Abstract
Evidence from cross-sectional human studies, and preliminary microbial-based intervention studies, have implicated the microbiota-gut-brain axis in the neurobiology of autism spectrum disorder (ASD). Using a prospective longitudinal study design, we investigated the developmental profile of the fecal microbiota and metabolome in infants with (n = 16) and without (n = 19) a family history of ASD across the first 36 months of life. In addition, the general developmental levels of infants were evaluated using the Mullen Scales of Early Learning (MSEL) test at 5 and 36 months of age, and with ADOS-2 at 36 months of age. At 5 months of age, infants at elevated-likelihood of ASD (EL) harbored less Bifidobacterium and more Clostridium and Klebsiella species compared to the low-likelihood infants (LL). Untargeted metabolic profiling highlighted that LL infants excreted a greater amount of fecal γ-aminobutyric acid (GABA) at 5 months, which progressively declined with age. Similar age-dependent patterns were not observed in the EL group, with GABA being consistently low across all timepoints. Integrated microbiome-metabolome analysis showed a positive correlation between GABA and Bifidobacterium species and negative associations with Clostridium species. In vitro experiments supported these observations demonstrating that bifidobacteria can produce GABA while clostridia can consume it. At the behavioral level, there were no significant differences between the EL and LL groups at 5 months. However, at 36 months of age, the EL group had significantly lower MSEL and ADOS-2 scores compared to the LL group. Taken together, the present results reveal early life alterations in gut microbiota composition and functionality in infants at elevated-likelihood of ASD. These changes occur before any behavioral impairments can be detected, supporting a possible role for the gut microbiota in emerging behavioral variability later in life.
Similar content being viewed by others
Introduction
Autism Spectrum Disorder (ASD) is a persistent early-onset neurodevelopmental condition, defined by the presence of social communication and interaction challenges in conjunction with restricted, repetitive behaviors and atypical sensory processing [1]. The worldwide prevalence rate of ASD is currently estimated to be 1% [2]. Many individuals with ASD experience gastrointestinal (GI) and immune dysfunction [3,4,5], as well as a range of co-occurring somatic and psychiatric conditions including sleep disorders, epilepsy, and anxiety [1]. ASD is a heterogenous and multifactorial behavioral condition, involving genetic susceptibility, environmental risk factors, and gene-environmental interactions [6]. One such environmental risk factor is the gut microbiota (the trillions of microorganisms that colonize our GI tract), which plays a critical role in host physiology and health [7]. The gut microbiota is now recognized as an important modulator of brain and behavior, including the development and function of social brain networks [8,9,10], representing a new potential target for intervention in neurodevelopmental conditions such as ASD.
A potential link between the gut microbiota and ASD was suggested almost two decades ago, by observations showing that oral vancomycin treatment resulted in a short-term benefit in a small group of children with regressive-onset autism [11] and that children with ASD presented alterations in the gut microbiota composition (e.g., increased abundance of Clostridium species) [12,13,14]. Subsequently, several studies have shown that GI symptoms, such as abdominal pain, diarrhea, constipation, and flatulence, are more common in children with ASD than their neurotypically developing peers [15] and are positively associated with the severity of behavioral problems (irritability, aggressive behavior, and repetitive behaviors) [16,17,18]. Multiple cross-sectional studies have also reported an altered gut microbiota composition in children and adolescents with ASD, with lower gut bacterial diversity and an underrepresentation of potentially beneficial bacteria (e.g., Bifidobacterium species) [19,20,21,22]. However, a clear consensus regarding the specific bacterial taxa or magnitude of changes associated with ASD is lacking. Indeed, a recent multi-omics analysis has highlighted the limitations of cross-sectional cohort studies, thus advocating for longitudinal multi-omic studies in combination with comprehensive patient metadata to advance our understanding of the role that the gut microbiome plays in ASD [23]. At the metabolic level, several neuroactive gut-bacterial-derived metabolites such as 4-ethylphenyl sulfate, p-cresyl sulfate, and other structurally related phenolic molecules, have been found to be elevated in a subset of children with ASD [24, 25]. The causal potential of the microbiota in ASD has been suggested from murine studies transferring the fecal gut microbiota from autistic individuals into germ-free mice, resulting in behavioral and molecular changes relevant to this condition [26]. Furthermore, microbial-focused intervention studies like fecal microbiota transplantation (FMT) and novel therapeutic agents that prevents the absorption of microbial metabolites from the GI tract, such as AB-2004 have shown positive preliminary results in ameliorating both autistic traits and GI symptoms [27,28,29].
Yap and colleagues have recently challenged the notion that the gut microbiota is a key contributing factor in the etiology of ASD, providing evidence that ASD-associated gut microbiota changes can be attributed to low diet diversity [30]. It is indeed well-documented that autistic children often exhibit selective eating patterns [31], and that they are more frequently exposed to antibiotics during the first years of life [32, 33], which can affect the composition of the gut microbiota. However, it remains unclear whether the gut microbiota contributes to, or modifies the likelihood of ASD onset before any dietary changes occur. Emerging evidence suggests that the development of the infant gut microbiota is influenced by host genetics, but that this effect is subtle and affects only certain taxa (e.g., Veillonella and Bacteroides species) [34]. Prospective longitudinal studies of infants at elevated-likelihood of ASD (i.e., siblings of children with ASD, who have ~20% increased likelihood to develop ASD) can provide insights into the relationship between the early life gut microbiota and the development of ASD [35, 36]. In the present study, we applied an integrated shallow shotgun metagenomic sequencing and 1H nuclear magnetic resonance (NMR) spectroscopy-based untargeted metabolomics approach to characterize the gut microbiota developmental profile of infants at elevated versus low-likelihood (i.e., infants without a family history of ASD) of from 5 to 36 months of age.
Materials and methods
Participants
Infants were recruited from the Early Autism Sweden (EASE) project, an ongoing longitudinal study of infants at elevated-likelihood of ASD. EASE follows younger siblings of children with ASD (elevated-likelihood) and siblings of typically developing children (low-likelihood) from 5 to 36 months of age. Infants at elevated-likelihood (EL) of ASD in EASE have at least one older sibling with a community clinical diagnosis of ASD according to ICD-10 or DSM-5, which was confirmed by inspection of medical records and an interview with parents led by an experienced child psychologist. Infants were recruited through clinical units, advertisement, and the EASE project website. Infants at low-likelihood (LL) of ASD have no familial history of ASD (in first- or second-degree relatives) and at least one typically developing older full sibling. Both groups were primarily from the larger Stockholm area (Sweden). All infants were born full-term (≥ 36 weeks) and did not have any confirmed or suspected medical conditions, including epilepsy, genetic syndromes associated with ASD, or visual/auditory impairments. Potential participants were excluded if they had been exposed to antibiotics. A total of 35 infants met the eligibility criteria and were included in the study, consisting of 19 EL infants (9 females) and 16 LL infants (10 females). All infants were breastfed until at least 6 months of age. An experienced clinical staff assessed the infants developmental level using the Mullen Scales of Early Learning (MSEL) at 5 and 36 months of age [37]. In addition, the Autism Diagnostic Observation Schedule-Second Edition (ADOS-2) was used to assess symptoms of ASD (communication, social interaction, play and restrictive/repetitive behaviors) at 36 months. Informed written consent was obtained from all parents. This study was approved by the Ethics Board in Stockholm and conducted in accordance with the 1964 Declaration of Helsinki.
Fecal sample collection
Parents collected approximately 200 mg of fecal material from a single infant’s bowel movement at 5, 10, 14, 24, and 36 months of age at their homes. At each time point, parents were guided with both oral and written instructions to collect the fecal material into a sterile collection tube and contact a dedicated research project assistant, who coordinated the immediate transport of the samples to the laboratory. Samples were transported on dry ice within 30 min of collection through the help of a dedicated courier service. Samples were aliquoted into two sterile tubes under a biological safety cabinet for shallow shotgun metagenome sequencing (~30 mg) and 1H NMR spectroscopic analysis (~ 50 mg).
