Abstract
Caspase-11 is a key upstream modulator for activation of inflammatory response under pathological conditions. In this study, we investigated the roles of caspase-11 in the maturation of interleukin-1β (IL-1β) and development of renal interstitial fibrosis in vivo and in vitro. Mice were subjected to unilateral ureteral obstruction (UUO). The mice were treated with either caspase-11 inhibitor wedelolactone (Wed, 30 mg/kg/day, ig) for 7 days or caspase-11 siRNA (10 nmol/20 g body weight per day, iv) for 14 days. The mice were euthanized on day 14, their renal tissue and blood sample were collected. We found that the obstructed kidney had significantly higher caspase-11 levels and obvious tubular injury and interstitial fibrosis. Treatment with Wed or caspase-11 siRNA significantly mitigated renal fibrosis in UUO mice, evidenced by the improved histological changes. Furthermore, caspase-11 inhibition significantly blunted caspase-1 activation, IL-1β maturation, transforming growth factor-β (TGF-β), fibronectin, and collagen I expressions in the obstructed kidney. Renal tubular epithelial NRK-52E cells were treated in vitro with angiotensin (Ang, 1 μmol/L), which stimulated caspase-11 activation and IL-1β maturation. Treatment with IL-1β (20 ng/ml) significantly increased the expression of TGF-β, fibronectin, and collagen I in the cells. Ang II-induced expression of TGF-β, fibronectin, and collagen I were suppressed by caspase-11 siRNA or Wed. Finally, we revealed using co-immunoprecipitation that caspase-11 was able to interact with caspase-1 in NRK-52E cells. These results suggest that caspase-11 is involved in UUO-induced renal fibrosis. Elevation of caspase-11 in the obstructed kidney promotes renal fibrosis by stimulating caspase-1 activation and IL-1β maturation.
Similar content being viewed by others
Introduction
Renal fibrosis is a common pathological feature of various pathogenesis-related chronic kidney diseases (CKDs) [1, 2]. Mounting evidence has demonstrated that the recruitment of immune cells and excessive release of pro-inflammatory cytokines from intrinsic cells play a crucial role in modulating renal fibrosis [3,4,5]. Among the innumerable inflammatory cytokines, interleukin-1β (IL-1β) has been reported to be crucial in inflammation-mediated renal fibrosis in CKD and diabetic nephropathy [6,7,8]. Previous studies have shown that IL-1β expression is remarkably increased in unilateral ureteral obstruction (UUO) mice. Blockade of IL-1β effectively ameliorates UUO-induced renal inflammation and interstitial fibrosis [9,10,11].
Overactivation of the local renin–angiotensin (Ang) system (RAS) in kidney under pathological conditions plays a fundamental role in driving renal fibrosis [12]. Ang II, the most important effector in the RAS, exerts its pro-fibrotic effect through the Ang type I (AT1) receptor [13]. Therefore, AT1 receptor blockers and Ang-converting enzyme inhibitors, which were originally designed as anti-hypertensive agents, are used as first-line medications to prevent renal fibrosis. Ang II is upregulated in different renal fibrosis models including obstructive nephropathy. In addition, accumulating evidence suggests that Ang II plays an important role in renal inflammation [14]. Ang II promotes the expression of pro-inflammatory cytokines, such as IL-1β, IL-8, and tumor necrosis factor-α (TNF-α), in tubular epithelial cells (TECs) [15]. Therefore, activation of renal inflammation is a pillar of Ang II in promoting renal fibrosis.
Nod-like receptor protein 3 (NLRP3) inflammasome-dependent activation of caspase-1 is known to be essential for IL-1β maturation [16, 17]. However, as an alternative mechanism for IL-1β processing [18], the role of caspase-11 has not been fully elucidated. Caspase-11 is synthesized as 43-kDa and 38-kDa precursors [19]. Unlike many other caspase members, pro-caspase-11 can be activated independent of the NLRP3 inflammasome [20, 21]. Caspase-11 was originally identified as a novel driver of IL-1β maturation and release in response to microbial signals [20, 22]. Later, studies found that caspase-11 could also be activated by noninfectious factors [23, 24]. In contrast to NLRP3 inflammasome pathway, caspase-11 processing does not require the NLRP3 inflammasome [18, 25, 26]. More importantly, caspase-11 can directly activate caspase-1 from its inactive precursor [27, 28]. Thus, understanding the role of caspase-11 in initiating chronic sterile inflammation, which is essential to the progression of renal fibrosis, is of great interest. In this study, the role of caspase-11 in IL-1β maturation and renal interstitial fibrosis was investigated in UUO mice and Ang II-treated renal TECs.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), recombinant human Ang II and wedelolactone (Wed) were obtained from Sigma-Aldrich (Saint Louis, MO, USA). Fetal bovine serum (FBS) was from Gibco (Grand Island, NY, USA). The BCA Protein Assay Kit was purchased from Shenergy Biocolor BioScience and Technology (Shanghai, China). Anti-fibronectin antibody was obtained from Sigma-Aldrich (Saint Louis, MO, USA). Anti-collagen I and anti-transforming growth factor-β (TGF-β) antibodies were purchased from Abcam (Cambridge, MA, USA). Anti-caspase-11, anti-caspase-1, and anti-IL-1β antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody, Cy3-conjugated anti-rabbit IgG (1:20), Fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG, horseradish peroxidase (HRP)-conjugated secondary antibodies, and enhanced chemiluminescence (ECL) detection kits were obtained from Beyotime Institute of Biotechnology (Haimen, China). Anti-pro-IL-1β antibody was obtained from Proteintech (Chicago, MA, USA). Polyvinylidene difluoride membrane was purchased from Millipore (Billerica, MA, USA). Protease and phosphatase inhibitors were obtained from Roche (Mannheim, Germany). All chemicals and reagents were of analytical grade.
