Abstract
The global impact of SARS-CoV-2 infection has raised concerns about secondary diseases beyond acute illness. This review explores the significance and potential underlying mechanisms of how SARS-CoV-2 infection might elicit an immune response targeting N-methyl-D-aspartate (NMDA) receptors, and its implications for autoimmune-driven neuropsychiatric manifestations. We identified 19 published case reports of NMDA receptor encephalitis associated with SARS-CoV-2 infection or vaccination by a systematic literature search. The significance of these reports was limited since it is not clear if a coincidental or causal relationship exists between SARS-CoV-2 infection or vaccination and manifestation of NMDA receptor encephalitis. The included studies were hampered by difficulties in establishing if these patients had pre-existing NMDA receptor antibodies which entered the brain by infection- or vaccination-associated transient blood-brain barrier leakage. In addition, four cases had comorbid ovarian teratoma, which is a known trigger for development of NMDA receptor encephalitis. Considering that billions of people have contracted COVID-19 or have been vaccinated against this virus, the publication of only 19 case reports with a possible link to NMDA receptor encephalitis, indicates that it is rare. In conclusion, these findings do not support the case that SARS-CoV-2 infection or vaccination led to an increase of existing or de novo encephalitis mediated by an autoimmune response targeting NMDA receptor function. Nevertheless, this work underscores the importance of ongoing vigilance in monitoring viral outbreaks and their potential impact on the central nervous system through basic, epidemiological and translational research.
Similar content being viewed by others
Introduction
Background
Although most coronavirus-dedicated websites have stopped collecting data on COVID-19 cases caused by infection with the SARS-CoV-2 virus [1, 2], agencies such as the Institute for Health Metrics and Evaluation Model have estimated that more than half of the world population had been infected at least once, as of January 2022 [3]. In addition, a significant proportion of people worldwide have been re-infected by the virus, particularly during the more recent waves of the SARS-CoV-2 B.1.1.529 (Omicron) sub-variants [4,5,6,7]. This has been linked to the high number of mutations in the Omicron strains, which has resulted in a higher transmissibility rate and increased ability of the virus to evade the immune system and reduce vaccine efficacy [8,9,10,11]. Furthermore, 10–30% of SARS-CoV-2 cases have been found to suffer from long-term effects of infection, which has been termed post-COVID syndrome (PCS; also known as long COVID) [12,13,14]. According to the National Institute for Health and Care Excellence (NICE) guidelines, PCS is characterised as “signs and symptoms that develop during or after an infection consistent with COVID‑19, continue for more than 12 weeks and are not explained by an alternative diagnosis” [15]. Although the clinical manifestation is not homogeneous, PCS may occur as various overlapping symptoms and include chronic fatigue and physical complaints, as well as neurological and neuropsychiatric presentations [16, 17].
Before the advent of the vaccines, most countries in the world enacted similar strategies to reduce the prevalence of the SARS-COV-2 virus and to save lives, including different lockdown and quarantine approaches, as well as implementing social restriction policies [18,19,20]. Although these policies were mostly effective in reducing the infection rate, the side effect was an increase in neuropsychiatric symptoms such as stress, anxiety and depression [21,22,23,24,25]. For example, the Office for National Statistics in the United Kingdom estimated that there was a doubling of adults experiencing either moderate or severe depression symptoms from 10 to approximately 20% during the first year of the pandemic [26, 27]. In addition to being a response to social restriction measures, reports demonstrated that some of the neurological and psychiatric effects may have resulted directly from infection with the SARS-CoV-2 virus [28,29,30,31].
COVID-19 infection and autoimmune outcomes
Some severe cases of SARS-CoV-2 infection or PCS have demonstrated effects on organs and tissues of the body similar to autoimmune diseases marked by the presence of autoantibodies [32,33,34,35,36,37], interstitial pneumonia with autoimmune features (IPAF) [38], antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) [39], systemic lupus erythematosus (SLE) [40], rheumatic musculoskeletal diseases [41], and neurological conditions such as Guillain-Barré syndrome (GBS) [42]. In addition, exacerbation or deterioration has been observed in patients with psychiatric disorders, including autism, schizophrenia [43], depression and anxiety disorders [44].
We and others have proposed that mimicry of the accessory, structural and non-structural proteins of the SARS-CoV-2 virus may be one of the underlying causes of autoimmune response against peripheral and central nervous system (CNS) G protein-coupled and ion channel receptors [45, 46]. In the latter study, we performed a meta-analysis and identified 8 potential cases of N-methyl-D-aspartate (NMDA) receptor encephalitis associated with SARS-CoV-2 infection [45]. This autoimmune disorder can present with neurological and psychosis-like symptoms and is diagnosed according to the clinical signs, as well as findings of brain magnetic resonance imaging (MRI), electroencephalography (EEG) and the presence of autoantibodies against NMDA receptors in blood serum, plasma or cerebrospinal fluid (CSF) [47, 48].
Molecular mimicry and autoimmunity following SARS-CoV-2 infection
As described above, autoimmunity is common after SARS-CoV-2 infection [49], and molecular mimicry may play a crucial role in this.
Types of molecular mimicry
We highlight three types of molecular mimicry that could cause autoimmune responses following a viral infection, as reviewed in previous publications [49,50,51]. The first type is caused by structural similarities between viral and host proteins. In the second type, the foreign antigen on the infectious agent contains similar epitopes to a host antigen but is different enough to induce the release of pro-inflammatory cytokines and chemokines (e.g. by antigen-presenting dendritic cells, macrophages and CD4 + T helper cells, which orchestrate the immune response). A third type of molecular mimicry is recognition of dissimilar chemical structures on separate molecules by a single antibody by chance. Cross-reactive peptide epitopes have been identified [52] or theorized [53] for SARS-CoV-2 and other coronaviruses. Given the large size of the SARS-CoV-2 proteome and the potential for severe activation of the immune response, it is likely that one or more types of mimicry are involved.
SARS-CoV-2 structure
SARS-CoV-2 is an enveloped virus particle containing a positive-sense 30 kb RNA genome, which is stabilized by nucleocapsid proteins. This is surrounded by a membrane containing envelope, membrane proteins, and the membrane anchored spike proteins that enable the infection of host cells [54,55,56]. The RNA genome encodes 16 non-structural proteins which, when activated, are involved in the infection and replication processes [57, 58], as well as the spike, envelope, membrane and nucleocapsid structural proteins and 3a, 6, 7a, 7b, 8, 9b, and 10 accessory proteins [59, 60].
SARS-CoV-2 and molecular mimicry
Viruses such as SARS-CoV-2 are well-known for having the potential to initiate inflammatory and autoimmune responses in infected individuals [61]. Related to this, a study by Yapici-Eser et al. proposed that some of the psychiatric symptoms associated with SARS-CoV-2 infections may be caused by the ability of the viral proteins to mimic host protein interactions, such as those of G-protein-coupled receptors (GPCR) and ligand-gated ion channel receptor proteins involved in neuronal signalling [46]. This included the SARS-CoV-2 non-structural proteins 8 (NSP8) and 9 (NSP9) which may mimic interactions of the host NMDA receptor NR2A and NR1 subunits, respectively. This kind of mimicry may lead to bystander T cell activation and epitope spreading, giving rise to an autoimmune reaction [62].