Shallow shotgun metagenome sequencing
DNA extraction and sequencing was carried out by Diversigen® (Minneapolis, USA) using in-house developed, CLIA-approved BoosterShot® protocol for shallow shotgun sequencing. Briefly, MO BIO’s PowerFecal DNA Isolation Kit (Qiagen, Hilden, Germany) automated for high throughput on QIACube, along with 0.1 mm glass bead plates for bead beating, were used for genetic material extraction. Extracted DNA was quantified using Quant-iT™ PicoGreen™ dsDNA Assay Kit (Invitrogen, Carlsbad, CA). Libraries were then prepared using an adapted procedure from the Nextera XT DNA Library Preparation Kit (Illumina, San Diego, CA). Sequencing was carried out on an Illumina NextSeq using single-end 1 × 145 reads with a NextSeq 500/550 High Output v2 kit (Illumina, San Diego, CA). DNA sequences were aligned to a curated database including all representative genomes in RefSeq for bacteria with additional manually curated strains. Alignments were made at 97% identity against all reference genomes. Every input sequence was compared to each reference sequence within the CoreBiome Venti database using fully gapped alignment with BURST. Ties were broken by minimizing the overall number of unique operational taxonomic units (OTUs). For taxonomy assignment, each input sequence was assigned the lowest common ancestor that was consistent across at least 80% of all reference sequences tied for best hit. The number of counts for each OTU was normalized to the OTU’s genome length. OTUs accounting for less than one millionth of all species-level markers and those with less than 0.01% of their unique genome regions covered (and <1% of the whole genome) were discarded.
Microbiome analysis
A general overview of the infant gut microbiome composition was generated plotting the most abundant genera of each group at the different timepoints, after calculating the mean relative abundances. The Shannon diversity index, a measure of alpha diversity, was estimated at genus level at each timepoint using the `DivNet` v 0.4.0 package, which takes into consideration the compositional nature of microbiome data, the co-occurrence of the different taxa, and avoids rarefaction [38]. A compositional data analysis workflow was used to investigate the structure of the data and highlight group differences. Zeroes in the OTU count table were imputed using Bayesian-Multiplicative replacement before applying a center log ratio (CLR) transformation with the `CoDaSeq` v 0.99.6 package. Principal component analysis (PCA), from the `mixOmics` v 6.22.0 package, was used to performed dimensionality reduction and the adonis function from the `vegan` v 2.6-4 package was used to perform permutational multivariate analysis of variance (PERMANOVA) on the calculated Aitchison distances. Differential abundance analysis was performed using the `ALDEx2` v 1.30.0 package. ALDEx2 generates distribution probabilities for each analyzed taxon and converts them into distribution of log ratios to account for the compositional nature of microbiome data [39]. Differential abundance is calculated using the Wilcoxon rank-sum test, together with the standardized effect sizes. Obtained p values were corrected for multiple comparisons using Benjamini-Hochberg (BH) correction and only taxa with adjusted p values < 0.25 were considered significant and retained for visualization.
1H NMR spectroscopy
Fecal samples were defrosted on ice and combined with ten 1 mm diameter zirconia beads (BioSpec Products, US) and 700 µL of demineralized water. Samples were homogenized using a Precellys 24 homogenizer (Bertin Instruments, FR) with 2 × 6500 rpm in a 2-min program (2 × 40 s homogenization, with a 20 s interval). Homogenized samples were centrifuged at 10,000 g for 20 min at 4 °C. Fecal water (supernatant) was collected from each tube and 630 µL was transferred to a new 1.5 mL Eppendorf. Each tube was supplemented with 70 µL of phosphate buffer (1.5 M KH2PO4, 2 mM NaN3, 1% TSP solution, pH 7.4) and vortexed for ~ 30 s. Samples were centrifuged at 10,000 g for 5 min at 4 °C and 600 µL was transferred to 5 mm NMR tubes. Samples were loaded into a refrigerated Bruker SampleJet robot on a 600 MHz UltraShield spectrometer (Bruker Biospin, Karlsruhe, Germany). A standard one-dimensional pulse sequence with saturation of the water resonance was applied (RD-90°-t1-90°-tm-90°-acquire FID, with RD set at 2 s and tm at 100 ms) at 300 K. For each spectrum 8 dummy scans, followed by 64 scans with 32 K data points and a spectral width of 20,000 Hz were acquired.
1H NMR data processing and analysis
Spectra were manually corrected for phase and baseline distortion to the TSP singlet (δ 0.00) before data acquisition on TOPSPIN 3.2 (Bruker, Germany). The obtained spectra were digitized in MATLAB 2019b using IMPaCTS. TSP (-0.2 to 0.2 ppm) and water (4.7 to 4.9 ppm) regions were removed from the spectra. Peaks were manually checked and aligned using recursive sample-wise peak alignment (RSPA). Spectra were normalized using a probabilistic quotient normalization approach to account for potential dilution factors. PCA analysis with pareto scaling was used for preliminary unsupervised analysis to identify possible outliers. Four samples were categorized as outliers and excluded due to spectral anomalies, most likely derived from issues during spectral acquisition or sample preparation. Pair-wise supervised analysis between groups on full spectra was performed with projection to latent structures-discriminant analysis (PLS-DA) and univariate scaling in MATLAB. Using in-house databases, Chenomx 8.4 (Chenomx Inc, Edmonton, Canada) and HMDB (http://www.hmdb.ca/), all identified peaks were annotated. STOCSY was used to identify peaks belonging to same metabolite (Pearson correlation >0.8). Representative peaks from the different metabolites were integrated and exported for further analysis.
Integrated peaks from 1H NMR spectra were log10 transformed. PCA was used to observe the overall structure of the data and sparse PLS-DA from the ‘mixOmics’ package was used to extract the most relevant features that were responsible for differentiating the two groups at the different timepoints. Model performance was evaluated using the perf function of the ‘mixOmics’ package with leave-one-out (loo) cross-validation, given the small sample size. A variable importance projection score (VIP) > 1 was used as a cut-off to identify the most relevant metabolites influencing group separation.
Integration omics data
Spearman correlations between and within the two omics blocks (microbiome and metabolome data) were calculated using the ‘Hmisc’ v 4.8-0 package. P values obtained from multiple comparisons (>250,000) were then corrected for false discovery rate using BH and only correlations with adjusted p values < 0.25 were kept in the network. To improve visualization, a subset network was generated retaining only bacterial species directly correlating with GABA or with GABA-correlated metabolites and removing edges within each omics block. The final microbiome-metabolic network was built using the ‘ggraph’ v 2.1.0 and ‘igraph’ v 1.3.5 packages. The network was exported and edited in Cytoscape v 3.8.229.