Animals
Male C57BL/6J mice (20–25 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). All animal experiments were performed in accordance with the criteria of the Medical Laboratory Animal Administrative Committee of Shanghai and the Guide for Care and Use of Laboratory Animals of Fudan University. The protocols were approved by the Ethics Committee for Experimental Research, Shanghai Medical College, Fudan University. Mice were anesthetized with 5% pentobarbital sodium via intraperitoneal injection (4 mL/kg body weight). The left ureter was visualized by a flank incision and ligated with 4–0 silk. Mice in the sham group underwent the same surgery without ligation of the left ureter. Each group contained eight mice. Mice that underwent UUO surgery were treated with either vehicle (0.9% dimethylsulfoxide (DMSO)) or Wed (30 mg/kg body weight per day) by oral gavage at 0.1 mL per day or specific modification of 2′-O-methyl (2′-OMe)-caspase-11 small interfering RNA (siRNA) (5′-GGGCAACCUUGACGAGAUATT-3′ (sense), 5′-TTCCCGUUGGAACUGCUCUAU-3′ (anti-sense), 10 nmoL/20 g body weight per day, via tail vein injection. The mice were euthanized 14 days after surgery to harvest renal tissue and blood samples.
Cell culture
The NRK-52E rat renal TEC line was obtained from the Institute of Biochemistry and Cell Biology (Shanghai, China) and cultured in DMEM containing 10% FBS in a 5% CO2 atmosphere at 37 °C. Cells were starved in medium containing 0.5% FBS for 24 h to form quiescent cells. Cells were treated with Ang II at a concentration of 1 μM and were harvested at different time periods. Extracellular medium was collected after 24 h and used to detect cell secretion of IL-1β. Cells were treated with DMSO (1%) or Wed (30 μmol/L) for 2 h before Ang II application. Each experiment was repeated at least three times.
Western blotting
Proteins were extracted from homogenized frozen kidneys or cultured cells using Radio-Immunoprecipitation Assay (RIPA) lysis buffer (1% NP-40, 0.1% sodium dodecyl sulfate (SDS), 100 μg/mL phenylmethylsulfonyl fluoride, 0.5% sodium deoxycholate, in phosphate-buffered saline (PBS)) on ice. The supernatants were collected after centrifugation at 12,000 × g at 4 °C for 20 min. Protein concentrations were determined using a BCA Protein Assay Kit according to the manufacturer’s instructions, and whole lysates were mixed with an equal volume of 6 × SDS loading buffer. Samples were boiled for 5 min and then separated on SDS-polyacrylamide gels. After transfer, the polyvinylidene fluoride (PVDF) membranes were blocked with Tris-buffered saline containing 5% skim milk and 0.1% Tween (TBS/Tween) for 1 h at room temperature. The membranes were then incubated overnight with the following primary antibodies at 4 °C: anti-fibronectin, 1:20,000; anti-collagen I, 1:1000; anti-TGF-β, 1:2000; anti-caspase-11, 1:2000; anti-pro-IL-1β 1:500; anti-caspase-1, 1:500; and anti-IL-1β, 1:500. The PVDF membranes were washed three times with TBST for 15 min each. The membranes were then incubated with HRP-conjugated secondary antibodies for 2 h. After another three washes, the hybridizing bands were developed using an ECL detection kit according to the manufacturer’s instructions.
Caspase-11 siRNA
Caspase-11 siRNA was purchased from Ribobio Company (Guangzhou, China). A nonsilencing siRNA oligonucleotide that does not recognize any known homolog of mammalian genes (Ribobio, Guangzhou, China) was used as a negative control. NRK-52E cells were transfected with caspase-11 siRNA (50 nmol/L) or control siRNA (50 nmol/L) using lipofectamine 2000 Reagent (Invitrogen, USA) according to the manufacturer’s instructions. After 48 h, the cells were treated with Ang II for 24 h or 48 h.
