Abstract
According to several theories, people differ in their sensitivity to environmental influences with some more susceptible than others to both supportive and adverse contextual conditions. Such differences in environmental sensitivity have a genetic basis but are also shaped by environmental factors. Herein we narratively build on our previous work proposing that prenatal experiences contribute to the development of environmental sensitivity. This hypothesis of prenatal programming of postnatal plasticity has considerable empirical support. After presenting illustrative animal and human evidence consistent with this claim, we discuss a range of biological mechanisms likely involved in the pathway from prenatal stress exposure to postnatal environmental sensitivity. We also consider work suggesting that genetic differences, gender, as well as the timing, duration and intensity of prenatal exposures may moderate the effects of prenatal programming on postnatal environmental susceptibility or sensitivity. Before concluding, we highlight “unknowns in the prenatal programming of environmental sensitivity” and their practical implications. Ultimately, we conclude that prenatal stress does not necessarily predispose individuals to problematical development, but rather increases sensitivity to both adverse and supportive postnatal contexts. Thus, prenatal stress may actually foster positive development if paired with supportive and caring postnatal environments.
Similar content being viewed by others
Introduction
It can be widely observed that people differ in how they respond to experiences, both in the short and longer term; the emphasis herein is on the latter. Consider, for example, that while some seem generally unperturbed by their experiences and exposures, others appear deeply affected. One reason for such variation is that individuals differ in their general susceptibility or sensitivity to environmental influences, such that some prove more and others less sensitive or susceptible. Several theories advanced over recent decades independently made a case for the existence of such individual differences [1,2,3] and have been summarized in the integrative framework of environmental sensitivity [4]. Importantly, according to these theories, heightened sensitivity is not only associated with increased vulnerability to the negative effects of adversity, but also with greater propensity to benefit from supportive experiences [5]. Although differences in general sensitivity are understood to have a genetic basis [6], environmental factors play an important role, too, including as early as during pregnancy [7, 8], the focus of this paper. In what follows, we present theory and empirical evidence consistent with this assertion and thus prenatal programming of environmental sensitivity. After considering theories and mechanisms of susceptibility to environmental influences, we discuss theoretical perspectives regarding the role of prenatal factors implicated in the development of such sensitivity. This sets the stage for the presentation of illustrative empirical evidence for prenatal stress affecting environmental sensitivity, including discussion of potential biological mechanisms and moderating factors, all of which stimulates practical implications and directions for future research.
Theories of environmental sensitivity
The notion that people differ in their sensitivity to environmental influences is not new in the field of psychiatry. Indeed, the diathesis-stress model is well established [9, 10], stipulating that some people are more vulnerable to the negative effects of adverse or stressful experiences due to inherent personal characteristics (e.g., genetics, physiology, temperament). Notably, over recent decades new perspectives have emerged indicating that some of these personal attributes are associated with sensitivity to both negative and positive experiences [11, 12]. In other words, some people are not only more vulnerable when faced with stressful experiences, but also benefit disproportionately from positive ones, such as supportive parenting [13] and psychological intervention [14], as the function of a general heightened sensitivity to environmental quality.
Such differences in environmental sensitivity have been central to the theory of differential susceptibility [1, 15], according to which natural selection has produced variation in susceptibility to environmental influences: Whereas some children are generally more unaffected—over the longer term—to would-be environmental influences during development (i.e., they are relatively “fixed”), others are more sensitive to the quality of their context and therefore more impacted in their development by both negative and positive exposures (i.e., they are relatively more “plastic”). Notably, the framework of Biological Sensitivity to Context [2] describes similar variation in environmental sensitivity, but, distinctively, regards such variation as resulting from the early rearing environment: those growing up in particularly adverse or especially supportive contexts develop enhanced sensitivity resulting from heightened physiological reactivity compared to those raised in more intermediate environments. Whilst the former two theories are strongly based on developmental and evolutionary considerations [16], a third theory of Sensory Processing Sensitivity [3, 17] is based on a psychological approach without detailed consideration of actual origins, stipulating that a minority of the population is characterized by a stable personality trait reflecting heightened sensory sensitivity and deeper cognitive processing of internal and external stimuli.
What these three theories have in common, in contrast to traditional diathesis-stress thinking, is that they contend that individuals do not only differ in their response to negative but also in response to positive exposures. A fourth theory is the converse of the diathesis-stress model in that vantage sensitivity stipulates that some individuals benefit more and others less from supportive environments due to differences in their sensitivity [5]. Given that these theories describe differences in susceptibility to environmental influences, they have been combined into a broader integrative framework of Environmental Sensitivity (which also includes vantage sensitivity) [4]. For simplicity, we will refer to environmental sensitivity or susceptibility from here on rather than to the individual theories.
Mechanisms of environmental sensitivity
Multiple empirical studies [for review, see [1, 18, 19]] documenting environmental sensitivity have linked heightened sensitivity with diverse individual characteristics, including genetic [20, 21], physiological [22, 23], and behavioural-psychological factors [12, 24, 25]. The different theories of environmental sensitivity all agree that susceptibility is most likely driven by features of the central nervous system [3, 4, 16, 26], including structural [27, 28] and functional aspects of the brain [29,30,31], often the result of physiological reactivity, sensory sensitivity and cognitive processing of contextual information. According to this neurosensitivity hypothesis [32], an individual’s sensitivity of the central nervous system is shaped by the complex interplay of genetic and environmental factors and becomes manifest in physiological reactivity and behavioural traits (i.e., infant temperament). Indeed, findings from a large twin study indicates that 47% of differences in environmental sensitivity, measured in adolescents with the Highly Sensitive Child (HSC) scale [25], are explained by genetic factors and the remaining 53% by environmental influences [33]. Although not much is known yet regarding the specific environmental factors that contribute to the development of susceptibility to environmental influences, there is evidence that environmental influences during the prenatal period, such as maternal stress or exposure to traumatic experiences, may play an important role in shaping physiological and behavioural factors associated with environmental sensitivity [7, 34, 35]. The basic notion that prenatal experiences can shape stable phenotypic characteristics of an individual has been the focus of much work conducted under the terms of developmental origins of health and disease [36, 37] as well as prenatal or fetal programming [38, 39].
