Abstract
Currently, nonalcoholic steatohepatitis (NASH) is one of the most common forms of chronic hepatitis, increasing the burden of health care worldwide. In patients with NASH, the fibrosis stage is the most predictive factor of long-term events. However, there are still no drugs approved by the Food and Drug Administration of the United States for treating biopsy-proven NASH with fibrosis or cirrhosis. Although some novel drugs have shown promise in preclinical studies and led to improvement in terms of hepatic fat content and steatohepatitis, a considerable proportion of them have failed to achieve histological endpoints of fibrosis improvement. Due to the large number of NASH patients and adverse clinical outcomes, the search for novel drugs is necessary. In this review, we discuss current definitions for the evaluation of treatment efficacy in fibrosis improvement for NASH patients, and we summarize novel agents in the pipeline from different mechanisms and phases of trial. We also critically review the challenges we face in the development of novel agents for fibrotic NASH and NASH cirrhosis.
Similar content being viewed by others
Introduction
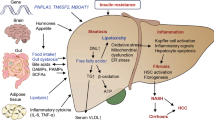
Nonalcoholic steatohepatitis (NASH) is a severe form of nonalcoholic fatty liver disease (NAFLD) presenting as steatosis-associated inflammation and liver injury [1]. With the global pandemic of obesity and metabolic syndrome, there has also been an increase in the prevalence of NAFLD and NASH in recent decades. It is estimated that NASH affects 1.5%–6.5% of the general population [2]. Excessive accumulation of lipids overwhelms the metabolic capacity of the liver, forming lipotoxic lipids, followed by oxidative stress, inflammation and hepatocellular damage through necrotic, apoptotic or pyroptotic pathways. These damaging signals comprehensively activate hepatic stellate cells and lead to fibrogenesis [3]. Approximately 25% of patients with NASH are accompanied by varying degrees of liver fibrosis, and the prevalence of NASH-related cirrhosis is ~1%–2% in the general population. Approximately 15%–25% of NASH patients develop cirrhosis in 10–15 years [4]. Moreover, NASH patients with advanced fibrosis could progress to a life-threatening outcome more rapidly, including decompensations, hepatocellular carcinoma, or the need for a liver transplant.
The NAFLD guidelines of the European Association for the Study of the Liver (EASL) define NASH patients with significant (≥F2) or advanced fibrosis (≥F3) as fibrotic NASH [5]. Compared with early NASH (with stage F0–F1 fibrosis), fibrotic NASH has a greater risk of all-cause mortality and worse long-term prognosis [6]. As the most important histological characteristic, liver fibrosis not only increases the risk of liver-related events [7] but also promotes the development of type 2 diabetes mellitus (T2DM), cardiovascular diseases, chronic kidney disease, and extrahepatic malignancy [8]. Currently, there are still no effective approved pharmacotherapies for the treatment of NASH and fibrosis. The huge unmet clinical needs have aroused wide public concern and attracted interest in drug discovery. In recent decades, numerous agents have progressed into the clinical development pipeline or have been tested in clinical trials. However, we are still not fully aware of the multiple pathways implicated in NASH and the related pathogenesis of fibrosis, and we do not know which agent would be effective among a broad range of targets. In this review, we will discuss the advances and challenges in developing novel drugs for reversing liver fibrosis in NASH patients.
How to define the treatment efficacy of fibrosis
Liver biopsy: gold is not pure gold
Evaluating the effectiveness in clinical trials may be a Gordian knot. Based on current evidence, there is a strong link between the resolution of steatohepatitis and improvement in fibrosis [9]. In addition, the fibrosis stage is the only histological marker predictive of the long-term outcomes of patients with NAFLD/NASH. The fibrosis stages serve as indicators for NASH patient enrollment and efficacy evaluation. As a gold standard for assessing liver fibrosis, biopsy is still recommended for patients who are at risk of significant or advanced fibrosis by the NAFLD guidelines of EASL and the American Association for the Study of Liver Diseases (AASLD) [1, 5]. The United States Food and Drug Administration (FDA), National Medical Products Administration of China and European Medicines Agency (EMA) all recommend a histological diagnosis of NASH with liver fibrosis before enrollment in NASH trials. For NASH patient enrollment, these agencies recommend phase 3 trials to enroll patients with stage >1 and < 4 fibrosis (F2–3), while EMA recommends that patients with stage 1 fibrosis (F1–3) be included in trials for exploratory purposes. For the treatment efficacy of fibrosis, these agencies all defined an improvement in liver fibrosis ≥1 stage with no worsening of NASH as the endpoint of treatment [10,11,12,13].
However, several issues are recognized in the application of these standards. First, we cannot avoid the randomness and bias of liver biopsies. Liver biopsy specimens cannot reflect the pathological changes of the whole liver, and sampling error is thus inevitable [14]. In addition, the scoring systems for fibrosis are semiquantitative and subjective. Interreader and intrareader inconsistency also added bias to the results [15]. Second, the progression or regression of fibrosis is continuous, while the fibrosis stage scores are categorical variables. The progression of fibrosis is not linear, and patients with advanced fibrosis or cirrhosis have exponentially higher risks of liver-related events than those without [16]. Therefore, one-stage improvement of fibrosis has different benefits for NASH patients with different stages. Third, the current definition of fibrosis improvement only focuses on the proportion of NASH patients with fibrosis improvement and neglects the proportion of patients with stable or worsened fibrosis. As a result, the current criteria cannot be used to compare the comprehensive efficacy among different novel agents [17].
Solutions and alternative approaches to assess fibrotic changes
Despite the limitations mentioned above, the current approaches for evaluating the efficacy of novel NASH drugs still relies on semiquantitative scoring systems. The results of histological assessment have a direct impact on the success or failure of novel drug trials. The low agreement of histological assessment can be partially explained by the lack of a standard scoring procedure. Consensus training for all pathologists is needed prior to specimen processing and histological assessment, which could increase the level of agreement [18]. To ensure the quality of the biopsies, protocols should define the size and location of liver biopsy specimens. Standard operation procedures for fixation and staining are also recommended [18]. Central reading of liver biopsy should include two or more pathologists blinded to the study design. Differences in observations can be evaluated and addressed in group review sessions or by pathology committees [19]. In addition, the sponsors of trials should also specify the details of liver biopsy interpretation in advance.