Measurement of Ang II in renal tissue and culture medium
Cytokine levels in cell culture supernatants were detected using commercial enzyme-linked immunosorbent assay (ELISA) kits for Ang II (Westang Biotech, Shanghai, China) and IL-1β (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Confocal fluorescence microscopy
NRK-52E cells were transfected with caspase-11 siRNA (50 nmol/L) or control siRNA (50 nmol/L) using lipofectamine 2000 Reagent (Invitrogen, USA) according to the manufacturer’s instructions. The cells were starved and treated with Ang II for 12 h, followed by fixation with 4% paraformaldehyde for 15 min at 4 °C. The cells were then incubated overnight with primary antibodies against caspase-11 and caspase-1 at 4 °C. After three washes, the samples were incubated with Cy3-conjugated anti-rabbit IgG (1:20) or FITC-conjugated anti-mouse IgG (1:30) secondary antibodies for 1 h at 37 °C. The cells were then incubated with 4,6-diamidino-2-phenylindole (DAPI) for 5 min. Cells were imaged on a laser scanning confocal microscope (Leica, Wetzlar, Germany).
Immunoprecipitation
NRK-52E (5 × 107) cells were starved and then stimulated with Ang II (1 ng/mL) for 12 h. Cells were subsequently lysed in NP-40 lysis buffer. For each immunoprecipitation, a 0.3 mL aliquot of the lysate was incubated for 2 h with 0.5 μg of caspase-1 antibody or control IgG and 25 μL of a 1:1 slurry of Protein G Sepharose (GE Healthcare, USA). The beads were washed two times with 1 mL of lysis buffer containing 0.5 mol/L NaCl and another two times with 1 mL of lysis buffer containing 2.5 mol/L NaCl. Samples were boiled for 5 min and then separated on SDS-polyacrylamide gels.
Renal histology
Kidneys were fixed in 10% formalin and embedded in paraffin. Sections were cut onto glass slides. Each section was 4 μm thick. The sections were then dewaxed in xylene and rehydrated in decreasing concentrations of ethanol. The sections were washed three times for 10 min each. Endogenous peroxidase was quenched for 45 min using a 0.6% methanol solution. After washing in filtered water and PBS, the sections were blocked with 1% bovine serum albumin supplemented with avidin and biotin blocking solution for 30 min. Sections were stained with hematoxylin–eosin (H&E) and Masson’s trichrome. Histological changes, such as the degrees of tubular atrophy and interstitial fibrosis, were observed at ×200 optical magnification. Immunohistochemistry of α-smooth muscle actin (α-SMA) was observed at ×100 optical magnification.
Statistical analysis
Data are presented as the mean ± SEM, with statistical analysis performed using SPSS 19.0. Column graphs were generated in GraphPad Prism 6.0. Student’s t-test was used for single comparisons and one-way analysis of variance with Tukey’s test were used for multiple comparisons. The Kruskal–Wallis test followed by the Mann–Whitney U-test was used for comparisons of nonparametric data. P < 0.05 was considered statistically significant.
Results
Caspase-11 was elevated in UUO mice, and caspase-11 blockade attenuated fibrosis in the obstructed kidney
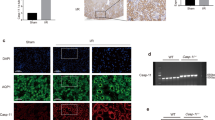
As shown in Figs. 1a, b, both pro-caspase-11 and caspase-11 were significantly increased in the obstructed kidneys of UUO mice. In addition, TGF-β and extracellular matrix proteins including fibronectin and collagen I were also significantly increased. Administration of the caspase-11 inhibitor Wed effectively attenuated UUO-induced activation of caspase-11. In the same time, TGF-β, fibronectin, and collagen I elevations were significantly blunted. UUO led to a substantial increase in Ang II concentration in renal tissue. Caspase-11 inhibition had no obvious effect on elevated Ang II levels in obstructed kidney (Fig. 1c). In sham mice, Wed treatment did not noticeably affect pro-caspase-11, TGF-β, fibronectin, and collagen I expression (Figs. 1d, e).
Wedelolactone (Wed) treatment reduced renal fibrosis in UUO mice. Control and UUO mice received vehicle or 30 mg/kg Wed by oral gavage once a day for 7 days. a Western blot of caspase-11, TGF-β, fibronectin, and collagen I. b Representative western blot quantification of caspase-11, TGF-β, fibronectin, and collagen I. c Ang II levels were detected using an ELISA kit. All values are presented as the mean ± SEM (n = 6). d Western blot of caspase-11, TGF-β, fibronectin, and collagen I. e Quantification of caspase-11, TGF-β, fibronectin, and collagen I. ***P < 0.001 versus sham. **P < 0.01 versus sham. #P < 0.05 versus UUO
Caspase-11 inhibition reduced caspase-1 activation and IL-1β maturation in UUO mice
As shown in Figs. 2a, b, compared with sham treatment, UUO markedly increased the expression and activation of caspase-1 in the kidney. Inhibition of caspase-11 by Wed attenuated the increased expression and activation of caspase-1. IL-1β maturation was also increased in obstructed kidney, which was suppressed by caspase-11 inhibition. Similarly, ELISA results showed that IL-1β concentrations were elevated in obstructed kidneys, whereas caspase-11 inhibition blunted this increase (Fig. 2c).
