angiogenesis may benefit histological and functional recovery, so we evaluated the effect of thioperamide on the infarct volume and motor function after focal cerebral ischemia. Compared with the ischemia group, mice given thioperamide displayed reduced injury volume at 28 d after ischemia, but not at d 14 (Fig. 2a, b). Grid test and cylinder tests were employed to evaluate the effect of antagonism of H3R on motor function after ischemia. The results showed that thioperamide significantly reduced the grid dislocation rate and asymmetry index at the late stage of focal cerebral ischemia (d 28), but had no effects from d 1 to d 21 (Fig. 2c, d). These results suggested that H3R antagonists subserved the recovery of neurological function following promoting angiogenesis after focal cerebral ischemia.
WT mice were treated with thioperamide after ischemia. a, b Representative microphotographs of toluidine blue stained lesion area and quantitative analysis of lesion volume on D14 (a) or D28 (b) after ischemia. c Time spent on right forelimb paw relative to left from D1 to D28 after ischemia. d Foot faults of forelimb relative to total steps taken from D1 to D28 after ischemia. n = 6, *P < 0.05, vs. Saline group. Scale bar, 500 μm. The lesion area was outlined by a dashed line.
The effect of H3R antagonism on angiogenesis is independent of activation of histaminergic neurons
Histamine H3R, as presynaptic autoreceptor, can negatively regulates the synthesis and release of histamine from histaminergic neurons, thereby acting on postsynaptic H1R or H2R [24]. To study whether the effect of thioperamide on promoting angiogenesis was related to the activation of histaminergic neurons, we first administered histidine decarboxylase inhibitor α-FMH to block histamine synthesis in the brain. Interestingly, α-FMH could not reverse the effect of thioperamide on promoting angiogenesis (Fig. 3a. b). Furthermore, HDC−/− mice lacking endogenous histamine was used, and the results indicated that the blood vessel density in the ischemic boundary zone in HDC−/− mice was comparable with that of the control group, and thioperamide was still capable of enhancing angiogenesis. We also administered H1R antagonist pyrilamine or H2R antagonist cimetidine together with thioperamide, and found that neither PYRI nor CIM could reverse the effect of thioperamide on angiogenesis (Fig. 3c, d). Therefore, the above results suggest that the effect of thioperamide on angiogenesis does not rely on the activation of histaminergic neurons.
WT or HDC−/− mice were treated with thioperamide or together with pyrilamine or cimetidine after ischemia. a Representative microphotographs of lectin labeled vessels at the ischemic boundary zone on D14 after ischemia. b Fold change of blood vessel density in a. n = 4–6. c representative microphotographs of lectin labeled blood vessels on D14 after ischemia. d Fold change of blood vessel density in c. n = 4. **P < 0.01, *P < 0.05. Scale bar, 100 μm. The lesion area was outlined by a dashed line.
Histamine H3R blockade promotes vascular endothelial cell migration and tube formation after oxygen-glucose deprivation (OGD)
Since the expression of H3R was detected in brain vascular endothelial cells [25] (Fig. 4f), we questioned whether the promotion of H3R antagonist on angiogenesis is due to its direct effect on vascular endothelial cells. Mouse brain vascular endothelial cells (bEnd.3) were subjected to OGD and treated with H3R antagonist thioperamide (10−9, 10−8 and 10−7 M) during reperfusion. The angiogenesis of bEnd.3 cells was evaluated by numbers of meshes, total tube length and number of branching points in tube formation assay. The results showed that the capillary-like tube formation was decreased after OGD, and thioperamide showed a concentration dependent effect to elevate the number of capillary-like structures, which can be abrogated by the combination of H3R agonist IMME administration (Fig. 4a, e).