Although with this standard training and education, pathologists have failed to improve interobserver agreement in some situations [20]. Therefore, the clinical need is to develop more quantitative and objective alternative approaches for histological assessment. In recent decades, the use of whole-slide imaging (WSI) has grown considerably [21]. WSI provides digitalization of entire histologic sections, offers highly detailed information about tissue morphology, and enables the development of digital pathology [22]. WSI detects potential steatotic hepatocytes through segregation of the overlapping steatosis component [23]. WSI could also detect fibrosis through quantification of both collagen and elastic fibers [24]. Another tool that accurately quantifies components of NASH histology is qFIBS, which can be used to distinguish differing stages of fibrosis and steatosis [25]. Thin perivessel collagen fibers, the solidity of collagen fibers and the area of vessel-bridging collagen fibers of qFIBS also had a high sensitivity and specificity in predicting regression of fibrosis [26]. Of the quantitative assessment approaches, machine learning-based histological assessment has been applied to measure antifibrotic treatment effects and histological disease progression in trials [27]. These novel techniques showed promise as the new gold standard for the quantitative measurement of liver fibrosis. This also emphasizes the pivotal role of liver biopsy in assessing the fibrotic efficacy for NASH treatment.
Investigational pharmacotherapies: no cross, no crown
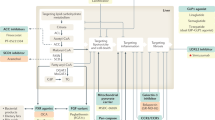
Despite favorable lifestyle modifications, only a small proportion of NASH patients can achieve fibrosis regression. In addition, long-term persistence with physical activity or dieting would be hard for most patients with NASH. It has been reported that nearly half of patients failed to achieve the goal of weight loss [28]. Hence, medications are more necessary for most NASH patients. The development of fibrosis in NASH has a complex pathogenesis involving genetic, epigenetic, metabolic and environmental factors. Treatment targeting insulin resistance and inflammatory injury would be effective in solving the key factors in NASH. As there are no approved pharmacotherapies for the treatment of NASH or even fibrotic NASH, traditional antioxidants, including vitamin E, pioglitazone, pentoxifylline and metformin, have been verified in NASH patients. However, most of these agents have uncertain benefits for treating fibrotic NASH and a lack evidence of long-term safety [29,30,31,32]. Numerous novel drugs in the pipeline for the treatment of NASH are being estimated in clinical trials, and many of them seem promising. Among them, we summarized the drugs using composite histological endpoints recommended by the FDA and compared their efficacy on fibrosis improvement and NASH resolution (Figs. 1 and 2).
Farnesoid X receptors: the agent closest to the approval
Obeticholic acid (OCA) is a semisynthetic derivative of chenodeoxycholic acid, a potent agonist of farnesoid X receptor (FXR). In the previous FLINT study, OCA achieved improved fibrosis stages (−0.2 vs. 0.1, P = 0.010) and a higher rate of fibrosis regression (35% vs. 19%, P = 0.004) than the placebo during 72 weeks of treatment. Among NASH patients with stage F2–F4 fibrosis, the rate of fibrosis regression was also higher than that of placebo (49% vs. 23%), suggesting that OCA might be a beneficial intervention in reversing fibrosis and cirrhosis in NASH patients [33]. In the following phase 3 trial (the REGENERATE study), which enrolled 56% patients with fibrosis stage F3, fibrosis improvement with no worsening of NASH was achieved in 23% of the 25 mg OCA group compared with 12% of the placebo group (P = 0.002). In the 25 mg OCA group, 38% of the patients achieved fibrosis improvement ≥1 stage, and 10% achieved fibrosis improvement ≥2 stages [34]. The significant efficacy in improving fibrosis demonstrates the effectiveness of the FXR target and makes OCA the most promising agent for FDA approval. However, considering the high incidence of pruritus and increased risk of dyslipidemia [35], the FDA has concerns about the risks and benefits of the long-term use of OCA and therefore did not accept the application.
Another FXR agonist, cilofexor (GS-9674), achieved reductions in hepatic steatosis assessed by magnetic resonance imaging-proton density fat fraction (MRI-PDFF) in NASH patients during 24 weeks of treatment, whereas the liver stiffness values estimated by magnetic resonance elastography (MRE) were not improved [36]. As NASH is a slow progressive disease, fibrosis regression in NASH patients requires a longer treatment time. Hence, this study was weak in treatment duration. Similar to other FXR agonists, cilofexor also had a high proportion (14%) of pruritus, which may lead to poor patient compliance. Other FXR agonists, including tropifexor, TERN-101, EDP-297, and EDP-305, were still in their earlier phases of NASH trials and lacked biopsy-based results [37,38,39]. The goal of FXR agonist development could be to find a balance between effectiveness in improving fibrosis and long-term safety.
Promising agents targeting glycolipid metabolism
Considering the association of NASH with obesity, insulin resistance and metabolic disorders, the international expert consensus statement presented a new definition of metabolic dysfunction-associated fatty liver disease (MAFLD) [40]. The new nomenclature calls for more recognition of metabolic disorders and associations within the spectrum of liver disease. Currently, many drugs for NASH treatment are based on glycolipid metabolism or used to treat T2DM. These drugs inherently address obesity and related glycolipid metabolism and are more likely to achieve hepatic and extrahepatic benefits. In addition, these drugs are often used in clinical trials in NASH patients with concomitant T2DM, which could reduce the heterogeneity of the patients.
Glucagon-like peptide-1 (GLP-1) agonists
Liraglutide is a long-acting GLP-1 analog that has been approved for the treatment of T2DM. Histological evidence of liraglutide improving fibrosis was first described in the LEAN study [41]. Although patients in the liraglutide group had a decrease in fibrosis score (−0.2 vs. 0.2, P = 0.11) and achieved more fibrosis regression (26% vs. 14%, P = 0.46) compared with the placebo, histological evidence of fibrosis improvement was not found. Another study on liraglutide (LEAN-J) focused on NAFLD patients with T2DM had similar findings [42]. As a representative GLP-1, liraglutide gained the attention of NAFLD guidelines from AASLD [1]. However, considering the insufficient evidence, it may be too early for liraglutide to be applied in treating fibrotic NASH.