Effect of Wed on UUO-induced inflammatory activation in the obstructed kidney. a Wed treatment reduced renal inflammation in a UUO model. Representative western blot of pro-caspase-1, caspase-1, pro-IL-1β, and IL-1β. b Quantification of pro-caspase-1, caspase-1, pro-IL-1β, and IL-1β. c IL-1β levels in the kidney cortex were detected using ELISA. All values are presented as the mean ± SEM (n = 6). *P < 0.05 versus sham. **P < 0.01 versus sham. ***P < 0.001 versus sham. #P < 0.05 versus UUO
Caspase-11 inhibition alleviated pathological changes in obstructed kidneys
HE staining showed that obstructed kidneys exhibited collapse and loss of renal tubules, whereas the cell number in the tubular interstitial space was increased (Figs. 3a, f). Masson’s trichrome staining and Sirius Red staining showed that collagen and other extracellular matrix deposition were also increased in obstructed kidneys (Figs. 3b–d, g). Immunohistochemistry showed that α-SMA expression was significantly increased in UUO mice (Figs. 3e, h). Caspase-11 inhibition significantly ameliorated the morphological changes in obstructed kidneys.
Wed treatment attenuated renal morphological changes in UUO mice. a Histological examination of renal injury by H&E staining for sham-operated, UUO and Wed-treated UUO mice, Magnification, × 100, Bar = 1 mm. b Assessment of interstitial fibrosis by Masson staining, Magnification, × 200, Bar = 500 μm. c Representative image of Sirius Red staining under standard illumination, Magnification, × 100, Bar = 1 mm. d Representative image of Sirius Red staining under polarized illumination, Magnification, × 100, Bar = 1 mm. e Immunohistochemistry of α-SMA. Magnification, × 200, Bar = 500 μm. f Quantitative analysis of tubular injury score. g Quantitative analysis of fibrosis area. h Quantitative analysis of α-SMA positive area. *P<0.05 versus sham. #P<0.05 versus UUO
Caspase-11 siRNA attenuated IL-1β maturation and pro-fibrotic molecule expression in UUO mice
UUO mice were injected with a 2′-OM modified caspase-11 siRNA (si-Casp-11 mice) through the caudal vein to knockdown caspase-11 expression (Figs. 4a, b). The increased expression of collagen I, fibronectin, and α-SMA in obstructed kidneys was attenuated by caspase-11 siRNA (Figs. 4c, d). The upregulation of pro-caspase-1, caspase-1, pro- IL-1β, and IL-1β was also significantly suppressed by caspase-11 siRNA (Figs. 4e, f).
IL-1β maturation and renal fibrosis were alleviated by caspase-11 siRNA in obstructed kidneys. Mice were subjected to UUO induction for 14 days (n = 6). UUO mice received caspase-11 siRNA (0.5 nmol/g) for 14 days. a Western blot of pro-caspase-11. b Representative western blot quantification of pro-caspase-11. c Representative western blot of TGF-β, fibronectin, and collagen I. d Quantification of TGF-β, fibronectin, and collagen I. e Representative western blot of pro-caspase-1, caspase-1, pro-IL-1β, and IL-1β. f Quantification of pro-caspase-1, caspase-1, pro-IL-1β, and IL-1β. All values are presented as the mean ± SEM (n = 6). *P < 0.05 versus sham + NC siRNA. #P < 0.05 versus UUO + NC siRNA
Ang II stimulated caspase-11 expression and IL-1β maturation in renal TECs
As revealed in our previous data, Ang II was significantly increased in obstructed kidneys. Ang II has been shown to play a crucial role in renal inflammation and fibrosis [29]. To identify the effect of Ang II on caspase-11 and IL-1β, caspase-11 expression and IL-1β maturation were measured in renal TECs upon Ang II treatment. As shown in Figs. 5a, b, caspase-11 expression was dramatically upregulated after Ang II treatment, whereas IL-1β maturation was also upregulated (Figs. 5c, d). We further observed the role of IL-1β in TGF-β and ECM production in renal TECs. In cultured renal TECs, IL-1β treatment significantly increased the expression of TGF-β, fibronectin, and collagen I (Figs. 5e, f).