Prenatal programming
According to the dominant view of prenatal programming, nutritional and hormonal cues reflective of the quality of the mother’s current and perhaps even childhood environment are passed on to the fetus in utero through the placenta. In so doing, they shape (i.e., “programme”) the fetus’ metabolism and stress reactivity, amongst other processes, in preparation for the conditions of the postnatal environment [40, 41]. The basic understanding is that this will (probabilistically) promote optimal adjustment by “fitting” the fetus to the postnatal environment it is most likely to encounter. When, however, there is a “mismatch” between prenatally “programmed” features of the child and the quality of the postnatal environment, such programming effects are expected to adversely affect the child’s development [40, 41]. This is likely to happen when the actual postnatal environment differs substantially from the environment the mother experienced during pregnancy or earlier. Thus, what was once evolutionarily adaptive under the assumption of prenatal-postnatal environmental stability is no longer so when the prenatal environment is not predictive of postnatal conditions. Similarly, more recent theories including Predictive Adaptive Response (PAR) [42] expand on this thinking to address the adaptive reasons of why organisms would adjust their development dependent on cues received in the prenatal environment.
A different explanation for such adverse prenatal programming effects is that the environmental “cues” that the fetus receives in utero affords “insight” into the degree of stability of the postnatal context [7, 8]. Should the environment be relatively unstable and more likely to change, it would make sense for the fetus to develop a higher degree of general environmental sensitivity in order to be better able to adapt to a wider range of postnatal environments [43]. Thus, with this line of thinking, prenatal stress may adversely affect offspring development under conditions of postnatal adversity but also foster more positive development when a supportive postnatal environment is encountered. In accordance with this hypothesis, several physiological and behavioural traits that have been associated with heightened environmental sensitivity (e.g., cortisol stress reactivity, difficult temperament) have also been shown to be shaped, at least partially, by prenatal factors such as maternal stress and other exposures during pregnancy [for review of empirical evidence, see [7, 8]].
In what follows, we review the strongest and most recent empirical evidence for the hypothesis that prenatal factors shape individual differences in environmental sensitivity, drawing on both animal and human studies. We then discuss several biological mechanisms that are likely involved in the prenatal programming of environmental sensitivity before considering whether such prenatal-programming effects are influenced by the intensity or timing of prenatal-stress exposure as well as child sex and genetic factors. It is important to note that this review is not intended to be a systematic examination of the field and although we present illustrative evidence for the proposed hypothesis, not all published studies are consistent with this thinking. Because of space constraints and the extensive literature that yields results consistent with environmental sensitivity, we decided this was the best way to construct this report. Thus, this paper builds upon previous research that provides empirical support for the aforementioned theoretical frameworks of sensitivity (e.g., differential susceptibility, diathesis-stress, vantage sensitivity) as well as for negative emotional temperament and increased stress reactivity as indicators of increased environmental sensitivity.
Empirical evidence of prenatal programming of environmental sensitivity
Prenatal stress has been associated with detrimental outcomes, especially increased risk for psychopathology [44]. Although a majority of studies control for potential postnatal confounds (e.g., postpartum maternal depression, parenting quality [45]), fewer examine effects of both the prenatal and postnatal environment which is needed when investigating prenatal effects on environmental sensitivity. We turn next to both animal models and humans in order to highlight associations between prenatal stress and individual differences in sensitivity to postnatal exposures.
Animal studies
Considering the numerous confounders present in human studies (e.g., comorbidities, strong pre/postnatal continuity) to say nothing of how unethical it would be to induce prenatal stress in humans, animal models are crucial to establish causal relationships between prenatal stress and postnatal functioning. Furthermore, animal models afford control over both the prenatal and postnatal environments which is critical considering that, in humans, prenatal and postnatal stress tend to co-occur [46]. In particular, cross-fostering experiments in which offspring are removed at birth from their biological parents and are reared by unrelated lactating dams or breeding pairs are a highly effective method for separating prenatal and genetic influence from postnatal conditions.
Prenatal stress has been shown to increase sensitivity, particularly vulnerability, to negative postnatal environments. Consider, for example, work using two different mouse strains, C57BL/6J and BALB/cJ, along with a pre- and postnatal cross-fostering design, to illuminate effects of prenatal and postnatal environments on adult anxiety-like behaviours [47]. C57BL/6J mice are known to have high levels of sociability, low levels of anxiety, and evince greater maternal care, whereas BALB/cJ mice are characterized by the opposite profile [47, 48]. In one study using prenatal cross-fostering, C57BL/6J embryos were transferred either to C57BL/6J (i.e., low anxiety) or BALB/cJ (i.e., high anxiety) females and, for postnatal cross-fostering, pups were moved after birth to either C57BL/6J (i.e., high care) or BALB/cJ (i.e., low care) dams. Although the investigators did not test behaviour or physiology during pregnancy, being cross fostered in utero to BALB/cJ mice can be considered a high prenatal stress condition given the strain’s characteristically high levels of anxiety-like behaviour compared to C57BL/6J. Results revealed that pups prenatally cross fostered to BALB/cJ females (i.e., prenatal stress) were more sensitive to postnatal rearing conditions. Specifically, prenatally stressed mice cross-fostered to BALB/cJ (i.e., low care) rearing mothers displayed the highest anxiety-like behaviour while pups not prenatally stressed were not affected by postnatal rearing condition.