Microbial cell culture and metabolomic analysis
Bacteria identified from the fecal microbiome analysis to be associated with GABA abundance were investigated for their potential to influence its presence. This included five different Bifidobacterium species, B. breve (DSM20091/ATCC15698), B. bifidum (DSM20215), B. longum subsp. infantis (ATCC17930/DSM20218), B. adolescentis (DSM20083), and B. scardovii (DSM13734), and two Clostridium related species, C. difficile (DSM1296), and C. bolteae (DSM15670) purchased from the DSMZ catalog (Leibniz-Institut DSMZ, Germany). The isolates were screened in anaerobic monocultures (CO2/N2 (80%/20%) at 1.7 atm) with three different substrates (glutamate, putrescine, and spermidine; 4.5 mM each), which can be converted to GABA, as well as GABA itself (4.5 mM). Bifidobacterium and Clostridium species were enumerated in MRS, and PYG broth respectively [40, 41]. A modified rich transoligosaccharide propionate medium was used as a base for culturing the above bacterial species anaerobically [41]. The medium consisted of glucose (120 mM, pH 6.2), yeast extract (1 g/L), potassium dihydrogen phosphate (3 g/L), dipotassium hydrogen phosphate (4.8 g/L), magnesium sulfate (0.2 g/L), sodium propionate (15 g/L), and l-cysteine hydrochloride (0.5 g/L). In addition, bacteriological peptone was added to the medium (1 g/L) to accommodate the growth of Clostridium species. Serum bottles were supplemented with filter-sterilized vitamin mix and trace metals (100x; originally designed for Lactobacillus lactis) [42]. All cultures were incubated for 24 hours at 37 °C. From each culture, 2 mL of bacterial growth medium were collected for 1H NMR spectroscopy. From the preliminary results based on growth levels and metabolic activity, B. breve, B. infantis, C. difficile, C. bolteae were selected for further analysis. These four strains were, after preculture enumeration, made equivalent in optical density under anaerobic conditions with sterile modified TOS, before transfer to 96-well plates containing 380 µL with a 1:100 inoculation. Media that contained either no supplement or supplemented with GABA was added to the wells. The chosen quadriculture ratios were (Bifidobacterium spp.:Clostridium spp.): 2:1; 1:1; 1:2. Following 24 h anaerobic incubation [CO2/N2 (80%/20%) at 1.5 atm], 1 ml of media was collected, centrifuged for 15 min at 10,000 g at 4 °C, and then 250 µL of supernatant was transferred into a 1.5 mL Eppendorf. After adding 350 µL of phosphate buffer solution, samples were vortex for ~ 30 s, centrifuged for 5 min at 10,000 g at 4 °C, and 600 µL of supernatant was transferred to 5 mm NMR tubes. For the quadricultures, 250 µL was retrieved and mixed with 350 µL of NMR Buffer (1.5 M KH2PO4, 1 g/L of TSP and 0.13 g/L of NaN3), before centrifugation at 10,000 g at 4 °C for 5 minutes and transfer to 5 mm NMR tubes. Spectral acquisition, processing, and integration were carried out as described above on a 700 MHz Bruker NMR spectrometer equipped with a cryoprobe. Peaks arising from metabolites of interest within the monoculture and quadriculture screenings were integrated from the NMR spectra and normalized to the respective TSP signals.
Statistical analysis
All statistical analysis was performed on R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Fisher’s exact test was used to evaluate differences between categorical variables, whereas Wilcoxon rank-sum test was used for numerical variables if not differently specified.
Results
General developmental profiles of infants at elevated- and low-likelihood of ASD
General developmental profiles of infants were evaluated using the MSEL test at 5 and 36 months of age, and with ADOS-2 at 36 months of age. Key confounding perinatal factors such as antibiotic exposure, preterm birth and medical conditions were excluded during enrollment. Additionally, maternal age, family income, and parental education level did not differ between the EL and LL groups (Supplementary Table I).
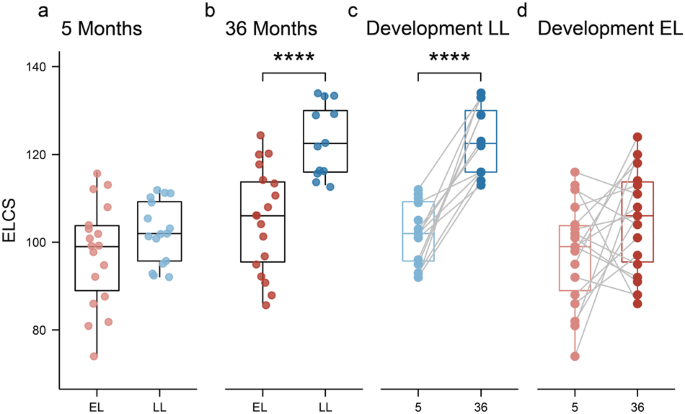
At 5 months, the Early Learning Composite Scores (ELCS, which is an age-adjusted standard score) of the MSEL were not significantly different between the EL and LL groups (Fig. 1a). However, at 36 months of age, the LL group had significantly higher ELCS compared to the EL group (Fig. 1b; Wilcoxon test, p = 0.0002). Additionally, the LL group had a significant increase in the ELCS between 5 and 36 months of age (Fig. 1c), while this was not the case for the EL group (Fig. 1d). In fact, the ELCS of seven infants in the EL group decreased with age, whereas all infants in the LL group increased with age. At 36 months of age, the overall ADOS-2 scores were significantly higher in the EL group compared to LL group (EL: 6.8 ± 2.484 vs. LL: 4.3 ± 2.720; mean ± SD; Wilcoxon test, p < 0.05). However, none of the infants were diagnosed with ASD according to the DSM-5 criteria at 36 months of age.
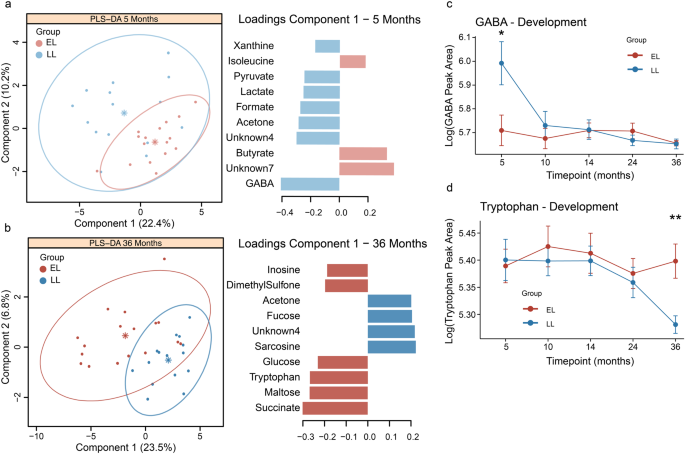
Infants were assessed using the MSEL test at 5- and 36-months of age. a At 5 months of age, no significant differences were observed between groups. b At 36 months of age, the elevated-likelihood group had a significantly lower ELC scores compared to the low-likelihood (p = 0.0002). c Infants in the low-likelihood group had a significant increase in their ELC scores between 5 and 36 months, while this developmental change was not observed in the elevated-likelihood group (d). Boxplots represent first (lower), median and third (upper) quartile. Wilcoxon test: ***p < 0.001. ELCS Early Learning Composite Scores, EL elevated-likelihood, LL low-likelihood.
Early-life differences in the gut microbiota composition and diversity between infants at elevated- and low-likelihood of ASD
Longitudinal fecal samples from 35 children (19 EL and 16 LL) from 5 to 36 months of age were analyzed by shallow shotgun metagenome sequencing yielding an average of 1,130,684 reads per sample. The number of unique OTUs detected after filtering was 1040. At 5 months, two samples from the LL group were identified as strong outliers and removed from downstream analysis. One sample was dominated by Clostridium neonatale (59% of the total relative abundance), while the other sample was dominated by Klebsiella oxytoca (44% of the total relative abundance).