bEnd.3 cells were subjected to OGD and treated with H3R antagonist thioperamide (THIO) at three concentrations (10−9, 10−8 and 10−7 M), or THIO 10−7 M combined with H3R agonist IMME during reperfusion. a Quantification of numbers of meshes, total tube length and numbers of branching points in tube formation tests after OGD. b Proliferation assay by MTT tests after OGD. c Migration assay by transwell and wound healing tests after OGD. d Proliferation assay by MTT tests and migration assay by transwell and wound healing tests for bEnd.3 cells without OGD exposure. e Representative microphotographs of tube formation, transwell and wound healing tests for bEnd.3 cells treated with THIO or IMME. f Western blot analysis of H3R expression of bEnd.3 cells transfected with empty vector (Vec), sh-Hrh3 or Hrh3-mCherry plasmids. g, h Quantification of numbers of meshes, total tube length and numbers of branching points in tube formation tests after OGD. i Migration assay by transwell and wound healing tests after OGD. j Representative microphotographs of tube formation, transwell and wound healing tests for bEnd.3 cells transfected with empty vector (Vec), sh-Hrh3 or Hrh3-mCherry plasmids. n = 3–6. ***P < 0.001, **P < 0.01, *P < 0.05. Scale bar, 100 μm.
It has been reported that vascular endothelial cells proliferation and migration contribute to cerebral angiogenesis [26]. Therefore, we analyzed whether thioperamide impacted the viability and migration of bEnd.3 cells. The results from MTT assay revealed that thioperamide had no effect on proliferation of bEnd.3 cells after OGD insult (Fig. 4b). The migration was evaluated by wound healing and transwell assay. The results demonstrated that the migration in both assays was inhibited after OGD, while thioperamide dose-dependently promoted migration, which was abrogated by IMME (Fig. 4c, e). Moreover, we found that thioperamide had no effect on viability or migration in bEnd.3 cells without OGD exposure (Fig. 4d). These data indicate that H3R antagonist thioperamide promoted the angiogenesis and migration of bEnd.3 cells following OGD insult.
To verify the role of H3R in angiogenesis, bEnd.3 cells were transfected with H3R knockdown plasmid (sh-Hrh3) or H3R overexpression plasmid (Hrh3-mCherry), followed by OGD exposure and reperfusion for 24 h. Both plasmids exhibited effective knockdown or overexpression in bEnd.3 cells (Fig. 4f). The results revealed that H3R knockdown notably elevated the number of capillary-like structures in terms of numbers of meshes, total tube length and number of branching points, however, H3R overexpression did not further reduce the tube formation compared with control group after OGD insult (Fig. 4g–j). In wound healing and ranswell assays, cell migration was increased in the H3R knockdown group, but not H3R overexpression group after OGD (Fig. 4i, j). Together, these results indicated that H3R blockage whether by antagonists or receptor knockdown can enhance the migration and tube formation of vascular endothelial cells to promote angiogenesis after OGD.
Histamine H3R antagonism impedes the interaction of H3R and ANXA2 to promote vascular endothelial cell migration and tube formation after OGD
To further investigate the mechanism underlying the H3R regulation of angiogenesis, we purified protein complexes using tandem affinity purification and identified the protein interactions in combination with mass spectrometry of H3R binding proteins [27]. We identified ANXA2 as a novel binding protein for H3R, which is located on the surface of endothelial cells and plays pro-angiogenic role [28]. To test whether H3R regulated angiogenesis by interacting with ANXA2, the co-immunoprecipitation analysis was performed to detect the interaction of H3R with ANXA2 after OGD exposure and thioperamide treatment. The results showed that interaction of H3R with ANXA2 was present in bEnd.3 cells and increased in the context of OGD, which can be reduced by H3R antagonist thioperamide (Fig. 5a). We then knocked ANXA2 down to investigate the effect of ANXA2 on the promotion by thioperamide after OGD exposure (Fig. 5b). We observed that the effect of thioperamide on elevating the number of capillary-like structures in tube formation assay was significantly counteracted by ANXA2 knockdown (Fig. 5c, d). Simultaneously, ANXA2 knockdown also attenuated the increased migration conferred by thioperamide after OGD in both would healing and transwell assays (Fig. 5c, e). The results suggest that H3R antagonism impedes the interaction of H3R and ANXA2 to promote vascular endothelial cell migration and tube formation after OGD. Furthermore, Anxa2 knock-in mice (ANXA2+/+) were employed, that having overexpression of ANXA2 (Fig. 5f). We found that compared with wild-type mice, ANXA2+/+ mice displayed more blood vessels at the ischemic boundary zone, which was reversed by IMME treatment (Fig. 5g, h). It suggests that H3R modulates angiogenesis by interacting with ANXA2 after focal cerebral ischemia.