Similarly, Hougaard et al. [49] found that rats prenatally stressed by either chronic mild stress (i.e., a variable schedule of mild stressors like damp bedding or crowding) or dexamethasone (i.e., a pharmacological stressor) that were subjected postnatally to a “stressful event” (i.e., blood sampling) later displayed greater reactivity (i.e., startle response) compared to rats not prenatally stressed. This is consistent with other research showing prenatally stressed rats, compared to controls, display heightened reactivity to contextual cues after fear conditioning [50].
Regarding individual differences in response to positive environmental influences [i.e., vantage sensitivity, [5]], Smythe and colleagues [51] examined the interaction between prenatal stress, induced by restraint, and postnatal handling on hypothalamic–pituitary–adrenal axis (HPA) axis activity in rats. Postnatal handling for brief periods is considered to be a positive early life experience as maternal care increases during the reunion with the mother after the handling [52]. Study results showed significant interactions between prenatal and postnatal conditions such that prenatally stressed rats assigned to postnatal handling had significantly lower corticotrophin-releasing hormone (CRH) levels in the Median Eminence whereas handling had no effect on rats that were not prenatally stressed. Moreover, other work produced similar evidence that rats exposed to prenatal stress, induced by unpredictable noise and light, are more sensitive to postnatal handling conditions with regard to adult anxiety-like behaviour than control rats who proved to be unaffected by postnatal handling [53].
Especially notable is recent work showing that prenatal stress increases offspring susceptibility to both negative and positive postnatal exposures, in that experimentally induced prenatal stress increased prairie voles postnatal sensitivity to low or high parenting quality [54]. Design-wise, pregnant voles were exposed to either a social stressor or not and, shortly after birth, pups were cross fostered to either high-quality (i.e., positive environment) or low-quality (i.e., negative environment) rearing conditions. Exposure to prenatal stress increased sensitivity to parenting quality in that prenatally stressed voles cross-fostered to high-quality parenting displayed the least behavioural and physiological reactivity as adults and the most reactivity if cross-fostered to low-quality parenting. Once again there was no effect of postnatal rearing on voles not prenatally stressed, thereby indicating lower susceptibility to the postnatal conditions. Clearly, then, animal work repeatedly indicates that prenatal stress can enhance environmental sensitivity to both positive and negative experiences. However, it is important to note that although research is still limited, epigenetics—which is discussed in the later section on “Potential Mechanisms” —is likely to play an important mechanistic role in these relations.
Human studies
When Pluess and Belsky [7] first postulated their prenatal-programming-of-postnatal-plasticity hypothesis, they provided empirical evidence from the NICHD Study of Early Child Care that linked prenatal stress—indexed via low birth weight—to infant negative emotionality which, in turn, was associated with infants being more susceptible to effects of both low and high parenting quality on cognition and behaviour [7]. Relatedly, work by Sharp and associates [55] revealed that maternal prenatal anxiety, measured during late pregnancy, increased children’s developmental responsiveness to postnatal maternal stroking during the first few weeks of life on later anxious/depressive symptoms. In this case--children—and especially girls—exposed to high levels of prenatal maternal anxiety evinced greater anxious/depressive symptoms when they experienced limited maternal stroking postnatally, yet very little symptomology when exposed to a great deal of maternal stroking. The same was not true of children whose mothers experienced little anxiety during pregnancy; that is, they proved relatively immune to the effects of stroking.
Other research yielded similar results when exploring the interactive effects of prenatal stress and early caregiving on child functioning. For example, Grant et al. [56] found that the impact of maternal sensitivity on infant physiological reactivity during a stressful experience (i.e., still-face procedure) was greater for prenatally stressed infants (i.e., mothers with prenatal anxiety disorders) compared to less prenatally stressed infants. Specifically, prenatally stressed infants displayed the least reactivity to a stressful experience when maternal sensitivity was high but the most if maternal sensitivity was low, with response to maternal sensitivity diminished for infants not exposed to prenatal stress.
Especially notably is evidence from a natural experiment. Prenatal stress effects resulting from the Queensland flood in 2011 interacted with maternal emotional availability during infancy in predicting toddlers’ language development [57]: children of highly emotionally available mothers exposed to disaster-related stress during pregnancy and PTSD symptoms had better language development but, if mothers were low in emotional availability and been exposed to comparable stress their offspring had poorer language development. Yet again, parenting quality proved unrelated to toddler’s development for children exposed to low prenatal stress.
Additional research on prenatal programming of environmental sensitivity compares infants born pre-term versus full-term, low versus normal birth weight, and small-for versus normal gestational age. This substantial body of work shows that psychosocial stress is an aetiological risk factor for preterm birth, low birth weight, and being small-for-gestational age [58, 59]—even when controlling for other well-known risk factors [60, 61]. Thus, preterm birth, low birth weight, and being small-for-gestational age are considered markers of prenatal stress.
Pertinent to the issue of prenatal programming, then, is work which examined differential effects of postnatal caregiving on pre-term and full-term infants’ cognitive and social functioning [62]. Results revealed that preterm infants were more developmentally responsive to their caregiving environment, evincing the best and poorest social and cognitive functioning when exposed, respectively, to high- and low-quality caregiving, with full-term infants proving insensitive to these caregiving effects. Clearly, these findings are in line with those of earlier work which chronicled stronger associations between maternal responsiveness and cognitive growth in the case of preterm infants compared to full-term ones [63]. In fact, an intervention designed to promote maternal responsiveness proved successful in doing so but, consistent with the data summarized through this point. Notably, though, when it came to effects on children’s development, the benefits of being in the experimental group rather than the control group proved greater in the case of children born preterm [64].