Aims
In this paper, we have extended our previous meta-analysis [45] by searching the PubMed and Google Scholar databases to identify all cases reports of NMDA receptor encephalitis associated with SARS-CoV-2 infection or COVID-19 vaccination. We aimed to describe potential mechanisms on how SARS-CoV-2 infection can lead to the development or exacerbation of autoimmune NMDA receptor encephalitis and summarize a potential screening and treatment approach for this condition in acute and post-SARS-CoV-2 infected patients. This is followed by critical reflections on the evaluation of the case reports found, open questions on causality and limitations of our meta-analysis.
Methods
We searched PubMed and Google Scholar databases using the search terms “NMDA encephalitis” or “NMDA” and “SARS-CoV-2” or “COVID-19” to identify relevant cases. The last search was performed on May 3rd, 2023. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, http://prisma-statement.org) were applied. The flow diagram for identification, screening, eligibility and inclusion of studies is illustrated in Fig. 1. Studies were checked for eligibility and selected by two authors (VV and PCG). Initially, many more papers were identified in Google Scholar than in PubMed. However, most of these articles did not meet the search criteria as, on close inspection, these were found to be comments on already published articles or related meta-analyses. For the final evaluation, only case or original reports published in peer-reviewed international journals were considered. For a complete review, we also included the case reports described in our previous meta-analysis [45]. Quality was assessed according to the Case Report Guidelines (CARE; https://www.care-statement.org/). Articles in languages other than English or German were translated using DeepL Translator [63]. Studies were included if cases showed SARS-CoV-2 positivity in nasopharyngeal swab, blood or CSF tests and had clinical signs of NMDA receptor encephalitis [47]. Data on patient age, medical history, hospitalization reason, respiratory status, psychiatric symptoms, brain MRI analyses, EEG readings, and blood-or CSF-based biomarkers were mined as available. Information on attempted therapies and clinical outcomes were extracted if these were indicated.
Identification: The original database search resulted in 98 records from PubMed and 1699 records from Google Scholar (in total 1797 records). Screening: After removing 93 duplicates, there were 1704 unique citations eligible for screening of the publication content. Eligibility: In the first phase of the eligibility check, 1682 records were excluded because they did not meet the inclusion criteria (e.g. missing CSF examination, missing COVID-19 confirmation, reviews, comments on case reports). This process left 22 records to be reassessed for eligibility by a very thorough review of all available data in the full-text articles. The second stage of screening excluded 3 articles where the timing of SARS-CoV-2 infection was not clear. Included: Finally, 19 articles were included in our systematic review.
As a supplement, we checked whether the cases described fulfilled the criteria of Graus et al. [47] for definite or probable NMDA receptor encephalitis. If the publications did not contain the necessary information, the authors were contacted to obtain this data.
Results
A search of the PubMed and Google Scholar databases led to identification of 1797 total cases of NMDA receptor encephalitis using the search terms described in the Methods section. Of these, 93 cases were excluded which appeared in duplicate articles and 1682 were removed which did not include CSF examination or confirmation for COVID-19 infection, or they were reviews or comments on case reports (Fig. 1). The 8 cases that we reported on previously were included [45], leaving 22 articles. Finally, three further cases were excluded as the timing of SARS-CoV-2 infection in these was not clear [64,65,66]. This left a final 19 articles which met all criteria for this meta-analysis (Table 1). The strict criteria for NMDA receptor encephalitis proposed by Graus et al. [47] were definitely met in 13 of the 19 cases, while 3 cases met the criteria for probable NMDA receptor encephalitis. In 3 cases this was unclear, as not all data were available for this assessment (see Supplementary Table).
Demographics
Of the 19 cases, 12 were females ranging from 23 months to 50 years-old [67,68,69,70,71,72,73,74,75,76,77,78] and 7 were males ranging from 10 months to 50 years-old [79,80,81,82,83,84,85] (Table 1). Two articles were in Spanish [71, 76], one was in Russian [70], one in Ukrainian [81], and the rest were published in the English language. Sixteen of the 19 cases tested positive for COVID-19 either before or shortly after admission (Table 1), and three patients had received a COVID-19 vaccination <1 week before admission [68, 69, 72].
Reason for hospitalization
All patients described in the published case reports had psychiatric and/or neurological symptoms and had been hospitalized for various reasons. These included: seizures [67, 68, 77]; front-orbital syndrome characterized by catatonic symptoms [79]; fever [70, 73, 75, 78]; psychiatric symptoms [71, 72, 76, 78, 82,83,84]; urinary frequency [69]; poor feeding, irritability and convulsion [80]; disturbed sleep [74, 78, 82]; gait disorder [85]; and severe lethargy, speech and swallowing difficulty [81]. Six out of the 16 patients with COVID-19 positivity had no respiratory symptoms [74, 75, 78, 81, 83, 84], while 10 of them had mild, to severe respiratory symptoms (Table 1).
CSF and blood findings
CSF
All cases were positive for the presence of NMDA receptor antibodies in CSF (Table 1), while positive test results for antibodies in both CSF and blood samples were mentioned in two cases [68, 78]. In addition, two patients showed oligoclonal immunoglobulin G (IgG) bands in CSF [73, 83] and one of these had polyclonal IgG banding in blood [72]. Another patient was also positive for the presence of glutamate decarboxylase 65 kDa isoform (GAD-65) antibodies in CSF [79]. In addition, pleocytosis >5/μL was found in 10 patients [69, 70, 72, 73, 76,77,78, 80, 82, 83]. Four cases had high protein and pleocytosis [69, 73, 76, 79] and one patient had only high protein levels in their CSF [75]. All cases were negative for commonly used virological and microbiological tests in CSF (excluding SARS-CoV-2 testing).
Blood
Some cases also showed elevations in other blood measures. For example, four cases had leukocytosis [71, 73, 80, 82] and two had lymphopenia [77, 85]. Other cases showed elevations in liver aminotransferases [69]; C-reactive protein (CRP) [77, 82, 85]; and one had high creatinine [81].
EEG recordings
EEG abnormalities occurred in 13 patients and consisted of either frontal intermittent rhythmic delta activity (FIRDA) [67], spike-and-waves in bilateral temporal areas [68], subcortical dysfunction in frontal, temporal and occipital regions [79], diffuse, slow activity, intermittent delta waves [71], delta brush pattern [74], diffuse delta activity with extreme delta brush pattern and anterior subcontinuous periodic theta activity [83], encephalopathic pattern with disseminated delta waves [85], diffuse, slow activity, bilateral spikes and slow waves discharges in fronto-central regions [72], epileptic focus in the right parietal region [73], epileptic discharges in the left frontotemporal region [76, 77], theta activity, unstable and non-reactive to visual stimuli [82]. The remaining cases were either normal for EEG recordings [69], this analysis was not mentioned or performed, or the analysis was not mentioned but likely abnormal due to the presence of multiple seizures [70].
Imaging
Brain imaging
Brain imaging was performed all cases described. Eighteen cases had MRI and one had a computed tomography (CT) scan of the brain. Unfortunately, it was often not clear whether an examination with a contrast agent had also been carried out. Twelve MRI scans were classified as normal [67, 69,70,71,72,73,74, 77, 78, 80, 83, 85], and one patient presented normal findings except for some subtle signal changes on T2-weighted and FLAIR images [68]. Another case showed hypermetabolism in the basal nuclei and diffuse cortical hypometabolism in FDG-PET [70]. One patient had changes in brain morphology due to multiple gliosis of vascular origin [81], and two patients presented typical NMDA receptor encephalitis findings and hyperintensities in T2 and/or FLAIR sequences [76, 79]. The patient who had the CT scan was classified as normal [82].