Recently, Newsome et al. reported a high NASH resolution rate (59%) with no worsening of fibrosis in a phase 2 trial of semaglutide through 72 weeks of treatment in NASH patients with F1–F3 fibrosis [43]. Compared with a previous report, semaglutide had a significantly higher NASH resolution rate than liraglutide (39%). However, as the secondary endpoint, the proportion of fibrosis improvement and no worsening of NASH did not seem to be different between the 0.4 mg group and the placebo group (43% vs. 33%, P = 0.48). The authors speculated that a longer treatment duration was needed to clarify the outcome of fibrosis. Furthermore, we noticed that although the improvement in fibrosis among the groups was similar, the 0.4 mg group had a lower rate of fibrosis progression than the placebo group (4.9% vs. 31%). As the progression of fibrosis in NASH patients is not a one-way linear process, the current endpoint setting for the evaluation of fibrotic changes in clinical trials may have some limitations.
More novel GLP-1 agonists are being investigated in clinical research. Exenatide, tirzepatide and dulaglutide all had potential efficacy in reducing the liver fat content and serum liver enzyme levels as well as histological improvement of NASH [44, 45]. GLP-1 agonists also showed effectiveness in terms of weight loss, hypoglycemic effects and cardiovascular benefits [46]. As GLP-1 agonists can slow gastric emptying, gastrointestinal symptoms were reported, including loss of appetite and nausea during treatment. These events might prevent patients from overeating but would also reduce compliance. Considering compliance due to subcutaneous injection, modified dosage forms are trying to reduce the frequency to once a week. In addition, the FDA has approved the first oral GLP-1 agonist for the treatment of T2DM [47]. Although evidence on NASH treatment is still unavailable, we believe oral formulations may help improve compliance for the long-term treatment of NASH.
Peroxisome proliferator-activated receptor (PPAR) agonists
Lanifibranor, a pan-PPAR agonist, is one of the most recently developed potential NASH drugs. Lanifibranor has been proven to decrease the proinflammatory activation of macrophages and reduce steatosis, inflammation and fibrosis in NASH in mouse models [48, 49]. In a phase 2 randomized trial named NATIVE (NCT03008070), 24 weeks of lanifibranor treatment achieved the primary endpoint of a significant reduction in the steatosis activity fibrosis score with no worsening of fibrosis (49% vs. 27%, P = 0.004). Moreover, the 1200 mg group also achieved significant improvement of fibrosis without worsening of NASH compared with the placebo group (46% vs. 29%, P = 0.040) [50, 51]. To date, lanifibranor is the first agent to achieved the endpoints and to be recommended by both the FDA and EMA at the same time. Therefore, lanifibranor received FDA breakthrough therapy designation for the treatment of NASH. Similar to other insulin sensitizers, patients receiving lanifibranor had weight gain during treatment (2.6%–3.1% from baseline). A phase 3 study of lanifibranor (NATIVE) will be conducted in 2000 patients with NASH and stages F2–F3 for 72 weeks, and it is now recruiting (NCT04849728).
Elafibranor is a dual activator of PPAR α/δ. Previously, elafibranor did not meet the predefined endpoint in the intention-to-treat population in the GOLDEN-505 study. The difference in NASH resolution without a worsening fibrosis rate compared with the placebo was only seen in a post hoc analysis for the modified definition [52]. Although patients with NASH resolution in the elafibranor 120 mg group had a greater decrease in fibrosis stages than those without NASH resolution (0.65 ± 0.61 vs. 0.10 ± 0.98, P < 0.001), the phase 3 clinical trial (RESOLVE-IT) was not completed. In the results recently announced, elafibranor did not meet the predefined study endpoint, and the trial was terminated (NCT02704403).
Saroglitazar was the “first” approved drug for the treatment of NASH by the Drugs Controller General of India (known as DCGI) [53]. As a dual PPARα/γ agonist, saroglitazar was previously used in India for diabetic dyslipidemia. In a phase 2 trial, saroglitazar showed promising results in the treatment of NASH. It reduced the liver fat content and improved ALT and insulin resistance in patients with NAFLD/NASH [54]. However, the efficacy of saroglitazar in NASH is still limited. Further biopsy-proven studies with histological endpoints are now recruiting to accumulate more “hard” evidence (NCT05011305).
Pioglitazone is a PPAR-γ agonist used as a classic drug for the treatment of T2DM. In the PIVENS study, the pioglitazone group achieved a higher rate of NASH resolution than the placebo group (47% vs. 21%, P < 0.001) [29]. Therefore, pioglitazone was recommended by AASLD guidelines for biopsy-proven NASH patients with and without T2DM [1]. In terms of fibrosis improvement, the pioglitazone group failed to show difference in the decrease in fibrosis score compared with the placebo group (44% vs. 31%). However, a meta-analysis of thiazolidinediones revealed that pioglitazone was associated with improved fibrosis in NASH patients with advanced fibrosis with an odds ratio of 5.84 (95% CI: 2.04, 16.71) [55]. Even with these histological improvements, a considerable portion of patients had weight gain during treatment, which would make this agent less attractive in obese NAFLD patients.
Endocrine fibroblast growth factors (FGF) analogs
FGF19 and FGF21 are novel endocrine messengers regulating multiple aspects of energy metabolism. Analogs of FGF19 and FGF21 have shown therapeutic promise in NASH treatment [56, 57]. In the open‐label study of aldafermin (NGM282), an FGF19 analog, 12 weeks of treatment achieved successful histological improvement in NASH patients. Twenty-five percent and 42% of the patients in the 1 or 3 mg group showed improvement in fibrosis ≥1 stage without worsening of NASH, respectively [58]. In the phase 2 trial of aldafermin, fibrosis improvement without worsening of NASH was achieved in 38% of patients receiving aldafermin vs. 18% of placebo (P = 0.10) [59]. Although the promising results of aldafermin revealed a potential benefit for fibrotic NASH patients, the phase 2 trial (ALPINE 4) did not meet the primary endpoint of fibrosis improvement by >1 stage with no worsening of NASH versus the placebo (NCT04210245) [60].
Efruxifermin is a long-acting Fc-FGF21 fusion protein that can regulate lipid and glucagon metabolism systemically and promote weight loss. Recently, the phase 2a trial of efruxifermin (BALANCED study) reported the positive results of a significant reduction in hepatic fat content by MRI-PDFF and potential histological benefits during 16 weeks of treatment. In this study, only efruxifermin-treated responders received a second liver biopsy. Fibrosis improvement with no worsening of NASH was achieved in 48% of the patients receiving efruxifermin, of which 28% even achieved fibrosis improvement ≥2 stages [61]. Recently, the results of efruxifermin for NASH-related cirrhosis (F4 fibrosis, cohort C) were released at the International Liver Congress of EASL [62]. Following only 16 weeks of treatment, efruxifermin showed trends to reverse cirrhosis: 33% of the patients achieved fibrosis improvement without worsening of NASH. Efruxifermin also received priority medicines (PRIME) from EMA. In addition, considering the low selectivity of FGFs, the safety of efruxifermin still needs to be determined in further studies.