Another relevant investigation found, intriguingly, that the effects of maternal sensitivity during childhood on adult wealth were moderated by whether children were born small or appropriate for gestational age [65]. Specifically, children small for gestational age—reflecting greater prenatal stress—proved more susceptible, in terms of their adult wealth, to the quality of maternal care during childhood than those who were appropriate for gestational age. Critically, only for those small for gestational age, did greater childhood maternal sensitivity predicted greater adult wealth whereas less sensitive parenting predicted lower adult wealth. Finally, in another investigation, low birth-weight children proved to be especially sensitive to low, but not high, postnatal maternal sensitivity [66]: low birth-weight children had poorer academic achievement if exposed to low levels of maternal sensitivity but did not differ in academic achievement from normal birth weight children when maternal sensitivity was high.
Potential mechanisms
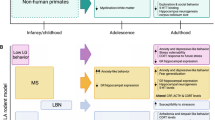
We now consider several biological systems by which maternal distress may alter fetal development potentially instantiating environmental sensitivity. Given the ubiquitous effects of prenatal stress and thus numerous possible mechanisms, the focus here is admittedly limited; a more detailed review can be found elsewhere [8]. Although the candidate mechanisms are discussed separately, they are likely all related, as indicated in Fig. 1.
Hypothalamic–pituitary–adrenal (HPA) axis
Two separate lines of research have highlighted the HPA axis as a key mediator of (1) environmental sensitivity and (2) prenatal stress effects on offspring. First, Boyce and Ellis [2] proposed heightened reactivity of the HPA system as the key mechanism for enhanced sensitivity to environmental effects. Indeed, heightened stress reactivity in children has been tied to greater sensitivity to both positive and negative experiences [8]. Second, prenatal programming of the offspring’s HPA axis functioning has been extensively investigated as a central mediator of maternal distress on offspring development in both human and animal models. For instance, a recent meta-analysis indicated that prenatal stress was associated with increased offspring glucocorticoids across 14 vertebrate species. Thus, not only is HPA axis sensitivity to prenatal stress highly conserved across evolution but, in addition, animal models of prenatal-HPA axis programming are appropriate guides for studying similar effects in humans [67].
In humans, various types of prenatal stress exposure are associated with altered child HPA axis functioning and stress reactivity [8]. For example, two meta-analyses found that dysregulation in child cortisol levels were predicted by (a) greater maternal cortisol during pregnancy [68] and (b) a variety of stressors experienced prenatally, including substance abuse and maternal distress [69]. This is consistent with animal studies finding that prenatal stress results in elevated levels of basal and reactive corticosteroids and decreased negative feedback of the HPA axis [70, 71]. Furthermore, in mice, prenatal stress affects stress neurocircuitry by increasing corticotropin-releasing factor in the amygdala and reducing hippocampal glucocorticoid receptor expression [72]. Similarly, in humans, prenatal stress is known to affect brain areas such as the prefrontal cortex, hippocampus, and amygdala that also regulate the HPA axis [73]. Although most of the reviewed evidence points to a pattern of heightened stress reactivity in response to prenatal stress, it is important to note that some work documents a more general dysregulation (i.e., blunting or over reactivity) in response to prenatal stress (e.g., [74]).
Given (a) the link between prenatal stress and stress reactivity and (b) that stress reactivity has been highlighted as an indicator of greater environmental sensitivity, it stands to reason that prenatal stress would foster greater physiological reactivity and, thereby, increased susceptibility to environmental influences (i.e., prenatal stress → greater physiological reactivity → increased sensitivity). Although portions of this process have been studied in isolation, the entirety of this potential mechanistic pathway has yet to be evaluated empirically.
Negative emotionality
Akin to physiological reactivity, there is considerable evidence that negative emotionality in infancy reflects increased environmental sensitivity such that more negatively emotional infants are more sensitive to their rearing experiences [7]. Like physiological reactivity, then, negative emotionality has been consistently associated with prenatal stress. Notably, there is extensive evidence that such negative emotionality is itself a consequence of prenatal stress. Consider, for example, another natural experiment, this one of pregnant women exposed to the 1998 Canadian ice storm and who experienced heightened subjective distress or illness/infection at various time points in their pregnancy. They had infants with more difficult temperaments than pregnant women also exposed to the storm but who experienced less subjective distress or illness/infection [75]. Then there is a recent meta-analysis showing that prenatal stress, indexed by maternal psychological distress or exposure to major life events and natural disasters, is associated with greater child negative affectivity [76].
Brain structure and function
Numerous studies have documented the effects of prenatal stress on both the structure and function of the brain [77]. Prenatal stress is associated with structural changes in the frontal and temporal lobes, including cortical thinning [78] and reductions in grey matter volume [34], as well as alterations in the limbic system, including increases in amygdala volume [79] and decreased hippocampal volume [80]. Functional brain activity is also affected by prenatal stress, with research chronicling reduced connectivity between the amygdala and prefrontal cortex in preterm infants exposed to prenatal maternal depression [81].
Importantly, differences in brain structure and function are thought to play a critical role in modulating environmental sensitivity [3, 31]. Evidence consistent with this claim comes from work showing that amygdala volume moderates the association between general environmental quality and child behaviour in boys [23]. Specifically, boys with larger amygdala volumes were more susceptible to beneficial effects of positive environmental features vis-à-vis their behaviour than boys with smaller amygdala volumes. Likewise, neonatal brain volume also moderates the association between parenting quality and cognitive development: newborns with larger brain volumes are more susceptible to effects of both positive and negative parenting on cognitive functioning [27].