Presence of ovarian teratoma
In the 12 female patients, four cases screened for ovarian teratoma showed the presence of this malignancy [68, 70, 73, 76]. However, 5 other cases were examined for the presence of ovarian teratomas and were negative [67, 69, 72, 74, 75]. Three publications did not mention a search for ovarian teratoma [71, 77, 78].
Outcome
Seventeen out of the 19 cases received intravenous immunoglobulin (IVIg) and corticosteroids after the NMDA receptor encephalitis diagnosis [67,68,69,70,71,72,73, 75,76,77,78,79,80, 82,83,84,85]. In addition, four cases also received plasmapheresis therapy [74, 75, 83, 85], 9 were administered rituximab [67,68,69, 71,72,73,74,75, 80], 12 cases were recorded as having received anti-seizure treatment [67, 70,71,72, 74, 75, 77,78,79, 82, 83, 85], and 5 were given antipsychotics [67, 69,70,71, 82]. One patient received corticosteroids, plasmapheresis and rituximab [74] and one was treated with amantadine and citicoline [81]. Nine patients were given antiviral medication [68, 70,71,72,73, 77, 82, 83, 85] and four had an ovarian teratoma removed [68, 70, 73, 76]. Finally, one patient was given metoprolol to manage tachycardia and hypertension [69], and one received azathioprine, cyclophosphamide and treatment for extrapyramidal movements [80].
Of the 19 cases, patient outcome was not mentioned in one study [67], two patients showed no improvement [74, 79], 15 improved or fully recovered [68,69,70,71,72, 75,76,77,78, 80,81,82,83,84,85] and one died [73].
Proposed mechanisms of NMDA receptor encephalitis following SARS-CoV-2
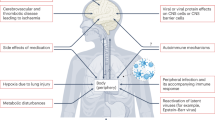
While general effects of SARS-CoV-2 on neuroinflammation are evident through peripheral inflammation and blood-brain barrier (BBB) disruption, autoimmune CNS effects of the virus may also play a role, although these are less well understood. Further research in both areas is essential for a more comprehensive understanding.
SARS-CoV-2, peripheral inflammation and BBB permeability
SARS-CoV-2 primarily targets respiratory epithelial cells but can stimulate a broad inflammatory response. Once the virus has been detected by the immune system, a cascade of inflammatory cytokines and chemokines, sometimes termed a “cytokine storm,” is released, which can instigate systemic inflammation [86]. Inflammatory mediators from the periphery can compromise the BBB, e.g. through the effects of pro-inflammatory cytokines or activation of the kynurenine pathway [87, 88]. Alternatively, direct effects of SARS-CoV-2 on the BBB have been proposed [89]. Finally, the breakdown of the BBB can trigger neuroinflammation, including the recruitment of macrophages and lymphocytes and the activation of astrocytes and microglia [88].
Induction of CNS autoimmunity by SARS-CoV-2
The precise mechanism of how SARS-CoV-2 viral infection can lead to autoimmunity in the CNS is not known. In cases of NMDA receptor encephalitis, it is possible that viral proteins such as NSP8 and NSP9 released during the infection cycle may lead to molecular mimicry of host interactions of the NR2A and NR1 subunits of the NMDA receptor [45]. In turn, this may lead to bystander T cell activation and epitope spreading, giving rise to an autoimmune reaction, as proposed by Pacheco et al. [90]. As an outcome of this, the virus-associated immune response in vascular endothelia can cause disruption of the BBB, permitting entry of these antibodies into the CNS in rare cases. This can lead to further BBB disruption and inflammation, along with a cellular immune response in the brain. Finally, auto-antibodies originating from the periphery or produced in the CNS can cross-react with NMDA receptor subunits leading to down regulation of this neuronal circuity.
Psychosis resulting from the presence of NMDA receptor antibodies is thought to result from (i) increased opening of synaptic NMDA receptor channels, and (ii) subsequent movement of these to extra-synaptic locations or via internalization [91]. As these channels open less frequently, this can lead to an imbalance between synaptic and extra-synaptic NMDA receptors. This imbalance is proposed to result in the multifaceted neurological presentations of NMDA receptor encephalitis [91]. A comparable effect can also be observed with administration of ketamine or phencyclidine as well as by antibodies against the NR1 and NR2 NMDA receptor subunits [92].
Diagnosis and treatment
To guide clinical decision making, step-wise schemes have been developed which can facilitate timely and accurate diagnostics to allow early initiation of the appropriate therapeutics for autoimmune encephalitis patients [48, 93,94,95]. If the patient presents with new-onset progressive psychiatric symptoms, together with focal neurological abnormalities, testing for the presence of autoantibodies should be considered depending on the results from the basic CSF analyses, in addition to EEG and MRI if warranted. To support this work, several multiplex immunoassay screening panels are available which can test for the presence of autoantibodies against NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and gamma-aminobutyric acid (GABAB) receptors (EUROIMMUN; Lübeck, Germany [96]) as well as the M1, M2 and M5 muscarinic acetylcholine, and α1- and α2- adrenergic receptors (CellTrend; Berlin, Germany [97]).
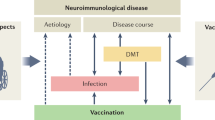
The standard first treatment for autoimmune encephalitis is high-dose steroids over a 3 to 5-day course, sometimes in combination with administration of intravenous immunoglobulins or plasmapheresis (Fig. 2). In case of the need for second line or escalation therapies, treatment with rituximab can be employed [94, 98]. In refractory cases, immunosuppressive agents such as cyclophosphamide, mycophenolate mofetil or methotrexate can be used to achieve an effective response [94, 98]. If there is no response to above line of therapy, treatment with the proteasome inhibitor and immunosuppressant bortezomib can be attempted [99].
Immunosuppression by corticosteroid therapy (1 g methylprednisolone/day for 5 days), intravenous human immunoglobulin (IVIg) administration (0.4 g/kg/day for 5 days) or immunoadsorption or plasmapheresis for rapid removal of pathogenic autoantibodies should be first-line therapy in patients with definite autoimmune encephalitis. If therapy fails, treatment should be extended within a few days, preferably with rituximab (2 × 1000 mg i.v. or s.c. at 2 to 4-week intervals). In refractory cases, combination treatment with cyclophosphamide (750 mg/m2 body surface area every 4 weeks), mycophenolate mofetil, or methotrexate may also be required to achieve a clinical response. Bortezomib can be used for escalation therapy (1–6 cycles of 1.3 mg/m2 body surface area for 21 days each cycle). In addition, antipsychotics with low extrapyramidal side effects (quetiapine, clozapine) should be prescribed for symptomatic pharmacotherapy of psychotic symptoms to reduce the risk of neuroleptic-induced dyskinesia or malignant neuroleptic syndrome. Short-acting benzodiazepines can be used for anxiolysis, sedation and to treat catatonic symptoms [91, 94, 98].
Critical considerations regarding relevance of SARS-CoV-2-induced molecular mimicry of NMDA receptors and questions of causality
At present, there are still open questions regarding classification of the clinical relevance of SARS-CoV-2-induced molecular mimicry of NMDA receptors and whether there is a causal relationship between SARS-CoV-2 infection or vaccination and the manifestation of NMDA receptor encephalitis. Based on the current literature this should be considered very rare, consistent with our finding of only 19 case reports worldwide during the COVID-19 pandemic.