Pegbelfermin (BMS-986036) is a pegylated FGF21 analog. In a 16-week phase 2a study, more than half of the NASH patients treated with pegbelfermin showed at least a 30% reduction in hepatic fat fraction, and nearly 1/3 of the patients showed decreased liver stiffness measured by MRE [63]. BIO89-100 is a glycol-pegylated FGF21 variant, and BIO89-100 also demonstrated a reduction in the hepatic fat fraction in NASH patients [64]. Although these FGF21 analogs showed promising improvements related to NASH, further trails are going on and will clarify fibrosis improvement and long-term safety (NCT04929483).
Thyroid hormone receptor-β (THR-β) agonist
THR-β is the most abundant thyroid hormone receptor isoform in the liver. Previous studies have demonstrated the association between low-normal thyroid function and advanced fibrosis in NASH [65]. THR-β agonist therapy may increase hepatic fat metabolism and reduce lipotoxicity and therefore reverse NASH and fibrosis. In a recent phase 2 study, resmetirom (MGL-3196) showed a potent reduction in hepatic fat content after 36 weeks of treatment. Approximately 37% of the resmetirom group had a reduction in liver fat compared with 9% of the placebo group (P < 0.0001) measured by MRI-PDFF [66]. Although there was no significant improvement of liver fibrosis on biopsy, in the extension of the trial, noninvasive fibrosis markers were significantly reduced [67]. VK2809 is another selective THR-β agonist that has been found to reduce liver fat through 12 weeks of treatment, as measured by MRI-PDFF [68]. A biopsy-based phase 2 trial is now recruiting (NCT04173065).
Investigational drugs targeting oxidative stress, inflammation, and fibrosis
Prevention and regression of cirrhosis are the ultimate goals in NASH treatment, and effective drug treatments are thus required. For NASH patients, the fibrosis stages F2–F3 are more cost effective for treatment, while the treatment for patients with cirrhosis is faced with more challenges.
Cenicriviroc is a dual antagonist of C–C chemokine receptor types 2 and 5 (CCR2/CCR5). In the phase 2b trial (CENTAUR), the cenicriviroc group demonstrated a higher rate of decreased fibrosis stage (29% vs. 19%), fibrosis resolution (7% vs. 3%), and fibrosis improvement ≥1 stage without steatohepatitis worsening (20% vs. 10%) than the placebo group [69]. In the final analysis of the CENTAUR study, patients with cenicriviroc treatment for 2 years had twice the proportion of fibrosis improvement than the placebo (60% vs. 30%), but the proportion of fibrosis improvement ≥1 stage with no worsening of NASH did not differ from that of the placebo (19.9% vs. 11.1%, P = 0.09) [70]. The authors suggested that the natural progression of fibrosis may lead to qualitative differences in fibrosis regression. The phase 3 trial of cenicriviroc (AURORA, NCT03028740) and its combination with tropifexor (TANDEM) is now ongoing to investigate their efficacy [71].
Unfortunately, many drugs targeting liver fibrosis and inflammation have reached late-phase studies but fall short of the endpoint. Selonsertib and emricasan both target cell apoptosis signal regulation but did not lead to fibrosis regression. Although selonsertib had promising effects on NASH patients with fibrosis stages F2–F3 [72], it failed to demonstrate a significant improvement in NASH patients with stage F3 (STELLAR-3 study) or cirrhosis (STELLAR 4 study) [73]. Emricasan is a pancaspase inhibitor and showed potential improvement in serum biomarkers of NAFLD in previous studies [74]. Emricasan did not achieve the primary endpoint and even had worse fibrosis outcomes [75]. Simtuzumab and belapectin (GR-MD-02) are both antifibrotic agents, but their antifibrotic efficacy is not satisfactory. Although belapectin showed potential efficacy in NASH with advanced fibrosis/cirrhosis [76], it failed to improve liver fibrosis in NASH patients with cirrhosis in a phase IIb study [77]. Simtuzumab is a monoclonal antibody against lysyl oxidase-like 2 (LOXL2) that also failed to improve fibrosis in two phase 2b trials [78]. Investigators considered this unexpected negative outcome to be due to inefficient antibodies. LOX family members play a key role in extracellular matrix crosslinking. LOX family members other than LOXL2 may also prove to be attractive therapeutic targets [79].
Despite so many failures, numerous therapeutic agents are still being developed. Potent agents targeting advanced fibrosis and cirrhosis still need time. We may need to consider the reasons from aspects of study design, clinical endpoints, patient recruitment and disease heterogeneity and learn lessons. Pathophysiological research and new drug pipelines would bring more possibilities.
Emerging strategies and perspectives for NASH therapies
Of the chronic liver diseases, NASH has the largest global market. The number of NASH trials is rapidly increasing according to clinical registrations. An increasing number of targets are being researched and discussed, and novel therapies in their early stage of study also offer hope for the cure of NASH. Modulators of fatty acid synthase could reduce excess liver fat and inhibit inflammatory and fibrogenic pathways. Recently, Ratziu et al. reported the results of 52 weeks of aramchol treatment. Although it did not reach the primary endpoint of liver fat reduction measured by magnetic resonance spectroscopy, aramchol achieved potential improvement in liver histology [80]. The phase 3 trial of aramchol is now recruiting (NCT04104321). TVB-2640 also showed a significant reduction in liver fat and improvements in metabolic and fibrotic markers in NASH patients in its phase 2 studies [81]. HTD1801, an ionic salt of berberine and ursodeoxycholic acid, achieved a reduction in liver fat content, improvement in liver enzymes, and significant weight loss in presumed NASH patients with T2DM [82]. ARO-HSD was the first investigational RNAi therapy to inhibit HSD17B13 mRNA and protein, which showed improvements in liver enzymes (NCT04202354) [83]. Volixibat, an inhibitor of the apical sodium-dependent bile acid transporter, has failed to show efficacy in improving the histological characteristics of NASH [84]. Altogether, the efficacy of these novel agents in improving fibrosis still needs further investigation.