Epigenetic modifications
Epigenetics is considered a central molecular pathway by which early life stress is biologically embedded, contributing to individual differences in environmental sensitivity [82]. The term epigenetics refers to a process that produces changes in gene activity (activation or suppression) without alterations to the DNA. Epigenetic mechanisms include many processes but the most widely studied is DNA methylation (which suppresses gene expression). Most epigenetic studies have focused on the programming effects of early postnatal life showing, for example, that early postnatal stress in rats influences offspring hippocampal DNA methylation in the promoter region of NR3C1, the gene coding the glucocorticoid receptor (GR), which, among many other factors, regulates the stress response [83]. Similarly, in humans, emerging evidence indicates that quality of maternal care is related to offspring methylation in N3CR1 as well as genes related to neuronal development (BDNF), the serotonergic system (SLC6A4), and social behaviour (OXTR) [84].
Notably, research in both humans and animals suggests that prenatal stress may induce the same epigenetic modifications in homologous promoter regions of NR3C1. For example, prenatal stress increases offspring stress reactivity and hypothalamic methylation in the promoter region of NR3C1 in mice [72]. Several human studies using neonatal cord blood find that prenatal anxiety [85], maternal exposure to interpartner violence [86], and depressive symptoms [87, 88] are associated with differential methylation patterns in the promoter region of NR3C1. In fact, one recent investigation examining pregnant mothers exposed to chronic stress found that placentas and neonatal cord blood had differential methylation patterns across several genes (i.e., CRH, CRHBP, NR3C1, and FKBP5) shown to regulate the HPA axis [89]. Notably, these methylation patterns were associated with lower infant birth weight, a marker of prenatal stress.
Placental functioning
The placenta plays a central role as a modulator of maternal-fetal physiology and is, thus, a key candidate for mediating effects of maternal distress on the fetus. Specifically, the placenta reduces fetal exposure to maternal glucocorticoids through the placental barrier enzyme 11β-hydroxysteroid dehydrogenase Type II (11β-HSD2) which converts glucocorticoids into inactive metabolites such as cortisone. In rats, prenatal stress, induced by chronic restraint, is associated with a reduction in the expression and activity of the placental 11β-HSD2 [90]. Consequently, these epigenetic changes in placental 11β-HSD2 are themselves related to 11β-HSD2 methylation in the fetal brain [90].
In humans, greater maternal anxiety measured one day prior to birth predicts lower gene expression of placental 11β-HSD2 [91]. In another study, self-reported maternal stress during mid-gestation was associated with increased placental DNA methylation of 11β-HSD2 which, in turn, was associated with a reduction in fetal coupling, a marker of neurobehavioral development [92]. Other work has also tied decreased activity of placental 11β-HSD2 with altered fetal development, including fetal growth restriction [93], prematurity [94] and low birth weight [95].
Considered together, it appears that prenatal stress may diminish the placental barrier via epigenetic changes in 11β-HSD2, thereby resulting in increased fetal exposure to maternal cortisol, with consequences for phenotypic outcomes.
Intestinal microbiota
There is ever more evidence that intestinal microbiota influence brain development and behaviour via the microbiome-gut-brain axis. For example, alterations in the microbiome have been linked to psychological disorders including depression and anxiety [96]. Other work has highlighted variations in the composition of the intestinal microbiome as a potential marker of environmental sensitivity [97].
Evidence also indicates that establishment and development of the intestinal microbiota begins early in life. Birth can significantly influence the microbiome with infants born via vaginal delivery showing a bacterial composition resembling their mothers’ vaginal microbiome; babies born via Caesarean section show more skin-like microbiomes [98]. Moreover, emerging rodent work indicates that microbiome transmission can happen prenatally, possibly through the placental barrier or fetal ingestion of amniotic fluid [99].
Findings from several studies indicate that prenatal stress may influence such early maternal-infant transmission. Prenatal stress is known to alter the composition of the maternal microbiota [100] and is associated with differences in infant intestinal microbiota [101, 102]. For example, preterm birth status, a marker of prenatal stress, is associated with alterations in the infant microbiome [101, 103]. Additionally, maternal prenatal anxiety is associated with differences in microbiota found in newborn meconium [104]. Furthermore, both subjective reports of stress and cortisol exposure during pregnancy predicted differences in infant microbiota diversity which, in turn, was linked to infant health [105].
Potential moderators
Having highlighted some mechanisms which may link prenatal stress and increased susceptibility to environmental influences, we now turn to potential moderators that may enhance or reduce the effect of prenatal exposures on environmental sensitivity. In other words, there is no claim that the link between prenatal stress and environmental sensitivity is inevitable. Like so much of development, “it depends”.
Genetic moderation
Ample evidence indicates that prenatal stress may affect offspring differently depending on their genetic make-up [106]. Candidate genes have been evaluated for their possible roles in this interaction with the gene encoding serotonin transporter (5-HTTLPR) being one of the most heavily studied. For example, Pluess et al. [35] found that prenatal anxiety predicted greater negatively emotional temperaments but only for children carrying the 5-HTTLPR short allele. Similarly, Babineau and colleagues [107] extended this work upon examining the interaction of prenatal depression and 5-HTTLPR in predicting infant and early childhood behavioural dysregulation. Results showed that greater prenatal depression measured mid- or late pregnancy predicted more infant and early childhood dysregulation from 3–36 months of age, but only for short-allele 5-HTTLPR carriers.
Other work has moved beyond examining variations in single candidate genes to a more systemic approach using polygenic scores based on multiple genetic variants [108, 109]. Belsky and Beaver [110] were the first to adopt this approach, discovering that the more would-be plasticity alleles—based on prior candidate gene work—moderated the effect of parenting on the self-control of teenage boys, such that the more plasticity alleles the adolescent carried, the more susceptible he appeared to parenting (in a manner consistent with differential susceptibility theorizing). For example, Silveira et al. [109] evaluated whether a polygenic score, based on genes co-expressed with the serotonin transporter gene (SLC6A4) in the hippocampus, moderated the relation between prenatal adversity and neurodevelopmental outcomes. Findings showed that exposure to greater prenatal adversity predicted a number of neurodevelopmental outcomes but only for children scoring high on the polygenic score. Additionally, Green et al. [111] observed that prenatal depression measured mid- to late pregnancy interacted with a polygenic profile score based on variants located in the 5-HTTLPR and the dopamine-receptor D4 (DRD4) genes in predicting infant negative temperament. Results indicated that prenatal depression only predicted greater infant negative emotionality for those with higher polygenic sensitivity. Moreover, Qiu et al. [112] reported that a polygenic risk score for major depressive disorder moderated the association between prenatal maternal symptoms of depression and children’s hippocampal and amygdala volume. Specifically, higher polygenic risk predicted greater right hippocampal and amygdala volumes when children were exposed to high levels of prenatal depression.