a Representative co-immunoprecipitation results showing the interaction of H3R with ANXA2 and the effect of H3R antagonist thioperamide (THIO) on the interaction following OGD. b Western blot analysis of ANXA2 expression of bEnd.3 cells transfected with empty vector (Vec) or sh-Anxa2 plasmids. c, d, e Representative photographs (c) and quantification of numbers of meshes, total tube length and numbers of branching points in tube formation tests (d) and migration in transwell and wound healing tests (e) for bEnd.3 cells subjected to OGD and transfected with empty vector (Vec) or sh-Anxa2 plasmids combined with THIO 10−7 M. f Western blot analysis of ANXA2 expression of WT or ANXA2+/+ mice. g, h Representative microphotographs and quantification of lectin labeled blood vessels in the ischemic boundary zone of WT or ANXA2+/+ mice treated immepip (IMME) at 14 d after ischemia. n = 3–5. Scale bar, 100 μm. ***P < 0.001, **P < 0.01, *P < 0.05. Scale bar, 100 μm. The lesion area was outlined by a dashed line.
Discussion
Ischemic stroke often leads to long-term neurological dysfunction, however, there is currently no effective drug treatment for late recovery besides rehabilitation training. Angiogenesis is a critical pathological event at the late stage of cerebral ischemia, which subserves neurogenesis and the reconstruction of neurovascular units [8]. In this study, we revealed that antagonism of H3R or knockdown of H3R promoted angiogenesis after focal cerebral ischemia, which also benefited the functional recovery. This action of H3R relied on H3R in vascular endothelial cells but not in histaminergic neurons. It suggests that H3R in endothelial cells can serve as a new target for the promotion of angiogenesis after ischemic stroke.
Angiogenesis is a complex multi-step process, which initiates from the proliferation and migration of vascular endothelial cells after the degradation of extracellular matrix [26]. It has been reported that rehabilitation training, or administration of growth factors such as VEGF, EPO, or statins can promote angiogenesis, however, these drugs have disadvantages such as side effects or limited efficacy [29]. In addition, transplantation of bone marrow mesenchymal stem cells or umbilical cord blood can produce vascular growth factors, and endothelial progenitor cells can either release vascular growth factor or be differentiated into vascular endothelial cells, which may reinforce angiogenesis, but the therapeutic value of these cell transplantation methods remains unknown [30, 31]. In addition, these approaches may have problems, such as inconvenient sources, stimulating the host’s immune inflammatory response, or possible tumorigenicity. In present study, we proved that blocking H3R enhanced angiogenic activity, which stems from a promotion on endothelial migration and tube formation in vitro and angiogenesis in vivo after antagonism, knockout or knockdown of H3R (Figs. 1 and 4). Importantly, the improvement of neurological function was observed following enhanced angiogenesis conferred by H3R antagonist (Fig. 2). So, antagonism of H3R is a convenient approach for angiogenesis and late recovery for patients suffering from ischemic stroke.
Our previous studies indicate that H3R antagonism reinforced autophagy to suppress neuronal apoptosis at the early phase after focal cerebral ischemia [17]. Moreover, H3R antagonist enhanced the neurogenesis at the late phase after a focal traumatic brain injury [32]. Besides that, activation of H1R or H2R elevated glutamate uptake by astrocytes and inhibited the formation of glial scars after cerebral ischemia [11, 12], which could be achieved by a blockade of H3R and subsequent increase of histamine release. Since cerebral ischemia captures complex pathological mechanism, drug interventions for a single pathway may not achieve multiple curative effects. Therefore, H3R antagonists may be viewed as promising candidates for the treatment of cerebral ischemia through multifaceted protection covering the entire progress after ischemic stroke. However, the protection function of H3R antagonist was not observed at the early stage in present study, as there was no alteration in infarct volume and motor function from d 1 to d 14 after the treatment of thioperamide (Fig. 2). It could be due to the discrepancy of cerebral ischemic models. The photothrombosis model used in current study, generates localized and reproducible ischemic infarcts that are useful for studying recovery mechanisms, but fails to produce a substantial ischemic penumbra, where the cells are potentially salvageable at the early stage [33].