The first question is whether antineuronal NMDA receptor antibodies are found more frequently in blood after acute COVID-19 disease or SARS-CoV-2 vaccination compared to other infections with similar severity. For example, these antibodies were present in blood more frequently if there was a serum scar from previous influenza infections [100]. However, a recent study of thousands of serum samples found no change in the positivity rate of NMDA receptor antibodies during the COVID 19 pandemic [101].
Notably, the presence of NMDA receptor antibodies in serum alone does not cause encephalitis, as CNS-reactive antibodies must be present in brain tissue to cause CNS symptoms. Approximately 10% of people without neuropsychiatric disease have low titers of NMDA receptor serum antibodies. This is age dependent as >20% of older individuals can show the presence of these antibodies, probably because their immune system matured further by contact with an increasing number of pathogens or antigens in general in the course of life [100]. This leads to the second question of whether encephalitis occurs more frequently after acute COVID-19 disease or SARS-CoV-2 vaccination compared to other infections with similar severity. For this to happen, the antibodies either reach the brain or are produced intrathecally [102].
Given that more than half of the world’s population had COVID-19 and more than 12.7 billion vaccinations have been performed [103], the publication of 19 case reports worldwide with a possible NMDA receptor antibody-mediated CNS disease suggests that this is exceedingly rare. Thus, the specific association between COVID-19 and NMDA receptor encephalitis is weak.
An important confounding factor is that ovarian teratoma was identified as a comorbidity in four of the 12 women. This tumor is a known trigger for development of NMDAR encephalitis [98]. Six other cases were examined and had no ovarian teratomas, while no search for this tumor was mentioned in two publications. As the current reports are not complete regarding screening for ovarian teratomas or potential pre-existence of NMDA receptor autoantibodies, it is not clear if a causal relationship existed between SARS-CoV-2 infection or vaccination and the NMDA receptor encephalitis diagnosis. Also, in the patient with focal right frontal lobe epilepsy [67], it is not clear if the NMDA receptor antibody formation was triggered by tissue destruction in the context of stroke or epileptic seizuresFootnote 1 rather than by SARS-CoV-2 infection. As far as vaccination-related causality is concerned, it should be noted that the interval between vaccination and hospitalisation was short (1 to 7 days). This raises the question of whether a transfer of pre-formed serum antibodies into the brain or CSF through BBB opening by pro-inflammatory cytokines during the immune response to vaccines played a role, rather than de novo NMDA receptor antibody formation induced by vaccination [102]. Notably, one of the cases with vaccination also had comorbid ovarian teratoma [68].
Nationwide studies including all SARS-CoV-2 PCR tests performed in Denmark showed that the risk of new-onset psychiatric and neurological disorders increased after COVID-19, particularly in association with disease severity [104, 105]. However, the risk was comparable to that observed after other infections of similar severity [104, 105]. An unbiased epidemiological analysis of the manifestation of NMDA receptor encephalitis after COVID-19 or vaccination is still lacking but this would be important for clarification regarding a causal link. Unfortunately, there is no specific ICD-10 code used only for autoimmune encephalitis or specifically for NMDA encephalitis, which makes it more difficult to extract such numbers from registers. Thus, there is a need to go into large-scale electronic health records to further shed light on the associations on a large-scale. However, in studies examining patients with severe COVID-19 and neuropsychiatric symptoms, NMDA receptor antibodies did not consistently appear in the CSF, so they are considered rare [106]. Also, CNS infection with SARS-CoV-2 have only been described on a case report level, whereas the CSF screening studies have not found clear evidence of intrathecal SARS-CoV-2 antibody production since levels in the peripheral blood were higher [106].
On the other hand, a Barcelona-based research network on autoimmune encephalitis found that the annualized mean number of patients screened for any type of antineuronal antibodies increased 1.3 times during the COVID-19 pandemic compared to the pre-pandemic period (p < 0.001) [107]. The overall positivity rate, controlled for the number of patients examined per year, did not change significantly during the COVID-19 pandemic (p = 0.51), but sub-analyses for different antibody specificities showed a potential increase in NMDA receptor positive serum and CSF findings (p = 0.03).
Important for assessing the incidence of autoimmune encephalitis after COVID-19 vaccination is the insight provided by large databases, such as the publicly available EudraVigilance database of the European Medicines Agency [108].Footnote 2 This database contains detailed safety information on all medicines approved in Europe, such as the COVID-19 vaccines. Although NMDA receptor encephalitis is not specifically listed, a search for reports of “autoimmune encephalitis” associated with COVID-19 vaccines on 28 August 2023 revealed the following numbers [108, 109]:
-
COVID-19 mRNA vaccine ORIGINAL (Elasomeran; Moderna): ~55 million doses were distributed in Europe. Overall ~380.000 reports of suspected side effects; 23 reports of suspected autoimmune encephalitis associated with this vaccine by healthcare professionals.
-
COVID-19 mRNA vaccine ORIGINAL/OMICRON BA.1 (Elasomeran/Imelasomeran; Moderna): ~12 million doses were distributed in Europe. Overall 675 reports of suspected side effects; 0 reports of suspected autoimmune encephalitis associated with this vaccine.
-
COVID-19 mRNA vaccine ORIGINAL (Tozinameran; Pfizer-Biontech): ~137 million doses were distributed in Europe. Overall ~1.2 Million reports of suspected side effects; 62 reports of suspected autoimmune encephalitis associated with this vaccine by healthcare professionals.
-
COVID-19 mRNA vaccine ORIGINAL/OMICRON BA.1 (Tozinameran/ Riltozinameran; Pfizer-Biontech): ~9.5 million doses were distributed in Europe. Overall ~6.000 reports of suspected side effects; 0 reports of suspected autoimmune encephalitis associated with this vaccine.
-
COVID-19 mRNA vaccine ORIGINAL/OMICRON BA.4-5 (Tozinameran/ Famtozinameran; Pfizer-Biontech): ~23.5 million doses were distributed in Europe. Overall ~9.400 reports of suspected side effects; 2 reports of suspected autoimmune encephalitis associated with this vaccine by healthcare professionals.
-
COVID-19 vector vaccine CHADOX1 NCOV-19 (AstraZeneca): ~12 million doses were distributed in Europe. Overall ~550.000 reports of suspected side effects; 28 reports of suspected autoimmune encephalitis associated with this vaccine by healthcare professionals.
-
COVID-19 vector vaccine AD26.COV2.S (Janssen): ~17.5 million doses were distributed in Europe. Overall ~71.000 reports of suspected side effects; 3 reports of suspected autoimmune encephalitis associated with this vaccine by healthcare professionals.
-
COVID-19 recombinant spike protein vaccine NVX-COV2373 (Novavax): ~3.2 million doses were distributed in Europe. Overall ~1.600 reports of suspected side effects; 0 reports of suspected autoimmune encephalitis associated with this vaccine.
-
COVID-19 inactivated virus vaccine VLA2001 (Valneva): ~150.000 doses were distributed in Europe. Overall 34 reports of suspected side effects; 0 reports of suspected autoimmune encephalitis associated with this vaccine.
-
COVID-19 recombinant spike protein vaccine VIDPREVTYN BETA (Sanofi): ~16.5 million doses were distributed in Europe. Overall 373 reports of suspected side effects; 0 reports of suspected autoimmune encephalitis associated with this vaccine.
In summary, reports of suspected autoimmune encephalitis in the context of COVID-19 vaccination are extremely rare. Importantly, it should be noted that vaccines containing mRNA for production of the spike protein, or those that contain the spike protein itself, cannot be affected by molecular mimicry of anti-NMDA receptor antibodies via NSP8 and NSP9 as these sequences come from a separate region of the virus (see: Induction of CNS autoimmunity by SARS-CoV-2). In the case of vector vaccines, immune reactions against the vector virus could play a separate role.