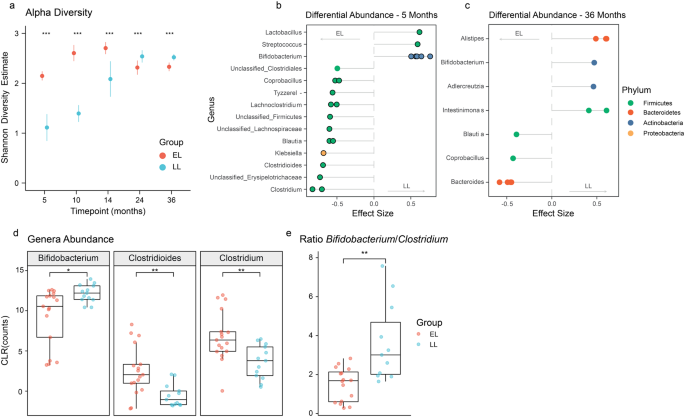
The overall gut microbiota composition at genus level of infants from the two different groups during the first 3 years of life is shown in Supplementary Fig. 1. Significant differences were observed in the estimated alpha-diversity (Shannon’s diversity index) between the two groups across all timepoints (DivNet, p < 0.001; Fig. 2a), with higher alpha diversity observed in the EL group during the first 14 months of life, but lower diversity thereafter (i.e., between 24 and 36 months). There were distinct differences in the developmental trajectory of alpha-diversity across the two groups. In the LL group, alpha-diversity gradually increased during the first year of life, reaching a plateau at 24 months. In contrast, the EL group showed a pronounced increase in alpha diversity between 5 and 10 months before a moderate decline between 24 and 36 months of age.
a Infants at elevated-likelihood of ASD showed higher alpha diversity during the first year of life, but lower diversity thereafter compared to the low-likelihood group. Dots represent mean estimate per group and whiskers show standard errors. b At 5 months of age, the low-likelihood group presented more Bifidobacterium species (B. breve, B. Bifidum, B. Longum, and B. kashiwanohense), while the elevated-likelihood group harbored more Clostridium related species (C. clostridioforme, C. neonatale, C. difficile, and C. bolteae), B. producta, R. gnavus, and K. variicola. Positive effect sizes indicate higher abundance in the low-likelihood group, while negative effect sizes indicate a greater presence of the taxon in the elevated-likelihood group. Displayed OTUs have p < 0.05 and a circle stroke indicates if the adjusted p value was <0.25. c At 36 months of age, none of the OTUs had an adjusted p value < 0.25, but several species that were more abundant in the low-likelihood group (A. senegalensis, A, timonensis, and I. butyriciproducens), as well as different Bacteroides species that were more abundant in the elevated-likelihood group had absolute effect sizes >0.5. d At 5 months of age, infants at elevated-likelihood of ASD presented significantly less Bifidobacterium, and more Clostridioides and Clostridium compared to infants at low-likelihood of autism. e At 5 months of age, infants at elevated-likelihood of autism had a significantly lower Bifidobacterium/Clostridium ratio compared to the low likelihood one. Boxplots (d, e) represent first (lower), median and third (upper) quartile. Wilcoxon test: *p < 0.05, **p < 0.01. ELCS Early Learning Composite Scores, EL elevated-likelihood, LL low-likelihood.
Infants at elevated-likelihood of ASD harbor less Bifidobacterium species and more Clostridium related species
Unconstrained ordination using PCA of CLR-transformed OTU counts showed a clear time-dependent development (PERMANOVA, R2 = 0.2 and p = 0.001) of the infant gut microbiota during the first three years of life (Supplementary Fig. 2). The variance between the individuals was greater during early life (5 and 10 months) than in the later sampling points (PERMDISPER, p = 3.59 × 10−15). Variation related to sampling age was captured in the first principal component. Inspection of the variables positively and negatively correlated with PC1 (absolute correlation > 0.7) indicated that bacteria belonging to the Ruminococcaceae, Lachnospiraceae, Clostridiaceae, Eubacteriaceae and Rikenellaceae families increased with age. Early timepoints were characterized by a higher abundance of taxa belonging to the Enterobacteriaceae and Veillonellaceae families (Supplementary Table 2).
Further analysis revealed significant differences in the microbial profiles between EL and LL groups at 5 and 36 months of age (PERMANOVA, R2 = 0.07 and p = 0.014, R2 = 0.04 and p = 0.015 respectively; Supplementary Fig. 3). To identify the OTUs driving the observed differences in the ordination analysis, differential abundance analysis was performed with ALDEx2. OTUs with p < 0.05 were retained and effect sizes were plotted. Taxa with adjusted p values < 0.25 after Benjamini–Hochberg correction were highlighted and considered significant. Effect sizes between 0.5 and 1 were considered of biological significance for group sample size of 20 [43]. At 5 months, the microbial profiles of infants in the EL group comprised significantly lower amounts of Bifidobacterium species (Bifidobacterium breve, Bifidobacterium bifidum, Bifidobacterium longum, Bifidobacterium kashiwanohense, and an unclassified species from the Bifidobacterium genus) compared to the LL group (Fig. 2b). Additionally, the EL group was characterized by a higher abundance of Clostridium related species including Clostridium clostridioforme, Clostridium neonatale, Clostridioides difficile and Clostridium bolteae, as well as Blautia producta, Ruminococcus gnavus and Klebsiella variicola, compared to the LL group. Consistent with the species level analysis, the analysis at the genus level showed significant changes in the Bifidobacterium, Lactobacillus, Clostridium and Clostridioides genera between the two groups. At 5 months of age, the EL group also had a significantly higher abundance of Coprobacillus and Erysipelatoclostridium, but a lower abundance of Alistipes and Parabacteroides compared to the LL group.
Although none of the differentially abundant OTUs at 36 months were significantly different between the two groups after BH correction (p adjusted > 0.25), an effect size >0.5 was observed for some of the taxa (Fig. 2c). The EL group presented a lower abundance of Alistipes senegalensis, Alistipes timonensis, Intestinimonas butyriciproducens, and Bifidobacterium bifidum compared to the LL group, but a greater abundance of several Bacteroides species.
Higher abundance of the Clostridium and Clostridioides genera and lower presence of Bifidobacterium in the EL group was also confirmed through univariate analysis (Wilcoxon test, p < 0.05). Boxplots highlighted the presence of 4 outliers within the EL group with a large abundance of Clostridium and Clostridioides (Fig. 2d). Three of the identified subjects also presented the lowest amount of Bifidobacterium compared to the other subjects in the same group. As expected, the Bifidobacterium/Clostridium ratio was also significantly lower in the EL group compared to the LL group (Fig. 2e).
Gut metabolome time-dependent development mirrors gut microbiome development
Analysis of the metabolites extracted from the longitudinal fecal samples of infants at elevated- and low-likelihood of ASD show a time-dependent development of the fecal metabolome during the first three years of life (PERMANOVA, R2 = 0.2 and p = 0.001; Supplementary Fig. 4). Variance within each timepoint progressively reduced through time. Fecal samples collected at earlier timepoints contained greater amounts of energy-related metabolites like formate, lactate, pyruvate, and metabolites derived from breast milk, including human milk oligosaccharides (HMOs), fructose, and galactose. No differences were noted in HMOs across the study groups, consistent with the parent reports, indicating no variation in breastfeeding between EL and LL infants. Samples collected at later timepoints contained greater amounts of isovalerate, valerate, urocanate, trimethylamine (TMA), and two unknown metabolites (doublet at 0.86 ppm and triplet at 0.85 ppm, both correlated to valerate) tentatively identified as isocaproate and valproate.
Infants at elevated-likelihood of ASD excrete lower amounts of fecal GABA at 5 months of age
Differences were observed in the fecal metabolomic profiles between elevated- and low-likelihood infants 5 to 36 months of age. Sparse PLS-DA models were generated with the integrated metabolites, and the loadings for metabolites with VIP > 1 were extracted. At 5 months, infants from the LL group were characterized by a greater presence of GABA and the energy-related metabolites, formate, lactate, and pyruvate, while the EL infants excreted greater amounts of butyrate and isoleucine (Fig. 3a). Univariate analysis confirmed these observations; with GABA abundance significantly lower in EL group compared to LL group (p < 0.05; Supplementary Fig. 5). In addition, butyrate appeared to be higher in the EL group, but not statistically significant (Supplementary Fig. 5).