Sex
Although some research indicates that sex is a significant moderating factor of prenatal stress effects, findings are mixed with regard to which sex proves more susceptible. One inquiry, using data from pregnant mothers who were exposed to the 2011 Queensland Flood, revealed that higher levels of hardship during pregnancy predicted greater infant irritability, but only for boys [113]. Additional work reveals that boys have an increased risk of developing childhood attention-deficit/hyperactivity disorder (ADHD) if their mother lost a spouse or child during pregnancy [114]. Other studies show girls as evincing more compromised mental health outcomes [55], perhaps especially in the case of evidence documenting a significant association between maternal prenatal depression and risk for adult depression in women but not men [115].
These mixed findings may partly be due to sex-dependent differences in the outcome of interest. For example, boys present more frequently with intellectual impairment and childhood behavioural disorders related to prenatal stress whereas girls may develop subtler, later-onset anxiety and affective disorders in response to the same exposure [116].
Type, timing, and intensity of prenatal exposure
There is an abundance of evidence that many different types of prenatal stress can influence child development, including maternal anxiety and depression [117, 118], pregnancy-specific anxiety [119] and exposure to acute disasters such as ice storms [120] or the 9/11 terrorist attacks [74]. This diversity of stressors also extends to animal work, some of which include repeated restraint [121], electric shock [122], chronic-unpredictable stress [72], and social stress [123]. These different types of stressors can vary in intensity, duration, and predictability, all of which may result in specific effects on the mother and fetus. For example, a review of animal studies [124] found that two types of prenatal stress—restraint stress and chronic variable stress—predicted generalized outcomes (e.g., increased stress-related behaviour, increased glucocorticoid stress response) and stress-type specific ones (e.g., increased CRF expression, increased NR3C1 DNA methylation for chronic variable stress only).
Relatedly, the timing of prenatal stress also represents an important consideration. Despite significant knowledge regarding the time course of fetal brain development, there are relatively few studies that investigate the gestational timing of prenatal stress and offspring functioning. Nevertheless, there are suggestions that perturbations early in pregnancy are likely to produce more severe neurological insults than later stressors, perhaps via effects on placental functions and neural organization [125]. Work by Davis and Sandman [126] shows that higher maternal cortisol levels early in gestation predicted poorer cognitive development in offspring but better development when occurring late in gestation. Intriguingly, the opposite seems true when it comes to prenatal-stress effects on emotional and behavioural problems during childhood [127]. Other work investigating administration of antenatal corticosteroid treatment (usually between the late second trimester and early third trimester) predicted greater incidences of mental and behavioural disorders among sibling pairs [128].
Most importantly perhaps given the focus of this report, yet other evidence indicates that environmental sensitivity in response to prenatal stress may depend on the intensity of the prenatal stress. Hartman et al. [129] examined the effects of prenatal stress (i.e., maternal anxiety/ depression and stressful life events during pregnancy) on postnatal sensitivity using data from a large and representative prospective study of Norwegians. When using extreme groups to quantify prenatal stress (i.e., very high/very low), children exposed to greater prenatal stress were more sensitive, compared to children less prenatally stressed, to effects on child internalizing behaviour of mother’s postnatal maternal depressive/anxiety symptoms [129]. However, and perhaps surprisingly, in an analysis using the full sample, children exposed to the least prenatal stress evinced greater susceptibility to postnatal maternal depressive/anxiety symptoms on externalizing and internalizing behaviour.
Animal work also documents that the effect of prenatal stress on offspring depends on stress intensity. In one such experiment, rats were subjected to the same prenatal stress paradigm (i.e., pregnant dam placed on elevated platform) but differing intensities (more or less platform time). Intensity predicted distinctive patterns of offspring development (e.g., brain weight, sensorimotor development, DNA methylation levels) [130]. Taken together, it appears that prenatal stress may have differing effects on sensitivity depending on the intensity of prenatal stress.
Unknowns in the prenatal programming equation
It should be clear that there is substantial evidence that environmental exposures as early as during the prenatal period appear to affect sensitivity to environmental effects. Critically, the work summarized herein for illustrative purposes suggests that individuals exposed to prenatal stress are not only more vulnerable to postnatal adversity but also respond more strongly to positive environmental quality such as high-quality maternal care, consistent with theories highlighted at the beginning of this report. Needless to say, multiple questions remain to be addressed in future research.
The reviewed work suggests that prenatal stress increases environmental sensitivity. However, most work on prenatal programming tends to examine direct effects of the prenatal environment on postnatal outcomes, not accounting for interactive effects with the postnatal environment. And even when interactive effects are examined, postnatal conditions are often limited to a negative or neutral condition instead of the full range of environmental quality from adverse to supportive (i.e., not just benign). This focus, which reflects the mainstream view, emphasizes pathological development (e.g., anxiety, depression, cognitive disorders, poor health), thereby leaving little opportunity to illuminate the postnatal conditions under which prenatal stress may promote more positive development rather than impede it. Clearly, more research is needed to investigate prenatal programming of sensitivity to both detrimental and beneficial aspects of the postnatal environment.