Limitations
This study has a number of limitations that should be considered before further studies in this area are attempted. First, we only identified 19 cases of SARS-CoV-2-related autoimmune NMDA receptor encephalitis which included 7 cases from our previous study [45]. Moreover, in some of these cases, the criteria for NMDA receptor encephalitis diagnosis were incomplete or not fully described [47], and most of these studies were heterogeneous in their reporting, particularly with regards to circulating and image-based parameters. Often, neither the test method used to determine COVID-19 positivity (antigen assay, PCR, antibody titre) nor the assay used to determine NMDA receptor antibodies, was stated in the case reports. However, there are differences in sensitivity and specificity depending on the test method used. Three of the cases had autoimmune encephalitis in association with a COVID-19 vaccination [68, 69, 72], four were linked with ovarian teratoma [68, 70, 73, 76] and one with focal epilepsy [67]. Another reason that makes it difficult to draw conclusions regarding these cases is that information is missing concerning how many of these patients had experienced a previous SARS-CoV-2 infection. In such cases, vaccination might represent a second exposure to SARS-CoV-2 antigens, especially for the one who had received the Sinopharm vaccine (non-replicating whole virus). One critical aspect is whether individuals with autoimmune encephalitis following SARS-CoV-2 infection are predisposed to this disease manifestation by an underlying compromised immune system or if it arises due to the actual impact of the SARS-CoV-2 virus on immune function. This raises the question of whether the occurrence of autoimmune encephalitis could be merely a non-specific indication of a pre-existing poor immune system or if it is directly linked to the viral infection. Regrettably, the immune function of the patients included in the case reports was largely unexplored. It should also be noted that we restricted our search to probable or definite NMDA receptor encephalitis cases linked with SARS-CoV-2 infection. However, it is possible that some cases were missed due to causes such as incomplete investigation, loss of records, or decisions not to publish due to difficulties encountered during the unprecedented pandemic situation.
Another limitation of our study concerns the point that we were unable to determine the most critical factors related to disease outcome. This is important as not all of the cases responded positively to the described therapeutic approaches, which mainly involved administration of corticosteroids and IVIg. Further studies are needed to address the above points in order to set standards for improved patient outcomes. This should include screening cases for blood and CSF biomarkers, such as inflammation-related molecules, antineuronal antibodies, in addition to brain imaging, EEG analyses and monitoring of neurological and psychiatric symptoms [47, 48, 110]. We suggest that there should be a consensus so that future studies collect the same complete data as well as patient histories. For SARS-CoV-2 infections, this will be difficult as most people who had the virus were never tested. The usual test for the presence of SARS-CoV-2 nucleocapsid protein antibodies would be required to establish this.
Conclusions and future perspectives
Although our findings do not support a definitive link between SARS-CoV-2 infection or vaccination and neuronal autoantibody-mediated encephalitis, we hope that this meta-analysis has helped to increase our capacity for differential diagnosis of the neurological and psychiatric symptoms associated with SARS-CoV-2 infections. We anticipate that this will also increase our attentiveness regarding the development, diagnosis and therapeutic management of NMDA receptor encephalitis associated with infections by this virus or by COVID-19 vaccination. This latter point may be important as more 70% of the world population has now received at least one dose of a COVID-19 vaccine [103]. In this study, we identified 19 cases of NMDA receptor encephalitis associated with SARS-CoV-2 infection or vaccination which included 7 cases identified in our previous investigation [45]. In contrast with our previous report which showed favourable responses of all patients to either the first- or second-line treatments, two out of the newly-identified cases showed no improvement and one died. This underlines the need for further research into the pathomechanisms involved in this disorder, combined with standardised procedures put in place to guide predictions of NMDA receptor encephalitis patient outcomes. One way this can be assessed is through the use of clinical, psychological and apparative testing in combination with screening tools for molecular markers, such as specific autoantibody panels. We also suggest the use of artificial intelligence approaches, such as machine and deep learning, to aid in the deconvolution of the expected high dimensionality in the resulting data associated with these approaches, although this approach would first be reasonable if large cohorts are collected.
Even though the World Health Organization has declared that COVID-19 is no longer a global health emergency, vigilance is required to detect the emergence of a potentially novel SARS-CoV-2 variant or a new virus that poses a similar or more deadly threat [111]. In addition, up to 10-30% of COVID-19 cases have resulted in PCS which can include chronic symptoms of fatigue, impairment of cognition, as well as both physical and neurological/neuropsychiatric deficits, as can be observed after other severe infections and medical conditions [112, 113]. Based on these findings and those in the current review, we suggest that further studies are conducted to determine if there is a subgroup of post-COVID syndrome sufferers with autoimmune-mediated dysfunction of the CNS circuitry, as in NMDA receptor encephalitis. It will also be important to incorporate screening procedures such as those described here to increase our understanding of both the acute and chronic effects of viral infection on neurological dysfunction and to aid in our preparedness in case of future coronavirus outbreaks.
Notes
Some seizures can be so severe they lead to excessive uncontrolled discharge of neurons. These discharges can lead to increased metabolism and energy demand in affected brain regions. Repeated and intense seizure episodes can lead to excitotoxic damage of neurons [Meldrum BS. Prog Brain Res 2002;135:3-11].
Additional safety information for authorised COVID-19 vaccines is available from the European Medicines Agency (EMA) [https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/covid-19-medicines#safety-information-for-authorised-covid-19-vaccines-section].
References
Worldometer; COVID-19 Coronavirus Pandemic https://www.worldometers.info/coronavirus.
COVID-19 Dashboard - by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. COVID-19 Map. https://coronavirus.jhu.edu/map.html. In: Center JHCR (ed).
Davis A COVID Evaluation Model Estimates 57 Percent of World Population Infected at Least Once. https://www.newsweek.com/covid-evaluation-model-estimates-57-percent-world-population-infected-least-once-1672440. Newsweek, Jan 24, edn. (2022).
Pulliam JRC, van Schalkwyk C, Govender N, von Gottberg A, Cohen C, Groome MJ, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. 2022;376:eabn4947.
Phan TT, Nguyen TB, Phung QT, Tran VT, Ho TT, Pho SP, et al. Incidence of SARS-CoV-2 infection during the Omicron variant emergence in Southern Vietnam: prior infection versus third-dose vaccination. Microbiol Spectr. 2022;10:e0117522.
Kovanen PT, Vuorio A. SARS-CoV-2 reinfection: adding insult to dysfunctional endothelium in patients with atherosclerotic cardiovascular disease. Atherosclerosis. 2023;53:1–5.
Ma KC, Dorabawila V, Leon TM, Henry H, Johnson AG, Rosenberg E, et al. Trends in laboratory-confirmed SARS-CoV-2 reinfections and associated hospitalizations and deaths among adults aged >/=18 years - 18 U.S. Jurisdictions, September 2021-December 2022. MMWR Morb Mortal Wkly Rep. 2023;72:683–9.
Dejnirattisai W, Huo J, Zhou D, Zahradnik J, Supasa P, Liu C, et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185:467–84.
Wei Z, He J, Wang C, Bao J, Leng T, Chen F. The importance of booster vaccination in the context of Omicron wave. Front Immunol. 2022;13:977972.