Sparse PLS-DA models at 5- and 36-months of age were constructed with integrated metabolites from the full 1H NMR spectra. Metabolites with VIP > 1 were retained for the visualization of the loading plots of the first principal component. a GABA was identified as the main discriminatory metabolite driving the separation between infants at elevated- and low-likelihood of ASD at 5 months of age. b At 36 months, infants at elevated-likelihood of ASD presented more succinate, tryptophan, maltose, and glucose and less sarcosine compared to infants in the low-likelihood group. c Developmental changes in GABA and d tryptophan concentrations in infants at elevated- and low-likelihood of ASD. Group centroids are represented as * and ellipses represent 95% confidence interval. EL elevated-likelihood, LL low-likelihood.
At 36 months, the fecal metabolomes of the two study groups were also found to differ. A greater excretion of succinate, tryptophan, maltose, and glucose was observed in the EL infants whereas the LL infants excreted greater amounts of sarcosine (Fig. 3b). Observations were also confirmed through univariate analysis (Supplementary Fig. 5).
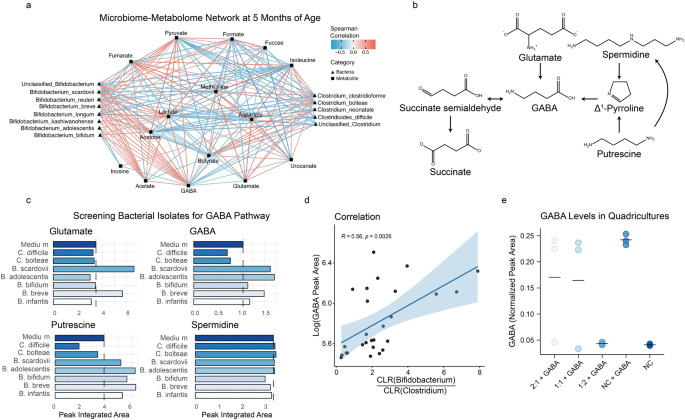
A microbiome-metabolome network was constructed using Spearman correlations between CLR-transformed OTUs and log-transformed integrated metabolites at 5 months. Only correlations with adjusted p value < 0.25 were retained during the construction of the full network (Supplementary Fig. 6). To simplify visualization, only Bifidobacterium and Clostridium related species of interest were plotted, together with the significantly correlating metabolites (Fig. 4a). Bifidobacterium species showed a strong positive correlation with GABA, acetate, and acetone. Some of the bifidobacteria were also positively associated with pyruvate and formate. Clostridium related species were strongly correlated with butyrate and glutamate and negatively correlated with acetone and lactate. Interestingly, GABA was positively correlated with acetate, acetone, lactate, and pyruvate and negatively correlated with glutamate and butyrate.
a Network constructed on CLR transformed OTUs and log transformed metabolites at 5 months of age using Spearman correlations. The full network was initially generated using all available OTUs and metabolites and correlations with adjusted p values < 0.25 were retained (full network available in Supplementary Fig. 6). To improve visualization, only Bifidobacterium and Clostridium species of interest and their correlated metabolites are displayed. GABA and acetate were positively correlated to the Bifidobacterium species of interest, while the Clostridium related species correlated with butyrate and glutamate. Red edges indicate positive correlations and blue edges indicate negative correlations. Edge width scaled on the absolute correlation values. b GABA can be produced through the GABA shunt, a closed-loop process that converts the α-ketoglutarate from the TCA cycle into glutamate, then GABA and finally into succinate, which re-enter the TCA cycle. GABA can also be produced from polyamines (e.g., spermidine and putrescine). Three different substrates (glutamate, putrescine, and spermidine) that can be converted into GABA were used in the Bifidobacterium and Clostridium cultures. c Bacterial isolates were cultured for 24 h in monocultures with the added substrate. B. breve and B. scardovii produced glutamate. GABA was produced by B. breve, B. adolescentis and B. scardovii, only when already present in the medium, and consumed by C. difficile and C. bolteae. Putrescine was consumed by Clostridium related species and produced by Bifidobacterium species, only when already present in the medium. No interactions with spermidine were observed. d A positive correlation was found between Bifidobacterium/Clostridium ratio and GABA concentration in fecal samples. e To determine whether the ratio of Bifidobacterium to Clostridium species. influenced the abundance of GABA, different growth conditions were investigated in vitro (Bifidobacterium spp.: Clostridium spp.; 2:1, 1:1, 1:2). GABA abundance was reduced to values comparable to that of the negative control (NC) when a greater proportion (2:1 Clostridium spp.: Bifidobacterium spp.) of Clostridium spp. were present in the cultures. Group means are plotted as line.
Regulation of GABA synthesis by Bifidobacterium and Clostridium species in vitro
To validate the statistical correlations between GABA and the discriminatory bacterial species, human isolates were grown in the presence of four different metabolites involved in the GABA pathway (Fig. 4b). Specifically, B. breve, B. bifidum, B. longum subsp. infantis, B. adolescentis, B. scardovii, C. difficile, and C. bolteae were cultured under anaerobic conditions in cell culture medium supplemented with either GABA, glutamate, putrescine, or spermidine.
Supernatants were collected after 24 h and analyzed by 1H NMR spectroscopy to assess the production and consumption of metabolites of interest (Fig. 4c). Both B. breve and B. scardovii produced glutamate when this amino acid was added to the culture medium (Fig. 4c). Consistent with the in vivo correlations, B. breve, B. scardovii, and B. adolescentis produced GABA, but exclusively when this neurotransmitter was added to the media (Fig. 4c), while the Clostridia related species. (e.g., C. difficile and C. bolteae) consumed it. All Bifidobacterium spp. produced putrescine, but only if this metabolite was supplemented to the medium. In contrast, Clostridia related species consumed putrescine. No interactions with spermidine were observed. Consistent with these in vitro observations, there was a positive correlation between GABA abundance and the Bifidobacterium/Clostridium ratio in the fecal microbiome of infants at 5 months of age (R = 0.56, p = 0.0026; Fig. 4d).
To assess whether the ratio of Bifidobacterium to Clostridium species influenced the abundance of GABA in the cultures, three growth conditions were studied (Bifidobacterium spp.: Clostridium spp.; 2:1, 1:1, 1:2). The species selected after initial experiments were B. breve, B. Infantis, C. difficile, and C. bolteae. To maintain GABA abundance around 4.5 mM, a higher initial ratio of Bifidobacterium species. was required (2:1; 1:1, Fig. 4e). Although this response was variable, GABA abundance was reduced to values comparable to that of the negative control (NC) when a greater proportion (2:1 Clostridium spp.: Bifidobacterium spp.) of Clostridium species were inoculated (Fig. 4e).
Discussion
The gut microbiota is increasingly recognized as a modulator of brain development and behavior, but its role in the etiology of common neurodevelopmental conditions such as ASD is poorly understood. Here, we report for the first time early life alterations in the gut microbiota composition and metabolic profile of infants at elevated-likelihood of ASD, during a critical period when the gut microbiota and brain are both undergoing rapid development [44]. Specifically, we found that infants at elevated-likelihood of ASD harbored less Bifidobacterium and more Clostridium related species at 5 months of age compared to infants at low-likelihood of ASD. This was accompanied by a significantly lower abundance of GABA in fecal samples from infants at elevated-likelihood of ASD. These early differences in the gut microbiota profile were also associated with changes in receptive language and expressive language from 5 to 36 months of age. These findings suggest that the intestinal microbiota of infants at elevated-likelihood of ASD develop differently to those at low-likelihood, with consequences for the bioavailability of GABA in the gut, and its neuro-immune modulatory effects on the host in early life. Although none of the infants were diagnosed with ASD at 3 years of age, more subtle presentations of ASD might still be diagnosed later in life, when everyday functional demands and expectancies increase (e.g., in educational settings) [45]. Indeed, only a minority of ASD cases are diagnosed in Sweden before age 5 [46]. Furthermore, about 28% of infants at elevated likelihood of ASD will also experience a range of neurodevelopmental difficulties, which will not reach the threshold for a clinical diagnosis of ASD [47]. Therefore, it will be important to follow-up this cohort of infants until school age.
