Abstract
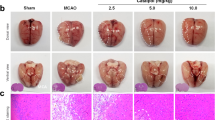
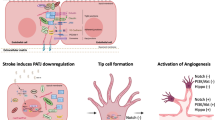
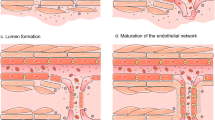
Dl-3-n-butylphthalide (NBP) is a small-molecule drug used in the treatment of ischemic stroke in China, which is proven to ameliorate the symptoms of ischemic stroke and improve the prognosis of patients. Previous studies have shown that NBP accelerates recovery after stroke by promoting angiogenesis. In this study, we investigated the mechanisms underlying the angiogenesis-promoting effects of NBP in ischemic stroke models in vitro and in vivo. OGD/R model was established in human umbilical vein endothelial cells (HUVECs) and human brain microvascular endothelial cells (HBMECs), while the tMCAO model was established in mice. The cells were pretreated with NBP (10, 50, 100 µM); the mice were administered NBP (4, 8 mg/kg, i.v.) twice after tMCAO. We showed that NBP treatment significantly stimulated angiogenesis by inducing massive production of angiogenic growth factors VEGFA and CD31 in both in vitro and in vivo models of ischemic stroke. NBP also increased the tubule formation rate and migration capability of HUVECs in vitro. By conducting the weighted gene co-expression network analysis, we found that these effects were achieved by upregulating the expression of a hedgehog signaling pathway. We demonstrated that NBP treatment not only changed the levels of regulators of the hedgehog signaling pathway but also activated the transcription factor Gli1. The pro-angiogenesis effect of NBP was abolished when the hedgehog signaling pathway was inhibited by GDC-0449 in HUVECs, by Sonic Hedgehog(Shh) knockdown in HUVECs, or by intracerebroventricular injection of AAV-shRNA(shh)-CMV in tMCAO mice. Furthermore, we found that HUVECs produced a pro-angiogenic response not only to autocrine Shh, but also to paracrine Shh secreted by astrocytes. Together, we demonstrate that NBP promotes angiogenesis via upregulating the hedgehog signaling pathway. Our results provide an experimental basis for the clinical use of NBP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wang J, Xing H, Wan L, Jiang X, Wang C, Wu Y. Treatment targets for M2 microglia polarization in ischemic stroke. Biomed Pharmacother. 2018;105:518–25.
GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20:795–820.
Xu R, Bai Y, Min S, Xu X, Tang T, Ju S. In vivo monitoring and assessment of exogenous mesenchymal stem cell-derived exosomes in mice with ischemic stroke by molecular imaging. Int J Nanomed. 2020;15:9011–23.
Papanagiotou P, White CJ. Endovascular reperfusion strategies for acute stroke. JACC Cardiovasc Inter. 2016;9:307–17.
Davis CK, Jain SA, Bae ON, Majid A, Rajanikant GK. Hypoxia mimetic agents for ischemic stroke. Front Cell Dev Biol. 2018;6:175.
Xu S, Lu J, Shao A, Zhang JH, Zhang J. Glial cells: role of the immune response in ischemic stroke. Front Immunol. 2020;11:294.
Dąbrowski J, Czajka A, Zielińska-Turek J, Jaroszyński J, Furtak-Niczyporuk M, Mela A, et al. Brain functional reserve in the context of neuroplasticity after stroke. Neural Plast. 2019;2019:9708905.
Barthels D, Das H. Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165–260.
Durukan A, Strbian D, Tatlisumak T. Rodent models of ischemic stroke: a useful tool for stroke drug development. Curr Pharm Des. 2008;14:59–70.
Paul S, Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol. 2021;335:113518.
Hatakeyama M, Ninomiya I, Kanazawa M. Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen Res. 2020;15:16–9.
Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117:481–96.
Shim JW, Madsen JR. VEGF signaling in neurological disorders. Int J Mol Sci. 2018;19:275.
Greenberg DA, Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci. 2013;70:1753–61.
Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–9.
Wang S, Ma F, Huang L, Zhang Y, Peng Y, Xing C, et al. Dl-3-n-Butylphthalide (NBP): a promising therapeutic agent for ischemic stroke. CNS Neurol Disord Drug Targets. 2018;17:338–47.
Chen XQ, Qiu K, Liu H, He Q, Bai JH, Lu W. Application and prospects of butylphthalide for the treatment of neurologic diseases. Chin Med J (Engl). 2019;132:1467–77.
Xing X, Huang L, Lv Y, Liu X, Su R, Li X, et al. DL-3-n-butylphthalide protected retinal Müller cells dysfunction from oxidative stress. Curr Eye Res. 2019;44:1112–20.
Li J, Liu Y, Zhang X, Chen R, Zhang L, Xue J, et al. Dl-3-N-butylphthalide alleviates the blood-brain barrier permeability of focal cerebral ischemia reperfusion in mice. Neuroscience. 2019;413:99–107.
Peng Y, Zeng X, Feng Y, Wang X. Antiplatelet and antithrombotic activity of L-3-n-butylphthalide in rats. J Cardiovasc Pharmacol. 2004;43:876–81.