As a disease with complex etiology and mechanisms, NASH could benefit from combination treatment. Combination therapies with complementary mechanisms are more likely to increase the effectiveness and reduce adverse events and are therefore recognized as promising standard NASH therapies in the future. Currently, the combination of an FXR agonist with a sodium-glucose cotransporter inhibitor (NCT04065841), a CCR2/5 antagonist (NCT03517540), acetyl-CoA carboxylase (ACC) inhibitors [85] and both an ACC inhibitor and a GLP1 receptor agonist (NCT03987074, NCT04971785) are being investigated in ongoing phase II clinical trials.
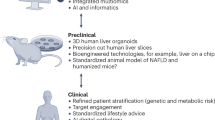
Major challenges in the development of novel drugs for fibrotic NASH
NASH slowly progresses from hepatic inflammation and fibrosis to cirrhosis and HCC [4]. With chronic histological changes, NASH patients may need potential lifelong treatment similar to other metabolic disorders. Patients with bland steatosis could be enrolled in early-stage studies evaluating lipid metabolism and hepatic fat changes. Lifestyle intervention and weight loss management may significantly impede the progression of simple steatosis. While for patients with fibrotic NASH, late-phase clinical trials should consider preliminary evidence of efficacy, assess potential time duration for treatment response and include histologically based endpoints or alternative approaches, including MRE and MRI-PDFF. In addition, agents for particular targets tend to enroll NASH patients with relative comorbidities to achieve a higher proportion of endpoints (Fig. 3).
Patient recruitment and disease heterogeneity
As the process of fibrosis progression varies greatly among NASH patients with or without cirrhois [86], the adequate duration of trials based on histological evidences could be different in these two groups of patients. Therefore, the FDA does not recommend merging noncirrhotic and cirrhotic NASH patients in the same analysis population [13]. Furthermore, there may also be differences in endpoint setting, management, and risk screening in the two subgroups of patients. In addition, the sample size should be sufficiently large to provide a presupposed security database. Considering the drug benefits and risks, NASH patients with different BMI classifications and concomitant metabolic disorders will be needed in the design of clinical trials to improve efficacy.
The FDA described a detailed definition for the enrollment of NASH patients for phase 3 trials. The heterogeneity of NASH mainly comprises body weight, metabolic factors, and genetic factors. For inclusion, NASH patients should have stable body weight for at least 3 months; T2DM patients should be on stable doses of antidiabetic medication for at least 3 months. These statements help reduce the heterogeneity of the metabolism status. The recent nomenclature of MAFLD emphasized the characteristics of metabolic disorders in NASH patients [87]. The MAFLD definition identifies a group of patients with significant fibrosis more precisely [88, 89]. With these positive criteria, patients who were previously diagnosed with cryptogenic cirrhosis could be correctly identified as MAFLD cirrhosis [90, 91]. Trials for NAFLD-related fibrosis added to the diagnosis of MAFLD would reduce the confounding factors and better evaluate novel agents.
The correlation of “hard endpoints” and fibrosis improvement
Similar to other types of chronic hepatitis, the ultimate goal of clinical benefit of NASH treatment should be reducing the “hard endpoints”, including complications of cirrhosis, liver transplantations, or even mortality [92]. Currently, fibrosis stages have been proven to be the most important characteristics associated with long-term outcomes, including liver-related events and mortality of NASH patients [7, 93]. The goal of NASH treatment is to improve the clinical outcome, and reversal of fibrosis is thus an appropriate surrogate endpoint predicting the clinical benefits to patients with NASH.
As recommended by major agencies worldwide, biopsy-proven NASH resolution and/or fibrosis improvement is essential for late-phase trials of NASH. For early-stage research, noninvasive biomarkers are also acceptable. For instance, MRI-PDFF acts as a quantitative, accurate and noninvasive measurement of liver fat content in early-phase NASH trials [94]. However, the core issue of surrogate endpoints is the correlation with “hard endpoints” but not with histology. Unlike other metabolic disorders, including T2DM, hypertension or gout, NASH lacks visualized and specific markers of disease severity. Setting up the correlation of clinical markers with clinical outcomes may require more resource inputs. Sometimes significant improvement in noninvasive markers is associated with the treatment of the particular agent [67]. In this case, the specific noninvasive marker could act as a tool for monitoring efficacy.
How to assess and control the placebo effect
At present, the main reason for the failure of new drug development for NASH is that only a small portion of patients respond to treatment. To illustrate the effectiveness of therapies for NASH, the standard used to be the evidence showing greater effectiveness than the placebo in randomized, placebo-controlled clinical trials. However, due to the heterogeneity of placebo patients, they cannot act as the standard references in many situations. A meta-analysis enrolled 39 studies and analyzed 1463 patients receiving placebo. Among them, as many as 21% of patients had improvement in fibrosis scores [95]. The variability in the placebo response created challenges for applying liver histology as the endpoint assessment in NASH trials. The potential reason for the placebo response in fibrosis improvement is partially caused by fibrosis regression or progression in its natural process, sampling variability and regression to the mean [96]. In addition, weight loss through lifestyle intervention also leads to improvements in liver histology in NASH [28, 97]. Therefore, defining standard care, including diet and physical recommendations, and characterizing and quantifying lifestyle variables for participants in NASH trials are feasible approaches [98].
Safety: considering risks and benefits in long-term treatment
For noncirrhotic patients, NASH is always in the early stages of the natural course and is largely asymptomatic. These patients need long-term or even lifelong treatment. Thus, safety should be emphasized, and drugs with severe adverse profiles may not be acceptable. For NASH patients with advanced fibrosis or cirrhosis, clinicians and patients balance the risks and benefits. Short-term use leads to higher tolerance to adverse events. Provided potent efficacy, adverse events would be more acceptable. NASH is the hepatic manifestation of metabolic syndromes. Cardiovascular diseases are the most common cause of mortality in NAFLD patients [99]. NAFLD patients also had a 2.2-fold greater risk of having T2DM [100]. Considering the susceptibility of extrahepatic cardiometabolic disorders, drugs for NASH should not increase the risk of these diseases.