The gut microbiota of infants at elevated-likelihood of ASD displayed a greater alpha diversity during the first year of life compared to the microbiota of low-likelihood infants, while the opposite was observed at 2 and 3 years of age. Previous studies have reported inconsistent differences in alpha diversity in infants with ASD compared to neurotypical siblings or healthy controls [12, 48,49,50,51,52,53]. However, these studies analyzed samples from children between the ages of 1 and 12 years, which is in alignment with the decrease in alpha diversity observed from the second year of life onwards. Differential abundance analysis showed a lower presence of B. breve, B. bifidum, B. longum, and B. kashiwanohense species in the elevated-likelihood of ASD group compared to the low-likelihood individuals. Decreased presence of Bifidobacterium has been previously observed in autistic children [21, 22]. Members of the genus Bifidobacterium (B. longum subsp. Infantis, B. bifidum, B. breve, and B. longum subsp. Longum) are among the first colonizers of the neonatal gut, and the most abundant taxa in vaginally born and breastfed infants [54]. Bifidobacteria have evolved a series of complex generic pathways to metabolize human milk oligosaccharides (HMOs), which support their growth [55]. The early life dominance of bifidobacteria have been associated with various health benefits, including folate production in the gut, protection against pathogens and development of the immune system, while their reduced levels have been associated with immune and metabolic disorders [56]. Moreover, we also found that infants at elevated-likelihood of ASD presented greater amounts of C. bolteae, C. difficile, C. clostridioforme, C. neonatale, B. producta, R. gnavus, and K. variicola compared to the low-likelihood group. Clostridium species are considered pathobionts and responsible for inflammation when homeostasis is disturbed [57]. Previous studies have shown higher abundance of Clostridium spp. (C. bolteae, C. difficile, and C. clostridioforme) in children with ASD [58,59,60]. Interestingly, the abundance of B. producta at 1 year of age has been associated with adverse social behavior at 3 years of age [61] while R. gnavus has been associated with depression [62] and was negatively correlated with cognitive functions in children [63]. Finally, K. variicola was found to be higher in pregnant women with gestational diabetes [64], a likelihood factor for ASD development [65]. When aggregated at the genus level, infants at elevated-likelihood of ASD presented less Alistipes and Parabacteroides and more Coprobacillus compared to infants in the low-likelihood group. Decreased abundance of Alistipes and Parabacteroides has been previously observed in children with ASD [66], while increased presence of Coprobacillus has been reported in ASD children with repetitive eating behavior disorders [67].
Metabolic profiling identified GABA as a discriminatory metabolite in feces driving the separation between infants at elevated- and low-likelihood of ASD at 5 months of age. GABA is the main inhibitory neurotransmitter in the adult central nervous system (CNS), and its imbalance has been implicated in different neurological and psychiatric disorders. Early in development, however, GABAergic synaptic transmission is excitatory, and plays an important role in neurodevelopment [68, 69]. Mounting evidence from human neuroimaging, postmortem, and genetic studies indicate an imbalance in excitatory/inhibitory neurotransmission ratio in ASD [70]. Moreover, lower amounts of GABA have also been detected in fecal samples of autistic children [49], suggesting a potential role of the GABA pathway in the neurobiology of ASD. The available evidence indicates that bacterial-derived GABA present in the blood does not enter the brain. Studies using germ-free mice mono-colonized with Bifidobacterium dentium, which possesses the enzymatic machinery to produce GABA from glutamate, glutamine, and succinate, have shown an increase in fecal GABA concentrations, but no changes in the brain [71]. This is consistent with the notion that GABA is unable to cross the blood–brain barrier. However, there is evidence that the central GABA pathway can be modulated via the vagus nerve and enteric nervous system. For instance, Bravo et al. demonstrated that ingestion of Lactobacillus reuteri, a probiotic with anti-inflammatory properties, can regulate emotional behavior and central GABA receptor expression in mice via the vagus nerve [72]. Moreover, recent findings have implicated GABA producing species such as Bifidobacterium infantis in the early life programming of the immune system [73]. Taken together, these observations raise the possibility that bacterial-derived GABA could modulate the central nervous system indirectly via neuronal (e.g., vagus nerve) and immune pathways.
In addition to the observed changes in GABA, infants at elevated-likelihood of ASD tended to excrete higher amounts of butyrate, which has previously been observed in greater concentrations in the stool samples of autistic children [74,75,76]. Although butyrate is considered a beneficial metabolite in the adult gut, animal studies indicate a dose-dependent effect on brain development and function, with higher doses causing a stress-like response [77]. At 36 months of age, the metabolic composition of the feces from the elevated-likelihood group was characterized by a greater presence of tryptophan and succinate, but lower amounts of sarcosine compared to the low-likelihood group. Previous studies have shown that children with ASD excrete higher amounts of tryptophan in the urine compared to their neurotypical peers [78]. In the gut, tryptophan can be metabolized into several compounds such as the neurotransmitter serotonin (5-HT), kynurenines, tryptamine, and indolic metabolites (under direct or indirect regulation by the gut microbiota). These compounds have been implicated in the communication along the microbiota-gut-brain axis [79]. Sarcosine, also known as N-methylglycine, can be converted into glycine, which has a fundamental role in the CNS homeostasis [80]. Interestingly, a previous study noted that fecal microbiota transplant (FMT) intervention in children with ASD improved their GI and ASD symptoms with a concomitant increase in plasma sarcosine [81, 82].
Integration of microbial and metabolic profiles showed a strong positive correlation between the identified Bifidobacterium species and GABA. In contrast, Clostridium species were correlated with glutamate and butyrate. Glutamate is a direct precursor of GABA, and the negative correlation between these two metabolites suggest an accumulation of this amino acid in the gut due to a lower conversion to GABA, given the reduced presence of bifidobacteria in the elevated-likelihood infants. Collectively, these observations raised the possibility that competition between Clostridium and Bifidobacterium species may exist and lead to reduced availability of GABA. To test this hypothesis, we used a simplified in vitro model system with different ratios of the identified Bifidobacterium and Clostridium species in the presence of GABA and its metabolic precursors. Fascinatingly, B. breve, B. scardovii, and B. adolescentis produced GABA when it was added to the medium, while C. difficile and C. bolteae degraded GABA. These results support the notion that a delicate balance exists between Bifidobacterium and Clostridium species in the infant gut, with clear consequences for the availability of GABA and its modulatory effects on the infant host.
The general development level of infants was assessed at 5- and 36-months of age using the MSEL. Consistent with previous findings, no significant differences were observed at 5 months of age between the elevated- and low-likelihood of ASD groups [83]. At 36 months, however, infants in the elevated-likelihood group had lower ELCS compared to those in the low-likelihood groups. However, none of the infants in our cohorts develop impairing ASD symptoms, leading to a clinical diagnosis. It is well-known that autistic individuals with good verbal and intellectual abilities can be diagnosed later in development, when daily demands increase. Moreover, other co-occurring mental health issues such as anxiety and depression may not be evident at this early age.
The absence of major perinatal complications in this cohort implicates host genetic risk factors in the observed differences in gut microbiota developmental profiles between the elevated- and low-likelihood groups at 5 months of age. Along this vein, recent studies have shown that many high-confidence risk genes for ASD are also expressed in the developing gut [84], suggesting that genetic variants previously linked to behavioral symptoms in ASD could contribute to host-microbial interactions early in life. In future prospective longitudinal studies of infants at elevated-likelihood of ASD, it will be important to include mother-father-child triads, coupled with the collection of genetic information of infants and their families, mother gut microbiota composition (from pregnancy until the first postnatal year of life), HMO content in breast milk, and relevant clinical outcomes.