Braeye T, Catteau L, Brondeel R, van Loenhout JAF, Proesmans K, Cornelissen L, et al. Vaccine effectiveness against transmission of alpha, delta and omicron SARS-COV-2-infection, Belgian contact tracing, 2021-2022. Vaccine. 2023;41:3292–3300.
Chimeddorj B, Bailie CR, Mandakh U, Price DJ, Bayartsogt B, Meagher N, et al. SARS-CoV-2 seroepidemiology in Mongolia, 2020-2021: a longitudinal national study. Lancet Reg Health West Pac. 2023;36:100760.
Tenforde MW, Kim SS, Lindsell CJ, Billig Rose E, Shapiro NI, Files DC, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993–8.
Afrisham R, Jadidi Y, Davoudi M, Moayedi K, Karami S, Sadegh-Nejadi S, et al. Renal, cardiac, neurological, cutaneous and coagulopathic long-term manifestations of COVID-19 after recovery: a review. Epidemiol Infect. 2022;150:e208.
Huang Y, Pinto MD, Borelli JL, Mehrabadi MA, Abrihim H, Dutt N, et al. COVID Symptoms, Symptom Clusters, and Predictors for Becoming a Long-Hauler Looking for Clarity in the Haze of the Pandemic. Clin Nurs Res. 2022;31:1390–98.
National Institute for Health and Care Excellence (NICE). COVID-19 rapid guideline: managing the longterm effects of COVID-19. https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742. (2022).
Renaud-Charest O, Lui LMW, Eskander S, Ceban F, Ho R, Di Vincenzo JD, et al. Onset and frequency of depression in post-COVID-19 syndrome: a systematic review. J Psychiatr Res. 2021;144:129–37.
SeyedAlinaghi S, Bagheri A, Razi A, Mojdeganlou P, Mojdeganlou H, Afsahi AM, et al. Late complications of COVID-19: an umbrella review on current systematic reviews. Arch Acad Emerg Med. 2023;11:e28.
Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–20.
Atalan A. Is the lockdown important to prevent the COVID-19 pandemic? Effects on psychology, environment and economy-perspective. Ann Med Surg. 2020;56:38–42.
Guedj E, Campion JY, Horowitz T, Barthelemy F, Cammilleri S, Ceccaldi M. The impact of COVID-19 lockdown on brain metabolism. Hum Brain Mapp. 2022;43:593–7.
Duan L, Zhu G. Psychological interventions for people affected by the COVID-19 epidemic. Lancet Psychiatry. 2020;7:300–2.
Park SC, Park YC. Mental health care measures in response to the 2019 novel coronavirus outbreak in Korea. Psychiatry Investig. 2020;17:85–86.
Liu S, Heinz A. Cross-cultural validity of psychological distress measurement during the coronavirus pandemic. Pharmacopsychiatry. 2020;53:237–8.
Moayed MS, Vahedian-Azimi A, Mirmomeni G, Rahimi-Bashar F, Goharimoghadam K, Pourhoseingholi MA, et al. A Survey of psychological distress among the community in the COVID-19 epidemic: a cross-sectional study. Adv Exp Med Biol. 2021;1321:253–60.
Ashtari S, Rahimi-Bashar F, Karimi L, Salesi M, Guest PC, Riahi MM, et al. Psychological distress impact of coronavirus disease (COVID-19) outbreak on three continents: a systematic review and meta-analysis. Adv Exp Med Biol. 2023;1412:73–95.
Office for National Statistics; Coronavirus and depression in adults in Great Britain. https://www.ons.gov.uk/peoplepopulationandcommunity/wellbeing/datasets/coronavirusanddepressioninadultsingreatbritain.
Office for National Statistics; Cost of living and depression in adults, Great Britain. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/mentalhealth/datasets/costoflivinganddepressioninadultsgreatbritain.
Rochmawati E, Iskandar AC, Kamilah F. Persistent symptoms among post-COVID-19 survivors: A systematic review and meta-analysis. J Clin Nursing. 2024;33:29–39.
Patel UK, Mehta N, Patel A, Patel N, Ortiz JF, Khurana M, et al. Long-term neurological sequelae among severe COVID-19 patients: a systematic review and meta-analysis. Cureus. 2022;14:e29694.
Ma Y, Deng J, Liu Q, Du M, Liu M, Liu J. Long-term consequences of COVID-19 at 6 months and above: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19:6865.
Mazza MG, Palladini M, Villa G, Agnoletto E, Harrington Y, Vai B, et al. Prevalence of depression in SARS-CoV-2 infected patients: an umbrella review of meta-analyses. Gen Hospital Psychiatry. 2023;80:17–25.
Ahmed S, Zimba O, Gasparyan AY. COVID-19 and the clinical course of rheumatic manifestations. Clin Rheumatol. 2021;40:2611–9.
Bornstein SR, Voit-Bak K, Donate T, Rodionov RN, Gainetdinov RR, Tselmin S, et al. Chronic post-COVID-19 syndrome and chronic fatigue syndrome: Is there a role for extracorporeal apheresis? Mol Psychiatry. 2022;27:34–37.
Dotan A, David P, Arnheim D, Shoenfeld Y. The autonomic aspects of the post-COVID19 syndrome. Autoimmun Rev. 2022;21:103071.
Mobasheri L, Nasirpour MH, Masoumi E, Azarnaminy AF, Jafari M, Esmaeili SA. SARS-CoV-2 triggering autoimmune diseases. Cytokine. 2022;154:155873.
Jammoul M, Naddour J, Madi A, Reslan MA, Hatoum F, Zeineddine J, et al. Investigating the possible mechanisms of autonomic dysfunction post-COVID-19. Auton Neurosci. 2023;245:103071.
Dobrowolska K, Zarebska-Michaluk D, Poniedzialek B, Jaroszewicz J, Flisiak R, Rzymski P. Overview of autoantibodies in COVID-19 convalescents. J Med Virol. 2023;95:e28864.
Steinestel K, Czech A, Hackenbroch C, Bloch W, Gagiannis D. Clinical, radiological, and histopathological features of pulmonary post-COVID syndrome : A form of autoimmune-mediated interstitial lung disease? Der Pathologe. 2021;42:160–4.
Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol online J. 2022;20:65.
Plazak W, Drabik L. SARS-CoV-2 infection and SLE: endothelial dysfunction, atherosclerosis, and thrombosis. Clin Rheumatol. 2023;42:2691–2702.
Ciaffi J, Vanni E, Mancarella L, Brusi V, Lisi L, Pignatti F, et al. Post-acute COVID-19 joint pain and new onset of rheumatic musculoskeletal diseases: a systematic review. Diagnostics. 2023;13:1850.
Malekpour M, Khanmohammadi S, Meybodi MJE, Shekouh D, Rahmanian MR, Kardeh S, et al. COVID-19 as a trigger of Guillain-Barre syndrome: a review of the molecular mechanism. Immun Inflamm Dis. 2023;11:e875.
Prikazchikov SV, Generalov VO, Sadykov TR, Mamedov LA. Neurological Complications of Covid-19: Review of Literature and Own Experience. Problemy Sotsial’noi Gigieny, Zdravookhraneniia I Istorii Meditsiny. 2021;29:1034–10.
Graham EL, Clark JR, Orban ZS, Lim PH, Szymanski AL, Taylor C, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann Clin Transl Neurol. 2021;8:1073–85.
Vasilevska V, Guest PC, Bernstein HG, Schroeter ML, Geis C, Steiner J. Molecular mimicry of NMDA receptors may contribute to neuropsychiatric symptoms in severe COVID‑19 cases. J Neuroinflam. 2021;18:245.