Summary
The pharmacological efficacy of novel NASH drugs involves multiple mechanisms, which could partially explain the reason for the failure of the majority of phase 2 or 3 NASH drug trials in achieving fibrosis improvement in fibrotic NASH patients, especially those with advanced fibrosis or cirrhosis. Due to the heterogeneity of NASH patients, the influence of confounding factors, and the consistency of diagnosis of quantitative evaluation indicators, clinical trials of new drugs for NASH have become more difficult, which also raises huge challenges for clinical study design and implementation. Nevertheless, with the development of our knowledge of the pathophysiology of NASH and research on novel therapeutic drugs, currently ongoing phase 3 trials would provide more solutions for patients with fibrotic NASH in the near future and finally moving to clinical application.
References
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57.
Kanwal F, Shubrook JH, Younossi Z, Natarajan Y, Bugianesi E, Rinella ME, et al. Preparing for the NASH Epidemic: a call to action. Gastroenterology. 2021;161:1030–42.
Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–24.
Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–72.
European Association for the Study of the Liver (EASL)., European Association for the Study of Diabetes (EASD)., European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402.
Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–65.
Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–97. e310.
Ampuero J, Aller R, Gallego-Durán R, Crespo J, Calleja JL, García-Monzón C, et al. Significant fibrosis predicts new-onset diabetes mellitus and arterial hypertension in patients with NASH. J Hepatol. 2020;73:17–25.
Brunt EM, Kleiner DE, Wilson LA, Sanyal AJ, Neuschwander-Tetri BA. Improvements in histologic features and diagnosis associated with improvement in fibrosis in nonalcoholic steatohepatitis: results from the nonalcoholic steatohepatitis clinical research network treatment trials. Hepatology. 2019;70:522–31.
National Medical Products Administration of China, Guiding principles for clinical trials of nonalcoholic steatohepatitis drugs (for trial implementation)(No. 92 of 2019), updated 2019. Accessed 21 Sep 2021. https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20191220141201879.html.
(EMA) EMA. Draft reflection paper on regulatory requirements for the development of medicinal products for chronic non-infectious liver diseases (PBC, PSC, NASH) Committee for Medicinal Products for Human Use (CHMP), https://www.emaeuropaeu/en/draft-reflection-paper-regulatory-requirementsdevelopment-medicinal-products-chronic-non-infectious. 2018. Accessed 5 Aug 2021.
U.S. Food and Drug Administration (FDA) Guidance for Industry. Noncirrhotic nonalcoholic steatohepatitis with liver fibrosis: developing drugs for treatment. Department of Health and Human Services, Center for Drug Evaluation and Research (CDER). 2018. https://www.federalregister.gov/documents/2018/12/04/2018-26333/noncirrhotic-nonalcoholic-steatohepatitis-with-liver-fibrosisdeveloping-drugs-for-treatment-draft:Last. Accessed 5 Aug 2021.
U.S. Food and Drug Administration (FDA) Guidance for Industry. Nonalcoholic Steatohepatitis with Compensated Cirrhosis: Developing Drugs for Treatment Guidance for Industry. Department of Health and Human Services, Center for Drug Evaluation and Research (CDER). 2019. https://www.federalregister.gov/documents/2019/06/07/2019-11951/nonalcoholic-steatohepatitis-withcompensated-cirrhosis-developing-drugs-for-treatment-draft:Last. Accessed 5 Aug 2021.
Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–906.
Davison BA, Harrison SA, Cotter G, Alkhouri N, Sanyal A, Edwards C, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. 2020;73:1322–32.
Cheung A, Neuschwander-Tetri BA, Kleiner DE, Schabel E, Rinella M, Harrison S, et al. Defining improvement in nonalcoholic steatohepatitis for treatment trial endpoints: recommendations from the liver forum. Hepatology. 2019;70:1841–55.
Ratziu V. A critical review of endpoints for non-cirrhotic NASH therapeutic trials. J Hepatol. 2018;68:353–61.
Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–44.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21.
Gawrieh S, Knoedler DM, Saeian K, Wallace JR, Komorowski RA. Effects of interventions on intra- and interobserver agreement on interpretation of nonalcoholic fatty liver disease histology. Ann Diagn Pathol. 2011;15:19–24.
Melo RCN, Raas MWD, Palazzi C, Neves VH, Malta KK, Silva TP. Whole slide imaging and its applications to histopathological studies of liver disorders. Front Med. 2019;6:310.
Marti-Aguado D, Rodríguez-Ortega A, Mestre-Alagarda C, Bauza M, Valero-Pérez E, Alfaro-Cervello C, et al. Digital pathology: accurate technique for quantitative assessment of histological features in metabolic-associated fatty liver disease. Aliment Pharmacol Ther. 2021;53:160–71.
Munsterman ID, van Erp M, Weijers G, Bronkhorst C, de Korte CL, Drenth JPH, et al. A novel automatic digital algorithm that accurately quantifies steatosis in NAFLD on histopathological whole-slide images. Cytom B Clin Cytom. 2019;96:521–8.
Masugi Y, Abe T, Tsujikawa H, Effendi K, Hashiguchi A, Abe M, et al. Quantitative assessment of liver fibrosis reveals a nonlinear association with fibrosis stage in nonalcoholic fatty liver disease. Hepatol Commun. 2018;2:58–68.
Liu F, Goh GB, Tiniakos D, Wee A, Leow WQ, Zhao JM, et al. qFIBS: an automated technique for quantitative evaluation of fibrosis, inflammation, ballooning, and steatosis in patients with nonalcoholic steatohepatitis. Hepatology. 2020;71:1953–66.
Wang Y, Vincent R, Yang J, Asgharpour A, Liang X, Idowu MO, et al. Dual-photon microscopy-based quantitation of fibrosis-related parameters (q-FP) to model disease progression in steatohepatitis. Hepatology. 2017;65:1891–903.
Taylor-Weiner A, Pokkalla H, Han L, Jia C, Huss R, Chung C, et al. A machine learning approach enables quantitative measurement of liver histology and disease monitoring in NASH. Hepatology. 2021;74:133–47.
Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–78. e365quize314-365
Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85.
Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610–9.
Bril F, Biernacki DM, Kalavalapalli S, Lomonaco R, Subbarayan SK, Lai J, et al. Role of vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2019;42:1481–8.
Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104.
Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for noncirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65.
Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–96.