Ang II stimulated caspase-11 and IL-1β expression. a Cells were stimulated with 1 μmol/L Ang II for 0–24 h. Caspase-11 was analyzed by western blotting. b Quantification of pro-caspase-11 and caspase-11. c Cells were treated with Ang II (1 μmol/L) for 24 h. Cleaved IL-1β was analyzed by western blotting. d Quantification of IL-1β. *P < 0.05 versus control. e Cells were stimulated with IL-1β (20 ng/mL) for 48 h. The levels of TGF-β, fibronectin, and collagen I were analyzed by western blotting. f Quantification of TGF-β, fibronectin and collagen I. Data are presented as the mean ± SEM (n = 6). *P < 0.05 versus control. **P < 0.01 versus control
Inhibition of caspase-11 suppressed Ang II-induced caspase-1 activation and IL-1β maturation
To investigate whether caspase-11 was required for Ang II-induced IL-1β secretion, specific siRNA (caspase-11 siRNA) was used to knockdown caspase-11 expression prior to Ang II stimulation in tubular cells. As shown in Figs. 6a, b, compared with the negative control siRNA (NC siRNA), caspase-11 siRNA significantly suppressed the expression of both pro-caspase-11 and caspase-11. Caspase-11 siRNA did not influence either the basal expression or the activation of caspase-1 and IL-1β. In Ang II-treated NRK-52E cells, pretreatment with caspase-11 siRNA largely suppressed the Ang II-induced increase in caspase-1 and IL-1β expression (Figs. 6c, d). ELISA results also showed that Ang II increased the concentration of IL-1β in the culture medium. Pretreatment with caspase-11 siRNA significantly blunted the increase in IL-1β in the culture medium (Fig. 6e).
Caspase-11 knockdown reduced Ang II-induced inflammatory activation in renal TECs. Cells were transfected with caspase-11 siRNA (50 nmol/L) or NC siRNA (50 nmol/L) for 48 h and then treated with Ang II (1 μmol/L) for 24 h. a Pro-caspase-11 and caspase-11 were detected by western blotting. b Quantification of pro-caspase-11 and caspase-11. c Pro-caspase-1, pro-IL-1β, caspase-1, and IL-1β were detected by western blotting. d Quantification of pro-caspase-1, caspase-1, pro-IL-1β, and IL-1β. e IL-1β concentrations in the supernatants were measured by ELISA. Data are presented as the mean ± SEM (n = 6). *P < 0.05 versus NC siRNA. **P < 0.01 versus NC siRNA. #P < 0.05 versus Ang II + NC siRNA
Caspase-11 formed a protein complex with caspase-1
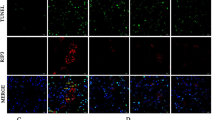
As shown in Fig. 7a, the expression of both caspase-11 and caspase-1 was detected in cultured renal TECs, and caspase-11 and caspase-1 both colocalized in the cytoplasm. Ang II treatment enhanced the colocalization signal, whereas transfection with caspase-11 siRNA weakened the colocalization. Co-immunoprecipitation experiments suggested that caspase-11 was able to interact with caspase-1, and the interaction was increased after Ang II stimulation (Fig. 7b).
Caspase-11 formed a protein complex with caspase-1. a NRK-52E cells were transfected with caspase-11 siRNA (50 nmol/L) or NC siRNA (50 nmol/L) for 48 h and then treated with Ang II (1 μmol/L) for 24 h. Levels of intracellular caspase-11 and caspase-1 were examined via immunofluorescence using anti-caspase-11 (red), anti-caspase-1 (green), and DAPI (blue). Magnification: × 200, Bar = 45 μm. b NRK-52E cells were treated with Ang II for 12 h. Cell lysates were immunoprecipitated with anti-caspase-1 antibody and analyzed by immunoblotting with anti-caspase-11 antibody
Inhibition of caspase-11 suppressed Ang II-induced TGF-β and extracellular matrix (ECM) production
Ang II led to the substantial upregulation of TGF-β, fibronectin, and collagen I. Knocking down caspase-11 via siRNA significantly blunted the Ang II-induced elevations in TGF-β, fibronectin, and collagen I expression (Figs. 8a, b). Inhibition of caspase-11 using Wed showed a similar effect on Ang II-induced TGF-β, fibronectin, and collagen I expression (Figs. 8c, d).
Caspase-11 siRNA and wedelolactone (Wed) suppressed Ang II-induced TGF-β and extracellular matrix (ECM) expression in renal TECs. a Cells were transfected with caspase-11 siRNA (50 nmol/L) or NC siRNA (50 nmol/L) for 48 h and then treated with Ang II (1 μmol/L) for 48 h. TGF-β, fibronectin, and collagen I expression were detected by western blotting. b Quantification of TGF-β, fibronectin, and collagen I. c After pretreatment with Wed, Ang II (1 μmol/L)-induced TGF-β, fibronectin, and collagen I levels were analyzed by western blotting. d Quantification of TGF-β, fibronectin, collagen I. Data are presented as the mean ± SEM (n = 6). *P < 0.05 versus NC siRNA. #P < 0.01 versus Ang II + NC siRNA. *P < 0.05 versus control. **P < 0.01 versus control. #P < 0.05 versus Ang II
Discussion
Dysfunctional inflammation plays a crucial role in aggravating renal fibrosis [7, 30, 31]. Caspase-11 was recently identified as an important inflammatory activator independent of the traditional inflammasome [18, 32]. Although caspase-11 was originally shown to be activated in immune cells in response to bacterial infection, subsequent work revealed that caspase-11 can be activated by different noninfectious pathological factors [27, 33]. In our study, we showed that caspase-11 expression and activation were upregulated in a traditional renal fibrosis animal model and TECs after Ang II treatment. Inhibition of caspase-11 significantly ameliorated renal morphological changes and fibrosis. These results suggested that caspase-11 activation is involved in UUO-induced renal deterioration.