In conclusion, the present study revealed distinct gut microbial and metabolic developmental profiles of typically developing infants with and without family history of ASD during the first 3 years of life. These differences were more pronounced at 5 months of age and characterized by lower abundance of beneficial Bifidobacterium species and GABA, and by an increased abundance of Clostridium related species and butyrate in the fecal samples of infants at elevated-likelihood of ASD. Further mechanistic studies are required to elucidate the impact of these species and metabolites on host development and their possible role in the etiology of ASD and associated comorbidities such as GI problems and anxiety. The supplementation of psychobiotic diet (e.g., prebiotics and probiotics) in early life should be explored as a strategy to promote healthy development of infants at EL of ASD.
References
Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder. Lancet 2018;392:508–20.
Zeidan J, Fombonne E, Scorah J, Ibrahim A, Durkin MS, Saxena S, et al. Global prevalence of autism: A systematic review update. Autism Res. 2022;15:778–90.
Hsiao EY. Gastrointestinal issues in autism spectrum disorder. Harv Rev Psychiatry. 2014;22:104–11.
Leader G, Abberton C, Cunningham S, Gilmartin K, Grudzien M, Higgins, E et al. Gastrointestinal symptoms in autism spectrum disorder: a systematic review. Nutrients. 2022;14:1471.
Madra M, Ringel R, Margolis KG. Gastrointestinal issues and autism spectrum disorder. Child Adolesc Psychiatr Clin N Am. 2020;29:501–13.
Bolte S, Girdler S, Marschik PB. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci. 2019;76:1275–97.
de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut 2022;71:1020–32.
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99:1877–2013.
Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–52.
Sherwin E, Bordenstein SR, Quinn JL, Dinan TG, Cryan JF. Microbiota and the social brain. Science 2019;366:eaar2016.
Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Vaisanen ML, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15:429–35.
Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010;16:444–53.
Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35:S6–S16.
Song Y, Liu C, Finegold SM. Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol. 2004;70:6459–65.
Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord. 2014;44:1117–27.
Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22.
Chakraborty P, Carpenter KLH, Major S, Deaver M, Vermeer S, Herold B, et al. Gastrointestinal problems are associated with increased repetitive behaviors but not social communication difficulties in young children with autism spectrum disorders. Autism 2021;25:405–15.
Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr. 2011;32:351–60.
Andreo-Martinez P, Rubio-Aparicio M, Sanchez-Meca J, Veas A, Martinez-Gonzalez AE. A meta-analysis of gut microbiota in children with autism. J Autism Dev Disord. 2022;52:1374–87.
Bezawada N, Phang TH, Hold GL, Hansen R. Autism spectrum disorder and the gut microbiota in children: a systematic review. Ann Nutr Metab. 2020;76:16–29.
Iglesias-Vazquez L, Van Ginkel Riba G, Arija V, Canals J. Composition of gut microbiota in children with autism spectrum disorder: a systematic review and meta-analysis. Nutrients. 2020;12:792.
Xu M, Xu X, Li J, Li F. Association between gut microbiota and autism spectrum disorder: a systematic review and meta-analysis. Front Psychiatry. 2019;10:473.
Morton JT, Jin DM, Mills RH, Shao Y, Rahman G, McDonald D, et al. Multi-level analysis of the gut-brain axis shows autism spectrum disorder-associated molecular and microbial profiles. Nat Neurosci. 2023;26:1208–17.
Needham BD, Adame MD, Serena G, Rose DR, Preston GM, Conrad MC, et al. Plasma and fecal metabolite profiles in autism spectrum disorder. Biol Psychiatry. 2021;89:451–62.
Zheng Y, Bek MK, Prince NZ, Peralta Marzal LN, Garssen J, Perez Pardo P, et al. The role of bacterial-derived aromatic amino acids metabolites relevant in autism spectrum disorders: a comprehensive review. Front Neurosci. 2021;15:738220.
Sharon G, Cruz NJ, Kang DW, Gandal MJ, Wang B, Kim YM, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 2019;177:1600–1618 e1617.
Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, et al. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci Rep. 2019;9:5821.
Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 2017;5:10.
Stewart Campbell A, Needham BD, Meyer CR, Tan J, Conrad M, Preston GM, et al. Safety and target engagement of an oral small-molecule sequestrant in adolescents with autism spectrum disorder: an open-label phase 1b/2a trial. Nat Med. 2022;28:528–34.
Yap CX, Henders AK, Alvares GA, Wood DLA, Krause L, Tyson GW, et al. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell 2021;184:5916–5931 e5917.
Bourne L, Mandy W, Bryant-Waugh R. Avoidant/restrictive food intake disorder and severe food selectivity in children and young people with autism: a scoping review. Dev Med Child Neurol. 2022;64:691–700.
House SA, Goodman DC, Weinstein SJ, Chang CH, Wasserman JR, Morden NE. Prescription use among children with autism spectrum disorders in Northern New England: intensity and small area variation. J Pediatr. 2016;169:277–283 e272.
Diaz Heijtz R. Fetal, neonatal, and infant microbiome: perturbations and subsequent effects on brain development and behavior. Semin Fetal Neonatal Med. 2016;21:410–7.
Palmeira O, Matos LRB, Naslavsky MS, Bueno HMS, Soler JP, Setubal JC, et al. Longitudinal 16 S rRNA gut microbiota data of infant triplets show partial susceptibility to host genetics. iScience. 2022;25:103861.
Girault JB, Piven J. The neurodevelopment of autism from infancy through toddlerhood. Neuroimaging Clin N Am. 2020;30:97–114.
Jones EJ, Gliga T, Bedford R, Charman T, Johnson MH. Developmental pathways to autism: a review of prospective studies of infants at risk. Neurosci Biobehav Rev. 2014;39:1–33.
Mullen EM. Mullen Scales of Early Learning. (Am Guid Serv Inc: Circle Pines, 1995).
Willis AD, Martin BD. Estimating diversity in networked ecological communities. Biostatistics 2022;23:207–22.
Fernandes AD, Reid JN, Macklaim JM, McMurrough TA, Edgell DR, Gloor GB. Unifying the analysis of high-throughput sequencing datasets: characterizing RNA-seq, 16 S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome 2014;2:15.
Lawson PA, Citron DM, Tyrrell KL, Finegold SM. Reclassification of Clostridium difficile as Clostridioides difficile (Hall and O’Toole 1935) Prevot 1938. Anaerobe. 2016;40:95–99.
Margolles A, Ruiz L. Methods for isolation and recovery of bifidobacteria. Methods Mol Biol. 2021;2278:1–12.
Otto R, Tenbrink B, Veldkamp H, Konings WN. The relation between growth-rate and electrochemical proton gradient of streptococcus-cremoris. FEMS Microbiol Lett. 1983;16:69–74.
Fernandes AD, Michael THQ, Edward L-M, Macklaim JM, Gloor GB. A reproducible effect size is more useful than an irreproducible hypothesis test to analyze high throughput sequencing datasets. arXiv https://doi.org/10.48550/arXiv.1809.02623 (2019).
Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20:509–18.
Zander E, Bolte S. The new DSM-5 impairment criterion: a challenge to early autism spectrum disorder diagnosis? J Autism Dev Disord. 2015;45:3634–43.
Idring S, Lundberg M, Sturm H, Dalman C, Gumpert C, Rai D, et al. Changes in prevalence of autism spectrum disorders in 2001-2011: findings from the Stockholm youth cohort. J Autism Dev Disord. 2015;45:1766–73.
Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, et al. The broader autism phenotype in infancy: when does it emerge? J Am Acad Child Adolesc Psychiatry. 2014;53:398–407 e392.
De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS ONE. 2013;8:e76993.
Kang DW, Ilhan ZE, Isern NG, Hoyt DW, Howsmon DP, Shaffer M, et al. Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe 2018;49:121–31.
Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE. 2013;8:e68322.