Younossi ZM, Stepanova M, Nader F, Loomba R, Anstee QM, Ratziu V, et al. Obeticholic acid impact on quality of life in patients with nonalcoholic steatohepatitis: REGENERATE 18-month interim analysis. Clin Gastroenterol Hepatol. 2021;S1542-3565:00751-5. https://doi.org/10.1016/j.cgh.2021.07.020.
Patel K, Harrison SA, Elkhashab M, Trotter JF, Herring R, Rojter SE, et al. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology. 2020;72:58–71.
Klucher K, Wang Y, Halcomb R, Fenaux M, editors. A novel farnesoid X receptor agonist, TERN-101, reduces liver steatosis, inflammation, ballooning and fibrosis in a murine model of non-alcoholic steatohepatitis. Viena (Austria): European Associate for the Study of the Liver International Liver Conference. April 2019.
Sojoodi M, Wang Y, Erstad DJ, Caravan P, Lanuti M, Qadan M, et al. EDP-297, a novel and potent fxr agonist, exhibit robust anti-fibrotic effects with significant liver function in a rat model of non-alcoholic steatohepatitis. J Hepatol. 2020;73:S453.
Kowdley KV, Bonder A, Heneghan MA et al. Final data of the phase 2a INTREPID study with EDP-305, a non-bile acid farnesoid X receptor agonist. Hepatology. 2020;72.
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202–9.
Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–90.
Eguchi Y, Kitajima Y, Hyogo H, Takahashi H, Kojima M, Ono M, et al. Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J). Hepatol Res. 2015;45:269–78.
Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–24.
Hartman ML, Sanyal AJ, Loomba R, Wilson JM, Nikooienejad A, Bray R, et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care. 2020;43:1352–5.
Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Targher G. Glucagon-like peptide-1 receptor agonists for treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: an updated meta-analysis of randomized controlled trials. Metabolites. 2021;11:73.
Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6:105–13.
Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–51.
Boyer-Diaz Z, Aristu-Zabalza P, Andrés-Rozas M, Robert C, Ortega-Ribera M, Fernández-Iglesias A, et al. Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. J Hepatol. 2021;74:1188–99.
Lefere S, Puengel T, Hundertmark J, Penners C, Frank AK, Guillot A, et al. Differential effects of selective- and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages(☆). J Hepatol. 2020;73:757–70.
INVENTIVA. Inventiva’s lanifibranor meets the primary and key secondary endpoints in the Phase IIb NATIVE clinical trial in non‐alcoholic steatohepatitis (NASH). 2020. Accessed 21 Sep 2021. https://inventivapharma.com/wp-content/uploads/2020/06/Inventiva-PR-NATIVE-top-line-results-EN-15062020.pdf.
Boyer-Diaz Z, Aristu-Zabalza P, Andrés-Rozas M, Robert C, Ortega-Ribera M, Fernández-Iglesias A, et al. Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. J Hepatol. 2021;74:1188–99.
Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–59. e1145.
Siddiqui MS, Idowu MO, Parmar D, Borg BB, Denham D, Loo NM, et al. A Phase 2 Double Blinded, Randomized Controlled Trial of Saroglitazar in Patients With Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2021;19:2670–2.
Gawrieh S, Noureddin M, Loo N, Mohseni R, Awasty V, Cusi K, et al. Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology. 2021;74:1809–24.
Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med. 2017;177:633–40.
Somm E, Jornayvaz FR. Fibroblast growth factor 15/19: from basic functions to therapeutic perspectives. Endocr Rev. 2018;39:960–89.
BonDurant LD, Ameka M, Naber MC, Markan KR, Idiga SO, Acevedo MR, et al. FGF21 regulates metabolism through adipose-dependent and –independent mechanisms. Cell Metab. 2017;25:935–44. e934.
Harrison SA, Rossi SJ, Paredes AH, Trotter JF, Bashir MR, Guy CD, et al. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology. 2020;71:1198–212.
Harrison SA, Neff G, Guy CD, Bashir MR, Paredes AH, Frias JP, et al. Efficacy and safety of aldafermin, an engineered FGF19 analog, in a randomized, doubleblind, placebo-controlled trial of patients with nonalcoholic steatohepatitis. Gastroenterology. 2021;160:219–31. e211.
NGM Bio Reports Topline Results from 24-Week Phase 2b ALPINE 2/3 Study of Aldafermin in NASH. 2021. Accessed 21 Sep 2021. https://ir.ngmbio.com/newsreleases/news-release-details/ngm-bio-reports-topline-results-24-week-phase-2b-alpine-23-study.
Harrison SA, Ruane PJ, Freilich BL, Neff G, Patil R, Behling CA, et al. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial. Nat Med. 2021;27:1262–71.
Harrison S, Ruane P, Freilich B, Neff G, Patil R, Behling C, et al. Efruxifermin (EFX) improved markers of fibrosis, liver injury and metabolism in F4 NASH patients with compensated cirrhosis. J Hepatol. 2021;75:S204–S205.
Sanyal A, Charles ED, Neuschwander-Tetri BA, Loomba R, Harrison SA, Abdelmalek MF, et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2019;392:2705–17.
Loomba R, Lawitz E, Frias J, Lasanta GO, Franey BB, Morrow L, et al. BIO89-100 demonstrated robust reduction in liver fat fraction and liver fat volume (LFV) and favorable tolerability with weekly (QW) and every 2 weeks (Q2W) dosing in a Phase 1b/2a placebo-controlled proof of concept study in NASH. J Hepatol. 2021;75:S627–S627.
Kim D, Kim W, Joo SK, Bae JM, Kim JH, Ahmed A. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol. 2018;16:123–31. e121
Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394:2012–24.
Harrison SA, Bashir M, Moussa SE, McCarty K, Pablo Frias J, Taub R, et al. Effects of resmetirom on noninvasive endpoints in a 36-week phase 2 active treatment extension study in patients with NASH. Hepatol Commun. 2021;5:573–88.
Loomba R, Neutel J, Mohseni R, Bernard D, Severance R, Dao M, et al. LBP-20-VK2809, a Novel liver-directed thyroid receptor beta agonist, significantly reduces liver fat with both low and high doses in patients with non-alcoholic fatty liver disease: a phase 2 randomized, placebo-controlled. Trial. 2019;70:e150–e151.
Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754–67.
Ratziu V, Sanyal A, Harrison SA, Wong VW, Francque S, Goodman Z, et al. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: final analysis of the phase 2b CENTAUR study. Hepatology. 2020;72:892–905.