Yapici-Eser H, Koroglu YE, Oztop-Cakmak O, Keskin O, Gursoy A, Gursoy-Ozdemir Y. Neuropsychiatric symptoms of COVID-19 Explained by SARS-CoV-2 Proteins’ Mimicry of Human Protein Interactions. Front Hum Neurosci. 2021;15:656313.
Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404.
Pollak TA, Lennox BR, Müller S, Benros ME, Prüss H, Tebartz van Elst L, et al. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry. 2020;7:93–108.
Rojas M, Herran M, Ramirez-Santana C, Leung PSC, Anaya JM, Ridgway WM, et al. Molecular mimicry and autoimmunity in the time of COVID-19. J Autoimmun. 2023;139:103070.
Rose NR, Mackay IR. Molecular mimicry: a critical look at exemplary instances in human diseases. Cell Mol Life Sci. 2000;57:542–51.
Cusick MF, Libbey JE, Fujinami RS. Molecular mimicry as a mechanism of autoimmune disease. Clin Rev allergy Immunol. 2012;42:102–11.
Szardenings M, Delaroque N, Kern K, Ramirez-Caballero L, Puder M, Ehrentreich-Förster E, et al. Detection of antibodies against endemic and SARS-CoV-2 coronaviruses with short peptide epitopes. Vaccines. 2023;11:1403.
Nunez-Castilla J, Stebliankin V, Baral P, Balbin CA, Sobhan M, Cickovski T, et al. Potential autoimmunity resulting from molecular mimicry between SARS-CoV-2 spike and human proteins. Viruses. 2022;14:1415.
Kim D, Lee JY, Yang JS, Kim JW, Kim VN, Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181:914–921.e910.
Jamison DA Jr, Anand Narayanan S, Trovao NS, Guarnieri JW, Topper MJ, Moraes-Vieira PM, et al. A comprehensive SARS-CoV-2 and COVID-19 review, Part 1: intracellular overdrive for SARS-CoV-2 infection. Eur J Hum Genet. 2022;30:889–98.
Rashid F, Xie Z, Suleman M, Shah A, Khan S, Luo S. Roles and functions of SARS-CoV-2 proteins in host immune evasion. Front Immunol. 2022;13:940756.
Rohaim MA, El Naggar RF, Clayton E, Munir M. Structural and functional insights into non-structural proteins of coronaviruses. Microb Pathogen. 2021;150:104641.
Sun G, Xue L, He Q, Zhao Y, Xu W, Wang Z. Structural insights into SARS-CoV-2 infection and therapeutics development. Stem cell Res. 2021;52:102219.
Alexandersen S, Chamings A, Bhatta TR. SARS-CoV-2 genomic and subgenomic RNAs in diagnostic samples are not an indicator of active replication. Nat Commun. 2020;11:6059.
Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–68.
Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev. 2021;20:102792.
Getts DR, Chastain EM, Terry RL, Miller SD. Virus infection, antiviral immunity, and autoimmunity. Immunolog Rev. 2013;255:197–209.
DeepL Translator. https://www.deepl.com.
Moura J, Duarte S, Sardoeira A, Neves-Maia J, Damasio J, Taipa R, et al. Anti-NMDAr encephalitis and COVID-19 in a patient with systemic pANCA-vasculitis and recurrent varicella Zoster Infection. Neurohospitalist. 2022;12:383–7.
Derakhshani F, Ghazavi M, Hosseini N. Autoimmune encephalitis due to COVID-19 in a young patient. Iran J Child Neurol. 2023;17:135–42.
McHattie AW, Coebergh J, Khan F, Morgante F. Palilalia as a prominent feature of anti-NMDA receptor encephalitis in a woman with COVID-19. J Neurol. 2021;268:3995–97.
Sanchez-Larsen A, Rojas-Bartolome L, Fernandez-Valiente M, Sopelana D. Anti-NMDA-R encephalitis post-COVID-19: Case report and proposed physiopathologic mechanism. Neurologia (Engl Ed). 2023;38:513–6.
Lee HC, Kim BK, Kang K, Lee WW, Yoo I, Kim YS, et al. Anti-N-Methyl-D-Aspartate receptor encephalitis after BNT162b2 COVID-19 Vaccination. J epilepsy Res. 2022;12:71–73.
Flannery P, Yang I, Keyvani M, Sakoulas G. Acute psychosis due to Anti-N-Methyl D-Aspartate receptor encephalitis following COVID-19 vaccination: a case report. Front Neurol. 2021;12:764197.
Khoreva MA, Serikova IY, Smagina IV, Golenko AA, Smirnov KV, Zavyalov AE, et al. Clinical case of anti-NMDA receptor encephalitis associated with new coronaviral infection (COVID-19). Russ Neurolog J. 2022;27:106–12.
Bazalar G, Azañero-Haro J, Piscoya T, Chambi L, Soto A. Anti-NMDA-R autoinmune encephalitis in times of COVID-19. Rev De La Soc Peru De Med Interna. 2023;36:e724.
Hina Naz A, Mohamed HA, Noha S, Abdallah AA, Szolics M. Acute Anti-N-Methyl D-Aspartate receptor encephalitis following Covid-19 Vaccination. SVOA Neurol. 2022;3:143–5.
Gillentine R, Palasiewicz R, Walters R. Anti-NMDA receptor encephalitis in a patient with COVID-19 infection: a case report. J Miss State Med Assoc. 2021;62:286–9.
Alvi J, Sultan MH, Sultan T. Post COVID anti-NMDAR encephalitis in an adolescent girl. Pak J Neurolog Sci. 2022;17:16–20.
Naidu K, Tayler R. Anti N-Methyl-D-Aspartate receptor antibody associated Acute Demyelinating Encephalomyelitis in a patient with COVID-19: a case report. J Med Case Rep. 2023;17:247.
Alvarez Bravo G, Ramio ITL. Anti-NMDA receptor encephalitis secondary to SARS-CoV-2 infection. Neurologia (Engl Ed). 2020;35:699–700.
Allahyari F, Hosseinzadeh R, Nejad JH, Heiat M, Ranjbar R. A case report of simultaneous autoimmune and COVID-19 encephalitis. J Neurovirol. 2021;27:504–6.
Burr T, Barton C, Doll E, Lakhotia A, Sweeney M. N-Methyl-d-Aspartate receptor encephalitis associated with COVID-19 infection in a toddler. Pediatr Neurol. 2021;114:75–76.
Valadez-Calderon J, Ordinola Navarro A, Rodriguez-Chavez E, Vera-Lastra O. Co-expression of anti-NMDAR and anti-GAD65 antibodies. A case of autoimmune encephalitis in a post-COVID-19 patient. Neurologia (Engl Ed). 2022;37:503–4.
Kaur P, MV Vinay, Madarkar BS. Infantile Anti-N-Methyl-D-Aspartate receptor encephalitis Post-SARS-CoV-2 infection. Indian Pediatr. 2022;59:343–4.
Khubetova IV. Postcovid amiostatic syndrome. Bull Mar Med. 2022;3:9–12.
Panariello A, Bassetti R, Radice A, Rossotti R, Puoti M, Corradin M, et al. Anti-NMDA receptor encephalitis in a psychiatric Covid-19 patient: a case report. Brain Behav Immun. 2020;87:179–81.
Monti G, Giovannini G, Marudi A, Bedin R, Melegari A, Simone AM, et al. Anti-NMDA receptor encephalitis presenting as new onset refractory status epilepticus in COVID-19. Seizure : J Br Epilepsy Assoc. 2020;81:18–20.