Although H3R is mainly located in the presynapses in histaminergic neurons and worked as autoreceptor, its action as heteroreceptor cannot be neglected in ischemic injury. For example, H3R modulated the autophagy in the neurons of the penumbra area and the GABA release during ischemia induced excitotoxicity [17, 34]. In this study, we found that thioperamide was still able to facilitate angiogenesis after administration of α-FMH, H1R antagonist or H2R antagonist, or in HDC−/− mice (Fig. 3). It suggests that the action of H3R antagonism on angiogenesis is independent of activation of histaminergic neurons. Previous studies indicated that endogenous mast-cell histamine is angiogenic in a rat mesenteric window assay and histamine augments angiogenesis in the inflammatory granulation tissue by producing VEGF [35, 36]. However, other studies found that histamine was absent of direct effects on the processes of angiogenesis or even showed suppression due to the up-regulation of the potent angiogenesis inhibitor thrombospondin-1 after long time exposure [13]. So, the effects of histamine on angiogenesis may vary in different situations, tissues and exposure times, which could be the reason of histamine-independent action of H3R antagonists on angiogenesis after cerebral ischemia.
We interestingly found that H3R on vascular endothelial cells modulated their migration and the tube formation, which is quite critical for the angiogenesis. The constitutive activity of H3R has been reported, that can be inhibited by H3R antagonists [37]. Here, we found that the H3R can bind to ANXA2 in vascular endothelial cells, and this interaction was inhibited by thioperamide (Fig. 5a). We propose that the effect of H3R on angiogenesis may be related to constitutive activity of H3R through acting on ANXA2. ANXA2 is a calcium-dependent protein that binds to phospholipids and is highly expressed in vascular endothelial cells in the brain. ANXA2 interacts with plasminogen and tPA to accelerate plasmin generation and facilitate fibrinolysis, thereby mediating the extracellular matrix degradation and angiogenesis [28]. In addition, ANXA2 was found to regulate cytoskeletal reorganization to promote epithelial cell migration through Rho-related signaling [38]. ANXA2 amplified beneficial effects of tPA through the amelioration of blood-brain barrier damage and augmentation of cerebrovascular patency and microvessel density [39, 40]. Although hypoxia and HIF-1α directly modulated ANXA2 expression in oxygen-induced retinopathy [41], the additional mechanisms that might conspire to up-regulate ANXA2 is still unclear. Here, we found that H3R is a novel interaction partner for ANXA2 in microvascular endothelial cells and the binding of H3R and ANXA2 was increased in the context of OGD (Fig. 5a). ANXA2 knockdown abrogated the effect of H3R antagonist on tube formation and migration of vascular endothelial cells after OGD; while ANXA2 overexpression increased the blood vessels at the ischemic boundary zone, which was reversed by H3R agonist (Fig. 5b–h). We proposed that the increased interaction of H3R and ANXA2 after ischemia may hinder ANXA2 to exert pro-angiogenic action, while H3R antagonism reduces this interaction to release ANXA2 to mediate angiogenesis (Fig. 6). We also found that H3R overexpression did not augment angiogenesis after OGD. It could be due to saturation of ANXA2 binding with H3R. We also found that H3R antagonists had no effect on vascular endothelial cells without OGD exposure. It further bolsters that H3R antagonist is feasible for the therapy of ischemic damage of brain, since it may not induce aberrant angiogenesis in uncompromised area.
Specific knockout of endothelial cell-specific H3R by crossing Hrh3fl/fl mice with Cdh5-Cre or Tie2-Cre mice helps to further verify the important role of H3R on vascular endothelial cells after stroke. However, in lineage-tracing experiment using Cdh5-Cre;Ai9 and Tie2-Cre;Ai14 mice, we observed the leakage of tdTomato fluorescence in neurons. So, these Cre mouse lines should be used with caution, although there may be different origins of these lines. We will try other approach to specifically knockout H3R on vascular endothelial cells in future study. In addition, it is generally understood that the downstream cascade of H3R constitutive activity coupled to Gi/o proteins negatively regulated adenylyl cyclase (AC) and cAMP/PKA/CREB pathway, which serves as an important positive regulatory mechanism for angiogenesis by limiting autophagy [42] and activating mitogen-activated protein kinase signaling [43] in vascular endothelial cell. Further study is needed to verify whether the canonical cAMP/PKA/CREB pathway is involved in the action of H3R on angiogenesis.