Combined with the activation of caspase-11, the data obtained in our study showed that the maturation and release of IL-1β were significantly increased. Mounting evidence has demonstrated that the activation of renal inflammation is crucial in promoting renal fibrosis [2, 34]. As a powerful inflammatory cytokine, IL-1β has been noted to mediate the development of fibrosis and contribute to the pathogenesis of CKD [31, 34]. Our results showed that blockade of caspase-11 significantly suppressed the maturation and release of IL-1β in UUO mice and Ang II-treated renal TECs. ECM production was increased after treatment with IL-1β in renal TECs. These data suggested that elevated caspase-11 promoted renal fibrosis and functional deterioration by enhancing inflammation. Caspase-11 inhibition might therefore be beneficial in ameliorating inflammation and renal fibrosis.
IL-1β is originally synthesized as an inactive precursor, pro-IL-1β [35, 36]. The activation of pro-IL-1β relies on caspase-1, formerly called IL-1β-converting enzyme, which cleaves inactive pro-IL-1β into mature IL-1β [37]. Previous studies have demonstrated that NLRP3 inflammasome formation is essential for the activation of caspase-1 and subsequent IL-1β processing after Ang II or bovine serum albumin treatment [7, 16, 38]. In the present study, we reported that an alternative mechanism, caspase-11-dependent activation of caspase-1 and IL-1β maturation, was involved in renal fibrosis in UUO mice. Caspase-1 is also synthesized as an inactive precursor, pro-caspase-1 [39]. Traditionally, caspase-1 activation relies on the formation of the NLRP3 complex [7, 40]. However, very recent studies have shown that pro-caspase-1 can also be directly activated by caspase-11 [28, 41], representing an alternative way for caspase-1 activation other than the NLRP3 inflammasome. Consistent with previous studies, our co-immunoprecipitation results showed that caspase-11 was able to interact with caspase-1 and that the interaction between caspase-11 and caspase-1 was enhanced by Ang II treatment. Inhibiting caspase-11 significantly suppressed caspase-1 activation and IL-1β maturation. Based on these data, we speculated that in UUO mice or Ang II-treated tubular cells, caspase-1 might be activated by interacting with caspase-11 and in turn promote the maturation of IL-1β. Nevertheless, we cannot exclude the possibility that the NLRP3 inflammasome was also involved in the activation of caspase-1.
Interestingly, we found that inhibiting caspase-11 suppressed the synthesis of pro-caspase-1 and pro-IL-1β following Ang II treatment, though the mechanism is unclear. The maturation and release of inflammatory cytokine IL-1β has been reported to stimulate NF-κB and TNF-α expression, which in turn enhances the synthesis of pro-caspase-1 and pro-IL-1β [42,43,44]. Ultimately, the formation of such a vicious circle may even trigger cell apoptosis or pyroptosis [45, 46] and promote activation of the NF-κB pathway. Thus, the reduced expression of pro-caspase-1 and pro-IL-1β may depend on the suppression of NF-κB activation but not on the reduction of pro-caspase-1 and pro-IL-1β cleavage. Whether this vicious circle is initiated by caspase-11 requires further investigation.
In summary, our data demonstrate that the expression and activation of caspase-11, a novel caspase member in the inflammatory subfamily, is increased in UUO mice. The activation of caspase-11 was involved in the development of renal fibrosis. Caspase-11 interacts with caspase-1 and promotes caspase-1 activation, which in turn enhances the maturation of inflammatory cytokines and the progression of renal fibrosis. Inhibition of caspase-11 is a potential therapeutic target for preventing inflammatory activation and renal fibrosis.
References
Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;6:643–56.
Zhou QG, Zheng FL, Hou FF. Inhibition of tubulointerstitial fibrosis by pentoxifylline is associated with improvement of vascular endothelial growth factor expression. Acta Pharmacol Sin. 2009;30:98–106.
Impellizzeri D, Esposito E, Attley J, Cuzzocrea S. Targeting inflammation: new therapeutic approaches in chronic kidney disease (CKD). Pharmacol Res. 2014;81:91–102.
Jialal I, Major AM, Devaraj S. Global Toll-like receptor 4 knockout results in decreased renal inflammation, fibrosis and podocytopathy. J Diabetes Complicat. 2014;28:755–61.
Yiu WH, Lin M, Tang SC. Toll-like receptor activation: from renal inflammation to fibrosis. Kidney Int Suppl. 2014;4:20–5.