One important limitation of most human studies is that detected associations between prenatal exposure and child development are likely confounded by genes shared by mother and child. This is a particular concern when prenatal stress is based on maternal reports of depression and anxiety (or measures of physiological stress). Many investigations try to account for this by controlling for postnatal maternal depression. But this may not be sufficient in order to discount the alternative explanation that women who report more stress during pregnancy also tend to have more stress-reactive children due to shared genes. Indeed, a unique human cross-fostering study comparing women with their own versus those with donated eggs for in-vitro fertilization found that some prenatal effects reflect inheritance rather than programming, including effects of prenatal maternal stress on children’s postnatal anxiety [131, 132]. Future studies should further disentangle inherited and environmental effects in prenatal programming studies by considering measured genes (i.e., applying genome-wide methodologies) in both child and mother. This is especially important given that environmental sensitivity has been shown to have a substantial genetic component [6].
Some theories stipulate that exposure to different environmental quality gives rise to different types of sensitivity [2, 4, 133]. For example, being exposed to a predominately adverse environment may foster the development of a more vigilant and stress-reactive type of sensitivity, whereas growing up in a consistently supportive context may result in a vantage sensitivity type that tends to benefit disproportionately from positive aspects of the environment [4]. Although first evidence supports aspects of this hypothesis [134], further work is needed to explore the existence of different sensitivity types and investigate whether different qualities of the prenatal environment play a role in shaping different types of sensitivity.
Another issue concerns the stability of sensitivity over time, that is, whether prenatally programmed environmental sensitivity is stable across life or whether it can change in response to the postnatal context. The stability of sensitivity across development has not been investigated in depth yet. Intriguingly, first evidence suggests that observer-rated sensitivity in three year old children can change within a year as a function of environmental quality [135], a result not inconsistent with Biological Sensitivity to Context thinking. Future studies should investigate to what degree prenatally programmed sensitivity is stable across subsequent developmental periods and identify the postnatal exposures that are likely to reverse or modify prenatally programmed environmental sensitivity.
Another important area for future research is to determine what specific prenatal conditions lead to increased environmental sensitivity. As mentioned previously, there is evidence to suggest that type, timing, and intensity all matter for prenatal stress effects. However, most of these studies are limited and have not been designed to test prenatal programming of environmental sensitivity. For example, few inquiries have compared prenatal factors such as varying levels of intensity or different types of prenatal stress and their relative influences on development. Furthermore, there is no systematic review, to our knowledge, of specific features of prenatal stress and their association with increased environmental sensitivity. It would be an important endeavour to systematically catalogue these differences indicating the type, timing, and intensity of prenatal stressors, postnatal environmental measures, associated outcomes, and effect sizes in relation to environment sensitivity. In this way, researchers may determine whether effects of prenatal stress on environmental sensitivity are more generalized or specific. It might be the case that extreme levels of prenatal stress confer only negative effects on the fetus or that non-linear relations exist similar to Selye’s stress theory [136].
Implications
Besides providing a broader conceptualization regarding the developmental impact of prenatal exposures compared to the traditional perspective [40], the current review specifically suggests that prenatal adversity does not necessarily result in pathology but can actually contribute to positive development when paired with a nurturing postnatal context. This implies that early support for the child and his/her family in the postnatal period could be especially beneficial and effective for children with a history of prenatal distress. At the same time, these children are at heightened risk for maladaptive development if growing up under adverse postnatal conditions. Hence, early intervention (e.g., programmes promoting supportive parenting) for prenatally stressed children is of great importance because it may not only prevent development of problematic outcomes but could facilitate and promote positive development.
In conclusion and in contrast to the traditional pathology-based perspective on prenatal programming effects, exposure to prenatal stress can increase general environmental sensitivity to the quality of the postnatal environment. According to a growing literature focused on animals and humans, individuals with a history of prenatal distress have not only been found to be more vulnerable to the negative effects of postnatal adversity but also disproportionately sensitive to the developmental benefits of a supportive postnatal context.
References
Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908.
Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17:271–301.
Aron EN, Aron A, Jagiellowicz J. Sensory processing sensitivity: a review in the light of the evolution of biological responsivity. Personal Soc Psychol Rev. 2012;16:262–82.
Pluess M. Individual differences in environmental sensitivity. child development. Perspectives. 2015;9:138–43.
Pluess M, Belsky J. Vantage sensitivity: individual differences in response to positive experiences. Psychol Bull. 2013;139:901–16.
Assary E, Zavos HMS, Krapohl E, Keers R, Pluess M. Genetic architecture of environmental sensitivity reflects multiple heritable components: a twin study with adolescents. Mol Psychiatry. 2021;26:4896–4904.
Pluess M, Belsky J. Prenatal programming of postnatal plasticity? Dev Psychopathol. 2011;23:29–38.
Hartman S, Belsky J. Prenatal programming of postnatal plasticity revisited-And extended. Dev Psychopathol. 2018;30:825–42.
Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol Bull. 1991;110:406–25.
Zuckerman M. Diathesis-stress models, In: Zuckerman M, editor. Vulnerability to psychopathology: a biosocial model. Washington: American Psychological Association; 1999.
Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14:746–54.
Slagt M, Dubas JS, Dekovic M, van Aken MA. Differences in sensitivity to parenting depending on child temperament: a meta-analysis. Psychol Bull. 2016;142:1068–110.
Lionetti F, Aron EN, Aron A, Klein DN, Pluess M. Observer-rated environmental sensitivity moderates children’s response to parenting quality in early childhood. Dev Psychol. 2019;55:2389–402.
Pluess M, Boniwell I. Sensory-processing sensitivity predicts treatment response to a school-based depression prevention program: evidence of vantage sensitivity. Pers Individ Differ. 2015;82:40–5.