Sanchez-Morales AE, Urrutia-Osorio M, Camacho-Mendoza E, Rosales-Pedraza G, Davila-Maldonado L, Gonzalez-Duarte A, et al. Neurological manifestations temporally associated with SARS-CoV-2 infection in pediatric patients in Mexico. Childs Nerv Syst. 2021;37:2305–12.
Sarigecili E, Arslan I, Ucar HK, Celik U. Pediatric anti-NMDA receptor encephalitis associated with COVID-19. Childs Nerv Syst. 2021;37:3919–22.
Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, Tarhriz V, Farjami A, Ghasemian Sorbeni F, et al. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J. 2022;19:92.
Almulla AF, Supasitthumrong T, Tunvirachaisakul C, Algon AAA, Al-Hakeim HK, Maes M. The tryptophan catabolite or kynurenine pathway in COVID-19 and critical COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2022;22:615.
Hernandez-Parra H, Reyes-Hernandez OD, Figueroa-Gonzalez G, Gonzalez-Del Carmen M, Gonzalez-Torres M, Pena-Corona SI, et al. Alteration of the blood-brain barrier by COVID-19 and its implication in the permeation of drugs into the brain. Front Cell Neurosci. 2023;17:1125109.
Erickson MA, Rhea EM, Knopp RC, Banks WA. Interactions of SARS-CoV-2 with the blood-brain barrier. Int J Mol Sci. 2021;22:2681.
Pacheco Y, Acosta-Ampudia Y, Monsalve DM, Chang C, Gershwin ME, Anaya JM. Bystander activation and autoimmunity. J Autoimmun. 2019;103:102301.
Lynch DR, Rattelle A, Dong YN, Roslin K, Gleichman AJ, Panzer JA. Anti-NMDA receptor encephalitis: clinical features and basic mechanisms. Adv Pharmacol. 2018;82:235–60.
Kreye J, Wenke NK, Chayka M, Leubner J, Murugan R, Maier N, et al. Human cerebrospinal fluid monoclonal N-methyl-D-aspartate receptor autoantibodies are sufficient for encephalitis pathogenesis. Brain. 2016;139:2641–52.
Herken J, Prüss H. Red Flags: clinical signs for identifying autoimmune encephalitis in psychiatric patients. Front Psychiatry. 2017;8:25.
Steiner J, Prüss H, Köhler S, Frodl T, Hasan A, Falkai P. Autoimmune encephalitis with psychosis: Warning signs, step-by-step diagnostics and treatment. World J Biol Psychiatry. 2020;21:241–54.
Endres D, Leypoldt F, Bechter K, Hasan A, Steiner J, Domschke K, et al. Autoimmune encephalitis as a differential diagnosis of schizophreniform psychosis: clinical symptomatology, pathophysiology, diagnostic approach, and therapeutic considerations. Eur Arch Psychiatry Clin Neurosci. 2020;270:803–18.
EUROIMMUN Autoantibodies in neurological diseases. https://www.euroimmun.com/documents/Indications/Autoimmunity/Neurology/MAG_myelin_GAD/FA_1111_I_UK_A.pdf.
POTS / Long Covid-diagnostics; CellTrend; https://www.celltrend.de/en/pots-cfs-me-sfn/.
Dalmau J, Armangue T, Planaguma J, Radosevic M, Mannara F, Leypoldt F, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18:1045–57.
Scheibe F, Prüss H, Mengel AM, Köhler S, Numann A, Kohnlein M, et al. Bortezomib for treatment of therapy-refractory anti-NMDA receptor encephalitis. Neurology. 2017;88:366–70.
Hammer C, Stepniak B, Schneider A, Papiol S, Tantra M, Begemann M, et al. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol Psychiatry. 2014;19:1143–9.
Handel AE, Palace J, Bateman E, Waters P, Irani SR. Changes in the rate of Leucine-Rich Glioma-Inactivated 1 seropositivity during the COVID-19 lockdown. JAMA Neurol. 2023;80:419–20.
Ehrenreich H. Autoantibodies against the N-Methyl-d-Aspartate receptor subunit NR1: untangling apparent inconsistencies for clinical practice. Front Immunol. 2017;8:181.
Holder J Tracking Coronavirus Vaccinations Around the World. The New York Times. https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html.
Gronkjaer CS, Christensen RHB, Kondziella D, Benros ME. Long-term neurological outcome after COVID-19 using all SARS-CoV-2 test results and hospitalisations in Denmark with 22-month follow-up. Nat Commun. 2023;14:4235.
Nersesjan V, Christensen RHB, Kondziella D, Benros ME. COVID-19 and risk for mental disorders among adults in Denmark. JAMA Psychiatry. 2023;80:778–86.
Gronkjaer CS, Christensen RHB, Kondziella D, Benros ME, Nersesjan V, Amiri M, et al. SARS-CoV-2 and auto-antibodies in the cerebrospinal fluid of COVID-19 patients: prospective multicenter cohort study. Brain Commun. 2023;5:fcad274.
Arino H, Ruiz Garcia R, Rioseras B, Naranjo L, Martinez-Hernandez E, Saiz A, et al. Frequency and referral patterns of neural antibody studies during the COVID-19 pandemic: experience from an autoimmune neurology center. Neurol(R) Neuroimmunol Meuroinflam. 2023;10:4.
EudraVigilance – European database of suspected drug adverse reactions. European Medicines Agency. https://www.adrreports.eu/en/covid19_message.html.
ECDC Vaccine Tracker. https://vaccinetracker.ecdc.europa.eu.
Vasilevska V, Guest PC, Schlaaff K, Incesoy EI, Pruss H, Steiner J. Potential cross-links of inflammation with schizophreniform and affective symptoms: a review and outlook on autoimmune encephalitis and COVID-19. Front Psychiatry. 2021;12:729868.
WHO chief declares end to COVID-19 as a global health emergency. May 5, https://news.un.org/en/story/2023/05/1136367. (2023).
Nersesjan V, Fonsmark L, Christensen RHB, Amiri M, Merie C, Lebech AM, et al. Neuropsychiatric and cognitive outcomes in patients 6 months after COVID-19 requiring hospitalization compared with matched control patients hospitalized for Non-COVID-19 illness. JAMA Psychiatry. 2022;79:486–97.
Penninx B, Benros ME, Klein RS, Vinkers CH. How COVID-19 shaped mental health: from infection to pandemic effects. Nat Med. 2022;28:2027–37.
Acknowledgements
This work has been partly supported by the European Research Area Network (ERA-NET) NEURON “Translational Biomarkers in Brain Disorders” (Project NicAb, grant no. 01EW2012).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
VV and PCG performed the database search, screened the eligible studies, extracted the data, prepared the figures, drafted the first version of the manuscript and worked on revisions. MS contributed his knowledge in immunodiagnostics and epitope mapping, while MEB contributed his expertise in epidemiological studies of the COVID-19 pandemic and mental health consequences to drafting and revising the manuscript. The Critical Considerations and Limitations sections were written by JS and PCG and edited by all co-authors. JS conceived the meta-analysis and supervised the drafting and revisions of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vasilevska, V., Guest, P.C., Szardenings, M. et al. Possible temporal relationship between SARS-CoV-2 infection and anti-NMDA receptor encephalitis: a meta-analysis. Transl Psychiatry 14, 139 (2024). https://doi.org/10.1038/s41398-024-02831-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-02831-0