Huang L, Wang S, Ma F, Zhang Y, Peng Y, Xing C, et al. From stroke to neurodegenerative diseases: the multi-target neuroprotective effects of 3-n-butylphthalide and its derivatives. Pharmacol Res. 2018;135:201–11.
Abdoulaye IA, Guo YJ. A review of recent advances in neuroprotective potential of 3-N-butylphthalide and its derivatives. Biomed Res Int. 2016;2016:5012341.
Liao W, Zhong Y, Cheng W, Dong LF. 3-N-butylphthalide inhibits neuronal apoptosis in rats with cerebral infarction via targeting P38/MAPK. Eur Rev Med Pharmacol Sci. 2019;23:144–52.
Wei ZZ, Chen D, Lee MJH, Zhao Y, Gu X, Yu SP, et al. DL-3-n-butylphthalide increases collateriogenesis and functional recovery after focal ischemic stroke in mice. Aging Dis. 2021;12:1835–49.
Qin C, Zhou P, Wang L, Mamtilahun M, Li W, Zhang Z, et al. Dl-3-N- butylphthalide attenuates ischemic reperfusion injury by improving the function of cerebral artery and circulation. J Cereb Blood Flow Metab. 2019;39:2011–21.
Zhang T, Jia W, Sun X. 3-n-Butylphthalide (NBP) reduces apoptosis and enhances vascular endothelial growth factor (VEGF) up-regulation in diabetic rats. Neurol Res. 2010;32:390–6.
Zhu BL, Xie CL, Hu NN, Zhu XB, Liu CF. Inhibiting of GRASP65 phosphorylation by DL-3-N-Butylphthalide protects against cerebral ischemia-reperfusion injury via ERK signaling. Behav Neurol. 2018;2018:5701–19.
Liu S, Chang L, Wei C. The sonic hedgehog pathway mediates Tongxinluo capsule-induced protection against blood-brain barrier disruption after ischaemic stroke in mice. Basic Clin Pharmacol Toxicol. 2019;124:660–9.
Zhou PT, Wang LP, Qu MJ, Shen H, Zheng HR, Deng LD, et al. Dl-3-N-butylphthalide promotes angiogenesis and upregulates sonic hedgehog expression after cerebral ischemia in rats. CNS Neurosci Ther. 2019;25:748–58.
Kumari S, Chaurasia SN, Kumar K, Dash D. Anti-apoptotic role of sonic hedgehog on blood platelets. Thromb Res. 2014;134:1311–5.
Jin Y, Barnett A, Zhang Y, Yu X, Luo Y. Poststroke sonic hedgehog agonist treatment improves functional recovery by enhancing neurogenesis and angiogenesis. Stroke. 2017;48:1636–45.
Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener. 2011;6:11.
Meadows KL. Experimental models of focal and multifocal cerebral ischemia: a review. Rev Neurosci. 2018;29:661–74.
Tang H, Gamdzyk M, Huang L, Gao L, Lenahan C, Kang R, et al. Delayed recanalization after MCAO ameliorates ischemic stroke by inhibiting apoptosis via HGF/c-Met/STAT3/Bcl-2 pathway in rats. Exp Neurol. 2020;330:113359.
Yang J, Yan H, Li S, Zhang M. Berberine ameliorates MCAO induced cerebral ischemia/reperfusion injury via activation of the BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem Res. 2018;43:702–10.
Zhou Z, Xu N, Matei N, McBride DW, Ding Y, Liang H, et al. Sodium butyrate attenuated neuronal apoptosis via GPR41/Gβγ/PI3K/Akt pathway after MCAO in rats. J Cereb Blood Flow Metab. 2021;41:267–81.
Liao SJ, Lin JW, Pei Z, Liu CL, Zeng JS, Huang RX. Enhanced angiogenesis with dl-3n-butylphthalide treatment after focal cerebral ischemia in RHRSP. Brain Res. 2009;1289:69–78.
Langfelder P, Horvath S. Fast R functions for robust correlations and hierarchical clustering. J Stat Softw. 2012;46:i11.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47.
Dang R, Wang M, Li X, Wang H, Liu L, Wu Q, et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J Neuroinflammation. 2022;19:41.
Jha NK, Chen WC, Kumar S, Dubey R, Tsai LW, Kar R, et al. Molecular mechanisms of developmental pathways in neurological disorders: a pharmacological and therapeutic review. Open Biol. 2022;12:210289.
Liu L, Zhao B, Xiong X, Xia Z. The neuroprotective roles of sonic hedgehog signaling pathway in ischemic stroke. Neurochem Res. 2018;43:2199–211.
Brennan D, Chen X, Cheng L, Mahoney M, Riobo NA. Noncanonical hedgehog signaling. Vitam Horm. 2012;88:55–72.
Petrov K, Wierbowski BM, Salic A. Sending and receiving hedgehog signals. Annu Rev Cell Dev Biol. 2017;33:145–68.
Patel SS, Tomar S, Sharma D, Mahindroo N, Udayabanu M. Targeting sonic hedgehog signaling in neurological disorders. Neurosci Biobehav Rev. 2017;74:76–97.