In conclusion, this study indicates that antagonism of H3R promotes angiogenesis and functional recovery after focal cerebral ischemia, which is independent of histaminergic neurons, but related to the H3R on vascular endothelial cells and its interaction with Annexin A2. H3R on vascular endothelial cells can serve as a new target for the treatment of ischemic stroke at late stage. H3R antagonists might be superior drug candidates to improve angiogenesis and neurological recovery after ischemic stroke.
References
Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38:208–11.
Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, et al. Prevalence, incidence, and mortality of stroke in china: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135:759–71.
Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379:2364–72.
George PM, Steinberg GK. Novel stroke therapeutics: unraveling stroke pathophysiology and its impact on clinical treatments. Neuron. 2015;87:297–309.
Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117:481–96.
Hoyte L, Barber PA, Buchan AM, Hill MD. The rise and fall of NMDA antagonists for ischemic stroke. Curr Mol Med. 2004;4:131–6.
Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–8.
Ergul A, Alhusban A, Fagan SC. Angiogenesis: a harmonized target for recovery after stroke. Stroke. 2012;43:2270–4.
Hu WW, Chen Z. Role of histamine and its receptors in cerebral ischemia. ACS Chem Neurosci. 2012;3:238–47.
Hu W, Chen Z. The roles of histamine and its receptor ligands in central nervous system disorders: an update. Pharmacol Ther. 2017;175:116–32.
Fang Q, Hu WW, Wang XF, Yang Y, Lou GD, Jin MM, et al. Histamine up-regulates astrocytic glutamate transporter 1 and protects neurons against ischemic injury. Neuropharmacology. 2014;77:156–66.
Liao RJ, Jiang L, Wang RR, Zhao HW, Chen Y, Li Y, et al. Histidine provides long-term neuroprotection after cerebral ischemia through promoting astrocyte migration. Sci Rep. 2015;5:15356.
Qin L, Zhao D, Xu J, Ren X, Terwilliger EF, Parangi S, et al. The vascular permeabilizing factors histamine and serotonin induce angiogenesis through TR3/Nur77 and subsequently truncate it through thrombospondin-1. Blood. 2013;121:2154–64.
Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, Liu WL, et al. International union of basic and clinical pharmacology. XCVIII. Histamine receptors. Pharmacol Rev. 2015;67:601–55.
Schwartz JC. The histamine H3 receptor: from discovery to clinical trials with pitolisant. Br J Pharmacol. 2011;163:713–21.
Nomura H, Mizuta H, Norimoto H, Masuda F, Miura Y, Kubo A, et al. Central histamine boosts perirhinal cortex activity and restores forgotten object memories. Biol Psychiatry. 2019;86:230–9.
Yan H, Zhang X, Hu W, Ma J, Hou W, Zhang X, et al. Histamine H3 receptors aggravate cerebral ischaemic injury by histamine-independent mechanisms. Nat Commun. 2014;5:3334.
Liu L, Zhang S, Zhu Y, Fu Q, Gong Y, Ohtsu H, et al. Improved learning and memory of contextual fear conditioning and hippocampal CA1 long-term potentiation in histidine decarboxylase knock-out mice. Hippocampus. 2007;17:634–41.
Lin YH, Yao MC, Wu HY, Dong J, Ni HY, Kou XL, et al. HDAC2 (Histone deacetylase 2): a critical factor in environmental enrichment-mediated stroke recovery. J Neurochem. 2020;155:679–96.
Wu Y, Wu H, Zeng J, Pluimer B, Dong S, Xie X, et al. Mild traumatic brain injury induces microvascular injury and accelerates Alzheimer-like pathogenesis in mice. Acta Neuropathol Commun. 2021;9:74.
Winship IR. Laser speckle contrast imaging to measure changes in cerebral blood flow. Methods Mol Biol. 2014;1135:223–35.
Tang Y, Lin YH, Ni HY, Dong J, Yuan HJ, Zhang Y, et al. Inhibiting histone deacetylase 2 (HDAC2) promotes functional recovery from stroke. J Am Heart Assoc. 2017;6:e007236.
Jiang L, Cheng L, Chen H, Dai H, An D, Ma Q, et al. Histamine H2 receptor negatively regulates oligodendrocyte differentiation in neonatal hypoxic-ischemic white matter injury. J Exp Med. 2021;218:e20191365.
Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–72.
Karlstedt K, Jin C, Panula P. Expression of histamine receptor genes Hrh3 and Hrh4 in rat brain endothelial cells. Br J Pharmacol. 2013;170:58–66.
Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–87.
Xu X, Song Y, Li Y, Chang J, Zhang H, An L. The tandem affinity purification method: an efficient system for protein complex purification and protein interaction identification. Protein Expr Purif. 2010;72:149–56.
Liu W, Hajjar KA. The annexin A2 system and angiogenesis. Biol Chem. 2016;397:1005–16.
Tahergorabi Z, Khazaei M. A review on angiogenesis and its assays. Iran J Basic Med Sci. 2012;15:1110–26.
Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler RA, Lawton MT, et al. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog Neurobiol. 2014;115:138–56.
Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22.
Liao R, Chen Y, Cheng L, Fan L, Chen H, Wan Y, et al. Histamine H1 receptors in neural stem cells are required for the promotion of neurogenesis conferred by H3 receptor antagonism following traumatic brain injury. Stem Cell Rep. 2019;12:532–44.
Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396–409.
Dai H, Fu Q, Shen Y, Hu W, Zhang Z, Timmerman H, et al. The histamine H3 receptor antagonist clobenpropit enhances GABA release to protect against NMDA-induced excitotoxicity through the cAMP/protein kinase A pathway in cultured cortical neurons. Eur J Pharmacol. 2007;563:117–23.
Sorbo J, Jakobsson A, Norrby K. Mast-cell histamine is angiogenic through receptors for histamine1 and histamine2. Int J Exp Pathol. 1994;75:43–50.
Ghosh AK. Regulation by prostaglandin E2 and histamine of angiogenesis in inflammatory granulation tissue. Yakugaku Zasshi. 2003;123:295–303.
Bongers G, Sallmen T, Passani MB, Mariottini C, Wendelin D, Lozada A, et al. The Akt/GSK-3beta axis as a new signaling pathway of the histamine H(3) receptor. J Neurochem. 2007;103:248–58.
Babbin BA, Parkos CA, Mandell KJ, Winfree LM, Laur O, Ivanov AI, et al. Annexin 2 regulates intestinal epithelial cell spreading and wound closure through Rho-related signaling. Am J Pathol. 2007;170:951–66.
Fan X, Jiang Y, Yu Z, Liu Q, Guo S, Sun X, et al. Annexin A2 plus low-dose tissue plasminogen activator combination attenuates cerebrovascular dysfunction after focal embolic stroke of rats. Transl Stroke Res. 2017;8:549–59.
Wang X, Fan X, Yu Z, Liao Z, Zhao J, Mandeville E, et al. Effects of tissue plasminogen activator and annexin A2 combination therapy on long-term neurological outcomes of rat focal embolic stroke. Stroke. 2014;45:619–22.
Huang B, Deora AB, He KL, Chen K, Sui G, Jacovina AT, et al. Hypoxia-inducible factor-1 drives annexin A2 system-mediated perivascular fibrin clearance in oxygen-induced retinopathy in mice. Blood. 2011;118:2918–29.
Zhao X, Nedvetsky P, Stanchi F, Vion AC, Popp O, Zuhlke K, et al. Endothelial PKA activity regulates angiogenesis by limiting autophagy through phosphorylation of ATG16L1. Elife. 2019;8:e46380.
Mayo LD, Kessler KM, Pincheira R, Warren RS, Donner DB. Vascular endothelial cell growth factor activates CRE-binding protein by signaling through the KDR receptor tyrosine kinase. J Biol Chem. 2001;276:25184–9.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81722045 and 81973302), Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study (SN-ZJU-SIAS-0011) and the “Ten thousand plan”-high-level talents special support plan of Zhejiang Province (ZJWR0108003).
Author information
Authors and Affiliations
Contributions
LSF and YCC performed the experiments and analyzed the data; LJ, RJL and YYZ performed some of the experiments; WWH wrote the paper; WWH, LJ and ZC conceived the idea; YYZ and XNZ provided constructive advice. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Fan, Ls., Chen, Yc., Liao, Rj. et al. Antagonism of histamine H3 receptor promotes angiogenesis following focal cerebral ischemia. Acta Pharmacol Sin 43, 2807–2816 (2022). https://doi.org/10.1038/s41401-022-00916-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-022-00916-4
Keywords
This article is cited by
-
Angiogenesis after ischemic stroke
Acta Pharmacologica Sinica (2023)