Pedrosa M, Seyedkazemi S, Francque S, Sanyal A, Rinella M, Charlton M, et al. A randomized, double-blind, multicenter, phase 2b study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: Study design of the TANDEM trial. Contemp Clin Trials. 2020;88:105889.
Younossi ZM, Stepanova M, Lawitz E, Charlton M, Loomba R, Myers RP, et al. Improvement of hepatic fibrosis and patient-reported outcomes in nonalcoholic steatohepatitis treated with selonsertib. Liver Int. 2018;38:1849–59.
Harrison SA, Wong VW, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J Hepatol. 2020;73:26–39.
Shiffman M, Freilich B, Vuppalanchi R, Watt K, Chan JL, Spada A, et al. Randomised clinical trial: emricasan versus placebo significantly decreases ALT and caspase 3/7 activation in subjects with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2019;49:64–73.
Harrison SA, Goodman Z, Jabbar A, Vemulapalli R, Younes ZH, Freilich B, et al. A randomized, placebo-controlled trial of emricasan in patients with NASH and F1-F3 fibrosis. J Hepatol. 2020;72:816–27.
Harrison SA, Marri SR, Chalasani N, Kohli R, Aronstein W, Thompson GA, et al. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment Pharm Ther. 2016;44:1183–98.
Chalasani N, Abdelmalek MF, Garcia-Tsao G, Vuppalanchi R, Alkhouri N, Rinella M, et al. Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology. 2020;158:1334–45. e1335.
Harrison SA, Abdelmalek MF, Caldwell S, Shiffman ML, Diehl AM, Ghalib R, et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology. 2018;155:1140–53.
Chen W, Yang A, Jia J, Popov YV, Schuppan D, You H. Lysyl oxidase (LOX) family members: rationale and their potential as therapeutic targets for liver fibrosis. Hepatology. 2020;72:729–41.
Ratziu V, de Guevara L, Safadi R, Poordad F, Fuster F, Flores-Figueroa J, et al. Aramchol in patients with nonalcoholic steatohepatitis: a randomized, doubleblind, placebo-controlled phase 2b trial. Nat Med. 2021;27:1825–35.
Loomba R, Mohseni R, Lucas KJ, Gutierrez JA, Perry RG, Trotter JF, et al. TVB-2640 (FASN Inhibitor) for the treatment of nonalcoholic steatohepatitis: FASCINATE-1, a randomized, placebo-controlled phase 2a trial. Gastroenterology. 2021;161:1475–86.
Harrison SA, Gunn N, Neff GW, Kohli A, Liu L, Flyer A, et al. A phase 2, proof of concept, randomised controlled trial of berberine ursodeoxycholate in patients with presumed non-alcoholic steatohepatitis and type 2 diabetes. Nat Commun. 2021;12:5503.
Gane E, Schwabe C, Yoon KT, Heo J, Scott R, Lee J, et al., editors. ARO-HSD reduces hepatic HSD17B13 mRNA expression and protein levels in patients with suspected NASH. J Hepatol. 2021. Conference paper.
Newsome PN, Palmer M, Freilich B, Sheikh MY, Sheikh A, Sarles H, et al. Volixibat in adults with non-alcoholic steatohepatitis: 24-week interim analysis from a randomized, phase II study. J Hepatol. 2020;73:231–40.
Loomba R, Noureddin M, Kowdley KV, Kohli A, Sheikh A, Neff G, et al. Combination therapies including cilofexor and firsocostat for bridging fibrosis and cirrhosis attributable to NASH. Hepatology. 2021;73:625–43.
Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–54.
Eslam M, Sanyal AJ, George J. Toward more accurate nomenclature for fatty liver diseases. Gastroenterology. 2019;157:590–3.
Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T, Nakano D, Yoshinaga S, et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 2020;40:3018–30.
Park H, Yoon EL, Cho S, Jun DW, Nah EH. Diabetes is the strongest risk factor of hepatic fibrosis in lean patients with non-alcoholic fatty liver disease. Gut. 2021. https://doi.org/10.1136/gutjnl-2021-325102.
Zheng KI, Eslam M, George J, Zheng MH. When a new definition overhauls perceptions of MAFLD related cirrhosis care. Hepatobiliary Surg Nutr. 2020;9:801–4.
Zheng KI, Fan JG, Shi JP, Wong VW, Eslam M, George J, et al. From NAFLD to MAFLD: a “redefining” moment for fatty liver disease. Chin Med J. 2020;133:2271–3.
D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–31.
Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265–73.
Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH Trials. Hepatology. 2018;68:763–72.
Han MAT, Altayar O, Hamdeh S, Takyar V, Rotman Y, Etzion O, et al. Rates of and factors associated with placebo response in trials of pharmacotherapies for nonalcoholic steatohepatitis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17:616–29. e626.
Rowe IA, Parker R. The placebo response in randomized trials in nonalcoholic steatohepatitis simply explained. Clin Gastroenterol Hepatol. 2021;S1542-3565:00598-X. https://doi.org/10.1016/j.cgh.2021.05.059.
Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–9.
Glass O, Filozof C, Noureddin M, Berner-Hansen M, Schabel E, Omokaro SO, et al. Standardisation of diet and exercise in clinical trials of NAFLD-NASH: recommendations from the Liver Forum. J Hepatol. 2020;73:680–93.
Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–53.
Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated metaanalysis of 501 022 adult individuals. Gut. 2021;70:962–9.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81873565, 81900507, 82170593), Shanghai Leading Talent Plan 2017, Star Program of Shanghai Jiao Tong University (YG2021QN54), Hospital Funded Clinical Research, Clinical Research Unit, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (17CSK04), and SJTU Trans-med Awards Research (20190104).
Author information
Authors and Affiliations
Contributions
Formulation of conception and design: YWS, JGF; creation of the initial draft: YWS; oversight and critical revising: JGF.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Shi, Yw., Fan, Jg. Current status and challenges in the drug treatment for fibrotic nonalcoholic steatohepatitis. Acta Pharmacol Sin 43, 1191–1199 (2022). https://doi.org/10.1038/s41401-021-00822-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-021-00822-1
Keywords
This article is cited by
-
All about NASH: disease biology, targets, and opportunities on the road to NASH drugs
Acta Pharmacologica Sinica (2022)
-
Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH)
Signal Transduction and Targeted Therapy (2022)