Pola R, Ling LE, Aprahamian TR, Barban E, Bosch-Marce M, Curry C, et al. Postnatal recapitulation of embryonichedgehog pathway in response to skeletal muscle ischemia. Circulation. 2003;108:479–85.22
Byrd N, Grabel L. Hedgehog signaling in murine vasculogenesis andangiogenesis. Trends Cardiovasc Med. 2004;14:308–13.
Briscoe J, Therond PP. The mechanisms of Hedgehog signalling andits roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–29.
Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29:4351–5.
Liu X, Liu R, Fu D, Wu H, Zhao X, Sun Y, et al. Dl-3-n-butylphthalide inhibits neuroinflammation by stimulating foxp3 and Ki-67 in an ischemic stroke model. Aging (Albany NY). 2021;13:3763–78.
Huang Y, Pan L, Wu T. Improvement of cerebral ischemia-reperfusion injury by L-3-n-butylphthalide through promoting angiogenesis. J Exp Brain Res. 2021;239:341–50.
Wei H, Zhan LP, Zhang B, Li YP, Pei Z, Li L. dl-3n-butylphthalide reduces oxygen-glucose deprivation-induced endothelial cell damage by increasing PGC-1α. Eur Rev Med Pharmacol Sci. 2019;23:4481–90.
Chen N, Zhou Z, Li J, Li B, Feng J, He D, et al. 3-n-butylphthalide exerts neuroprotective effects by enhancing anti-oxidation and attenuating mitochondrial dysfunction in an in vitro model of ischemic stroke. Drug Des Dev Ther. 2018;12:4261–71.
Yang CS, Guo A, Li Y, Shi K, Shi FD, Li M. Dl-3-n-butylphthalide reduces neurovascular inflammation and ischemic brain injury in mice. Aging Dis. 2019;10:964–76.
Wang Y, Shen Y, Liu Z, Gu J, Xu C, Qian S. Dl-NBP (Dl-3-N-Butylphthalide) treatment promotes neurological functional recovery accompanied by the upregulation of white matter integrity and HIF-1α/VEGF/Notch/Dll4 expression. Front Pharmacol. 2019;10:1595.
Rakocevic J, Orlic D, Mitrovic-Ajtic O, Tomasevic M, Dobric M, Zlatic N, et al. Endothelial cell markers from clinician’s perspective. Exp Mol Pathol. 2017;102:303–13.
Wang YB, Yuan HF, Zhi W, Wang Q, Hao GZ, Jiang YF. The effect and mechanism of dl-3-n-butylphthalide on angiogenesis in a rat model of chronic myocardial ischemia. Am J Transl Res. 2022;14:4719–27.
Katoh M. Genomic testing, tumor microenvironment and targeted therapy of Hedgehog-related human cancers. Clin Sci (Lond). 2019;133:953–70.
Niu XL, Jiang X, Xu GD, Zheng GM, Tang ZP, Yin N, et al. DL-3-n-butylphthalide alleviates vascular cognitive impairment by regulating endoplasmic reticulum stress and the Shh/Ptch1 signaling-pathway in rats. J Cell Physiol. 2019;234:12604–14.
Pekny M, Pekna M, Messing A, Steinhäuser C, Lee JM, Parpura V, et al. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 2016;131:323–45.
Uchiyama M, Nakao A, Kurita Y, Fukushi I, Takeda K, Numata T, et al. O2-dependent protein internalization underlies astrocytic sensing of acute hypoxia by restricting multimodal TRPA1 channel responses. Curr Biol. 2020;30:3378–96.e7.
Tang T, Hu L, Liu Y, Fu X, Li J, Yan F, et al. Sex-associated differences in neurovascular dysfunction during ischemic stroke. Front Mol Neurosci. 2022;15:860959.
Shen D, Wu W, Liu J, Lan T, Xiao Z, Gai K, et al. Ferroptosis in oligodendrocyte progenitor cells mediates white matter injury after hemorrhagic stroke. Cell Death Dis. 2022;13:259.
Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, et al. Stromal cell-derived factor 1alpha mediates neural progenitor cell motilityafter focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–34.
Arai K, Lo EH. Wiring and plumbing: oligodendrocyte precursors and angiogenesis in the oligovascular niche. J Cereb Blood Flow Metab. 2021;41:2132–3.
Acknowledgements
This work was supported by grants from the key program of the Educational Department of Liao Ning Province (Nos. LJKZZ20220100).
Author information
Authors and Affiliations
Contributions
WC, MJD, and STL designed the research. MJD and XXG performed most of the experiments and data analyses. SMJ and STL performed some experiments. HD was responsible for bioinformatics analyses, and MJD wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, Mj., Gui, Xx., Jia, Sm. et al. Dl-3-n-butylphthalide promotes angiogenesis in ischemic stroke mice through upregulating autocrine and paracrine sonic hedgehog. Acta Pharmacol Sin 44, 2404–2417 (2023). https://doi.org/10.1038/s41401-023-01137-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01137-z