Wang M, Wan J, Rong H, He F, Wang H, Zhou J, et al. Alterations in gut glutamate metabolism associated with changes in gut microbiota composition in children with autism spectrum disorder. mSystems 2019;4:e00321–18.
Dan Z, Mao X, Liu Q, Guo M, Zhuang Y, Liu Z, et al. Altered gut microbial profile is associated with abnormal metabolism activity of Autism Spectrum Disorder. Gut Microbes. 2020;11:1246–67.
Wan Y, Zuo T, Xu Z, Zhang F, Zhan H, Chan D, et al. Underdevelopment of the gut microbiota and bacteria species as non-invasive markers of prediction in children with autism spectrum disorder. Gut 2022;71:910–8.
Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 2017;81:e00036–17.
Sprenger N, Tytgat HLP, Binia A, Austin S, Singhal A. Biology of human milk oligosaccharides: From basic science to clinical evidence. J Hum Nutr Diet. 2022;35:280–99.
Hidalgo-Cantabrana C, Delgado S, Ruiz L, Ruas-Madiedo P, Sanchez B, Margolles A. Bifidobacteria and their health-promoting effects. Microbiol Spectr. 2017;5. https://doi.org/10.1128/microbiolspec.BAD-0010-2016.
Lees EA, Miyajima F, Pirmohamed M, Carrol ED. The role of Clostridium difficile in the paediatric and neonatal gut - a narrative review. Eur J Clin Microbiol Infect Dis. 2016;35:1047–57.
Alshammari MK, AlKhulaifi MM, Al Farraj DA, Somily AM, Albarrag AM. Incidence of Clostridium perfringens and its toxin genes in the gut of children with autism spectrum disorder. Anaerobe 2020;61:102114.
Kandeel WA, Meguid NA, Bjorklund G, Eid EM, Farid M, Mohamed SK, et al. Impact of clostridium bacteria in children with autism spectrum disorder and their anthropometric measurements. J Mol Neurosci. 2020;70:897–907.
Liu F, Li J, Wu F, Zheng H, Peng Q, Zhou H. Altered composition and function of intestinal microbiota in autism spectrum disorders: a systematic review. Transl Psychiatry. 2019;9:43.
Laue HE, Korrick SA, Baker ER, Karagas MR, Madan JC. Prospective associations of the infant gut microbiome and microbial function with social behaviors related to autism at age 3 years. Sci Rep. 2020;10:15515.
Chahwan B, Kwan S, Isik A, van Hemert S, Burke C, Roberts L. Gut feelings: a randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J Affect Disord. 2019;253:317–26.
Bonham KS, Bruchhage MMK, Rowland S, Volpe AR, Dyer K, RESONANCE Consortium et al. Gut microbes and their genes are associated with brain development and cognitive function in healthy children. bioRxiv https://doi.org/10.1101/2020.02.13.944181 (2020).
Yao Y, Cai X, Chen C, Fang H, Zhao Y, Fei W, et al. The role of microbiomes in pregnant women and offspring: research progress of recent years. Front Pharm. 2020;11:643.
Rowland J, Wilson CA. The association between gestational diabetes and ASD and ADHD: a systematic review and meta-analysis. Sci Rep. 2021;11:5136.
Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017;5:24.
Berding K, Donovan SM. Diet can impact microbiota composition in children with autism spectrum disorder. Front Neurosci. 2018;12:515.
Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell 2001;105:521–32.
Marenco S, Meyer C, van der Veen JW, Zhang Y, Kelly R, Shen J, et al. Role of gamma-amino-butyric acid in the dorsal anterior cingulate in age-associated changes in cognition. Neuropsychopharmacology 2018;43:2285–91.
Zhao H, Mao X, Zhu C, Zou X, Peng F, Yang W, et al. GABAergic system dysfunction in autism spectrum disorders. Front Cell Dev Biol. 2021;9:781327.
Luck B, Horvath TD, Engevik KA, Ruan W, Haidacher SJ, Hoch KM, et al. Neurotransmitter Profiles Are Altered In The Gut And Brain Of Mice Mono-associated With Bifidobacterium dentium. Biomolecules 2021;11:1091.
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–5.
Henrick BM, Rodriguez L, Lakshmikanth T, Pou C, Henckel E, Arzoomand A, et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021;184:3884–3898 e3811.
Coretti L, Paparo L, Riccio MP, Amato F, Cuomo M, Natale A, et al. Gut microbiota features in young children with autism spectrum disorders. Front Microbiol. 2018;9:3146.
Liu S, Li E, Sun Z, Fu D, Duan G, Jiang M, et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci Rep. 2019;9:287.
Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci. 2012;57:2096–102.
Gagliano H, Delgado-Morales R, Sanz-Garcia A, Armario A. High doses of the histone deacetylase inhibitor sodium butyrate trigger a stress-like response. Neuropharmacology 2014;79:75–82.
Gevi F, Zolla L, Gabriele S, Persico AM. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Mol Autism. 2016;7:47.
O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48.
Wang W, Wu Z, Dai Z, Yang Y, Wang J, Wu G. Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids. 2013;45:463–77.
Qureshi F, Adams J, Hanagan K, Kang DW, Krajmalnik-Brown R, Hahn J. Multivariate analysis of fecal metabolites from children with autism spectrum disorder and gastrointestinal symptoms before and after microbiota transfer therapy. J Pers Med. 2020;10:152.
Kang DW, Adams JB, Vargason T, Santiago M, Hahn J, Krajmalnik-Brown R. Distinct fecal and plasma metabolites in children with autism spectrum disorders and their modulation after microbiota transfer therapy. mSphere 2020;5:e00314–20.
Gamliel I, Yirmiya N, Sigman M. The development of young siblings of children with autism from 4 to 54 months. J Autism Dev Disord. 2007;37:171–83.
Niesler B, Rappold GA. Emerging evidence for gene mutations driving both brain and gut dysfunction in autism spectrum disorder. Mol Psychiatry. 2021;26:1442–4.
Acknowledgements
This work was supported by the Swedish Research Council (2018-06232), Swedish Brain Foundation (FO2020-0088, FO2022-0199), Foundation Freemasons-Children’s house in Stockholm, and Foundation Olle Engkvists. J.R.S. is supported by the NIHR Southampton Biomedical Research Centre, Medical Research Council (MR/W003597/1), and BBSRC (BB/W00139X/1, BB/N005953/1). We thank the children and families who participated in this research.
Funding
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
RDH and HF conceived the project and coordinated the recruitment of infants with support from TF-Y and SB. RDH and JRS designed the experiments. RDH and AG-S were responsible for sample preparation. Microbial profiling (shotgun metagenomics and metabolomics) analyses and integration of omics data were performed by SZ and JRS. The culture studies were conducted by PS, SZ, and JRS with support from CB and JK. TF-Y and SB were responsible for behavioral and clinical assessments of the infants. SZ, PS, JRS, and RDH wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
PS and CB received funding from Danone Nutricia Research. JK is an employee of Danone Nutricia Research. Danone Nutricia Research was not involved in the design or funding of this research. SB discloses that he has in the last 5 years acted as an author, consultant, or lecturer for Medice and Roche. He receives royalties for textbooks and diagnostic tools from Hogrefe and Liber. SB is a partner of Neuro Support Solutions International AB.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zuffa, S., Schimmel, P., Gonzalez-Santana, A. et al. Early-life differences in the gut microbiota composition and functionality of infants at elevated likelihood of developing autism spectrum disorder. Transl Psychiatry 13, 257 (2023). https://doi.org/10.1038/s41398-023-02556-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02556-6
This article is cited by
-
From womb to world: mapping gut microbiota-related health literacy among Italian mothers, a cross-sectional study
BMC Public Health (2024)
-
Gamma-aminobutyric acid as a potential postbiotic mediator in the gut–brain axis
npj Science of Food (2024)
-
Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota–gut–brain axis
Nature Reviews Gastroenterology & Hepatology (2024)