Shahzad K, Bock F, Al-Dabet MM, Gadi I, Kohli S, Nazir S, et al. Caspase-1, but not caspase-3, promotes diabetic nephropathy. J Am Soc Nephrol. 2016;27:2270–5.
Ding LH, Liu D, Xu M, Liu H, Wu M, Tang RN, et al. Enalapril inhibits tubulointerstitial inflammation and NLRP3 inflammasome expression in BSA-overload nephropathy of rats. Acta Pharmacol Sin. 2014;35:1293–301.
Hu ZB, Ma KL, Zhang Y, Wang GH, Liu L, Lu J, et al. Inflammation-activated CXCL16 pathway contributes to tubulointerstitial injury in mouse diabetic nephropathy. Acta Pharmacol Sin. 2018;39:1022–33.
Li Q, Fu W, Yao J, Ji Z, Wang Y, Zhou Z, et al. Heme induces IL-1beta secretion through activating NLRP3 in kidney inflammation. Cell Biochem Biophys. 2014;69:495–502.
Yamagishi H, Yokoo T, Imasawa T, Mitarai T, Kawamura T, Utsunomiya Y. Genetically modified bone marrow-derived vehicle cells site specifically deliver an anti-inflammatory cytokine to inflamed interstitium of obstructive nephropathy. J Immunol. 2001;166:609–16.
Zhang M, Guo Y, Fu H, Hu S, Pan J, Wang Y, et al. Chop deficiency prevents UUO-induced renal fibrosis by attenuating fibrotic signals originated from Hmgb1/TLR4/NFkappaB/IL-1beta signaling. Cell Death Dis. 2015;6:e1847.
Ruiz-Ortega M, Egido J. Angiotensin II modulates cell growth-related events and synthesis of matrix proteins in renal interstitial fibroblasts. Kidney Int. 1997;52:1497–510.
Wolak T, Kim H, Ren Y, Kim J, Vaziri ND, Nicholas SB. Osteopontin modulates angiotensin II-induced inflammation, oxidative stress, and fibrosis of the kidney. Kidney Int. 2009;76:32–43.
Lin L, Phillips WE, Manning RD. Intrarenal angiotensin ii is associated with inflammation, renal damage and dysfunction in dahl salt-sensitive hypertension. J Am Soc Hypertens. 2009;3:306–14.
Xie P, Joladarashi D, Dudeja P, Sun L, Kanwar YS. Modulation of angiotensin II-induced inflammatory cytokines by the Epac1-Rap1A-NHE3 pathway: implications in renal tubular pathobiology. Am J Physiol Ren Physiol. 2014;306:F1260–74.
Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol. 2010;21:1732–44.
Haneklaus M, O’Neill LA. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265:53–62.
Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–21.
Kang SJ, Wang S, Kuida K, Yuan J. Distinct downstream pathways of caspase-11 in regulating apoptosis and cytokine maturation during septic shock response. Cell Death Differ. 2002;9:1115–25.
Vigano E, Mortellaro A. Caspase-11: the driving factor for noncanonical inflammasomes. Eur J Immunol. 2013;43:2240–5.
Meunier E, Dick MS, Dreier RF, Schurmann N, Kenzelmann Broz D, Warming S, et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509:366–70.
Stowe I, Lee B, Kayagaki N. Caspase-11: arming the guards against bacterial infection. Immunol Rev. 2015;265:75–84.
Wang J, Sahoo M, Lantier L, Warawa J, Cordero H, Deobald K, et al. Caspase-11-dependent pyroptosis of lung epithelial cells protects from melioidosis while caspase-1 mediates macrophage pyroptosis and production of IL-18. PLoS Pathog. 2018;14:e1007105.
Kerur N, Fukuda S, Banerjee D, Kim Y, Fu D, Apicella I, et al. cGAS drives noncanonical-inflammasome activation in age-related macular degeneration. Nat Med. 2018;24:50–61.
Bordon Y. Innate immunity: caspase 11 hunts down cytosolic bacteria. Nat Rev Immunol. 2013;13:154–5.
Broz P, Monack DM. Noncanonical inflammasomes: caspase-11 activation and effector mechanisms. PLoS Pathog. 2013;9:e1003144.
Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150:606–19.
Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–9.
Ruster C, Wolf G. Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J Am Soc Nephrol. 2011;22:1189–99.
Lee J, Hur J, Lee P, Kim JY, Cho N, Kim SY, et al. Dual role of inflammatory stimuli in activation-induced cell death of mouse microglial cells. Initiation of two separate apoptotic pathways via induction of interferon regulatory factor-1 and caspase-11. J Biol Chem. 2001;276:32956–65.
Zhang HF, Wang YL, Gao C, Gu YT, Huang J, Wang JH, et al. Salvianolic acid A attenuates kidney injury and inflammation by inhibiting NF-kappaB and p38 MAPK signaling pathways in 5/6 nephrectomized rats. Acta Pharmacol Sin. 2018. https://doi.org/10.1038/s41401-018-0026-6.
Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490:288–91.
Schauvliege R, Vanrobaeys J, Schotte P, Beyaert R. Caspase-11 gene expression in response to lipopolysaccharide and interferon-gamma requires nuclear factor-kappa B and signal transducer and activator of transcription (STAT) 1. J Biol Chem. 2002;277:41624–30.
Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–30.
Zheng XY, Mao CY, Qiao H, Zhang X, Yu L, Wang TY, et al. Plumbagin suppresses chronic periodontitis in rats via down-regulation of TNF-alpha, IL-1beta and IL-6 expression. Acta Pharmacol Sin. 2017;38:1150–60.
Krumm B, Xiang Y, Deng J. Structural biology of the IL-1 superfamily: key cytokines in the regulation of immune and inflammatory responses. Protein science: a publication of the Protein. Society. 2014;23:526–38.
Ng TM, Monack DM. Revisiting caspase-11 function in host defense. Cell Host Microbe. 2013;14:9–14.
Wang J, Wen Y, Lv LL, Liu H, Tang RN, Ma KL, et al. Involvement of endoplasmic reticulum stress in angiotensin II-induced NLRP3 inflammasome activation in human renal proximal tubular cells in vitro. Acta Pharmacol Sin. 2015;36:821–30.
Lopez-Pastrana J, Ferrer LM, Li YF, Xiong X, Xi H, Cueto R, et al. Inhibition of caspase-1 activation in endothelial cells improves angiogenesis: a novel therapeutic potential for ischemia. J Biol Chem. 2015;290:17485–94.
Song MT, Ruan J, Zhang RY, Deng J, Ma ZQ, Ma SP. Astragaloside IV ameliorates neuroinflammation-induced depressive-like behaviors in mice via the PPARgamma/NF-kappaB/NLRP3 inflammasome axis. Acta Pharmacol Sin 2018;39:1559–70.
Kang SJ, Wang S, Hara H, Peterson EP, Namura S, Amin-Hanjani S, et al. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J Cell Biol. 2000;149:613–22.
Hansen B, Dittrich-Breiholz O, Kracht M, Windheim M. Regulation of NF-kappaB-dependent gene expression by ligand-induced endocytosis of the interleukin-1 receptor. Cell Signal. 2013;25:214–28.
Tarantino N, Tinevez JY, Crowell EF, Boisson B, Henriques R, Mhlanga M, et al. TNF and IL-1 exhibit distinct ubiquitin requirements for inducing NEMO-IKK supramolecular structures. J Cell Biol. 2014;204:231–45.
Wang W, Guan WJ, Huang RQ, Xie YQ, Zheng JP, Zhu SX, et al. Carbocisteine attenuates TNF-alpha-induced inflammation in human alveolar epithelial cells in vitro through suppressing NF-kappaB and ERK1/2 MAPK signaling pathways. Acta Pharmacol Sin. 2016;37:629–36.
Cheng KT, Xiong S, Ye Z, Hong Z, Di A, Tsang KM, et al. Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J Clin Invest. 2017;127:4124–35.
Miao N, Wang B, Xu D, Wang Y, Gan X, Zhou L, et al. Caspase-11 promotes cisplatin-induced renal tubular apoptosis through a caspase-3-dependent pathway. Am J Physiol Ren Physiol. 2018;314:F269–F79.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 81470591 and 81670664) to Li-min Lu and (No. 81270814) to Xiao-xia Wang. This work was also supported by the Science and Technology Commission of Shanghai Municipality (14DZ2260200, the project of Shanghai Key Laboratory of Kidney and Blood Purification).
Author contributions
L-mL, JL, X-xW, WZ, and N-jM designed the research; N-jM, H-yX, DX and J-yY performed experiments; Y-zW, BW, FY, Z-lz, LZ, P-pC, and QC analyzed the data; L-mL, N-jM, and HX wrote the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Miao, Nj., Xie, Hy., Xu, D. et al. Caspase-11 promotes renal fibrosis by stimulating IL-1β maturation via activating caspase-1. Acta Pharmacol Sin 40, 790–800 (2019). https://doi.org/10.1038/s41401-018-0177-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-018-0177-5
Keywords
This article is cited by
-
Deubiquitinating enzyme USP11 promotes renal tubular cell senescence and fibrosis via inhibiting the ubiquitin degradation of TGF-β receptor II
Acta Pharmacologica Sinica (2023)
-
Caspase-11 promotes NLRP3 inflammasome activation via the cleavage of pannexin1 in acute kidney disease
Acta Pharmacologica Sinica (2022)
-
TLR2/caspase-5/Panx1 pathway mediates necrosis-induced NLRP3 inflammasome activation in macrophages during acute kidney injury
Cell Death Discovery (2022)
-
Disulfiram inhibits inflammation and fibrosis in a rat unilateral ureteral obstruction model by inhibiting gasdermin D cleavage and pyroptosis
Inflammation Research (2021)