Belsky J. Variation in susceptibility to rearing influences: an evolutionary argument. Psychol Inq. 1997;8:182–86.
Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, Van IJzendoorn MH. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Dev Psychopathol. 2011;23:7–28.
Aron EN, Aron A. Sensory-processing sensitivity and its relation to introversion and emotionality. J Pers Soc Psychol. 1997;73:345–68.
Belsky J, Pluess M. Beyond risk, resilience, and dysregulation: phenotypic plasticity and human development. Dev Psychopathol. 2013;25:1243–61.
Belsky J, Pluess M. Differential susceptibility to environmental influences, In: Cicchetti D, editor. Developmental psychopathology. New York: Wiley; 2016. p. 59.
Keers R, Coleman JR, Lester KJ, Roberts S, Breen G, Thastum M, et al. A genome-wide test of the differential susceptibility hypothesis reveals a genetic predictor of differential response to psychological treatments for child anxiety disorders. Psychother Psychosom. 2016;85:146–58.
van IJzendoorn MH, Belsky J, Bakermans-Kranenburg MJ. Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studies. Transl Psychiatry. 2012;2:e147.
Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: the interactive effects of stress reactivity and family adversity on socio-emotional behavior and school readiness. Child Dev. 2010;81:270–89.
Pluess M, De Brito SA, Bartoli AJ, McCrory E, Viding E. Individual differences in sensitivity to the early environment as a function of amygdala and hippocampus volumes: an exploratory analysis in 12-year-old boys. Dev Psychopathol. 2022;34:901–10.
Davies PT, Hentges RF, Coe JL, Parry LQ, Sturge-Apple ML. Children’s dove temperament as a differential susceptibility factor in child rearing contexts. Dev Psychol. 2021;57:1274–90.
Pluess M, Assary E, Lionetti F, Lester KJ, Krapohl E, Aron EN, et al. Environmental sensitivity in children: Development of the Highly Sensitive Child Scale and identification of sensitivity groups. Dev Psychol. 2018;54:51–70.
Moore SR, Depue RA. Neurobehavioral foundation of environmental reactivity. Psychol Bull. 2016;142:107–64.
Nolvi S, Rasmussen JM, Graham AM, Gilmore JH, Styner M, Fair DA, et al. Neonatal brain volume as a marker of differential susceptibility to parenting quality and its association with neurodevelopment across early childhood. Dev Cogn Neurosci. 2020;45:100826.
Schriber RA, Anbari Z, Robins RW, Conger RD, Hastings PD, Guyer AE. Hippocampal volume as an amplifier of the effect of social context on adolescent depression. Clin Psychol Sci. 2017;5:632–49.
Rudolph KD, Davis MM, Modi HH, Fowler C, Kim Y, Telzer EH. Differential susceptibility to parenting in adolescent girls: moderation by neural sensitivity to social cues. J Res Adolesc. 2020;30 Suppl 1:177–91.
Gard AM, Shaw DS, Forbes EE, Hyde LW. Amygdala reactivity as a marker of differential susceptibility to socioeconomic resources during early adulthood. Dev Psychol. 2018;54:2341–55.
Acevedo BP, Santander T, Marhenke R, Aron A, Aron E. Sensory processing sensitivity predicts individual differences in resting-state functional connectivity associated with depth of processing. Neuropsychobiology. 2021;80:185–200.
Pluess M, Stevens S, Belsky J. Differential susceptibility: developmental and evolutionary mechanisms of gene-environment interactions. In: Legerstee M, Haley DW, Bornstein MH, editors. The infant mind: origins of the social brain. New York: Guilford; 2013. p. 77–96.
Assary, E, Vincent, J, Machlitt-Northen, S, Keers, R, and Pluess, M, The role of gene-environment interaction in mental health and susceptibility to the development of psychiatric disorders. In: Teperino R, editor. Beyond our genes: pathophysiology of gene and environment interaction and epigenetic inheritance. Cham: Springer International Publishing; 2020. p. 117–38.
Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci USA. 2012;109:E1312–9.
Pluess M, Velders FP, Belsky J, van IMH, Bakermans-Kranenburg MJ, Jaddoe VW, et al. Serotonin transporter polymorphism moderates effects of prenatal maternal anxiety on infant negative emotionality. Biol Psychiatry. 2011;69:520–5.
Gluckman PD, Hanson MA. The developmental origins of health and disease. In: Early life origins of health and disease. Springer; 2006. p. 1–7.
O’Donnell KJ, Meaney MJ. Fetal origins of mental health: the developmental origins of health and disease hypothesis. Am J Psychiatry. 2017;174:319–28.
Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4:611–24.
Sandman CA, Davis EP, Buss C, Glynn LM. Prenatal programming of human neurological function. Int J Pept. 2011;2011:837596.
Barker DJ. In utero programming of chronic disease. Clin Sci. 1998;95:115–28.
Gluckman P, Hanson M. The fetal matrix: evolution, development and disease. Cambridge: Cambridge University Press; 2005.
Bateson P, Gluckman P, Hanson M. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. J Physiol. 2014;592:2357–68.
Frankenhuis WE, Panchanathan K. Individual differences in developmental plasticity may result from stochastic sampling. Perspect Psychol Sci. 2011;6:336–47.
Glover V, O’Donnell KJ, O’Connor TG, Fisher J. Prenatal maternal stress, fetal programming, and mechanisms underlying later psychopathology-a global perspective. Dev Psychopathol. 2018;30:843–54.
Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, et al. Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci Biobehav Rev. 2020;117:26–64.
Glover V, Capron L. Prenatal parenting. Curr Opin Psychol. 2017;15:66–70.
Francis DD, Szegda K, Campbell G, Martin WD, Insel TR. Epigenetic sources of behavioral differences in mice. Nat Neurosci. 2003;6:445–6.
An XL, Zou JX, Wu RY, Yang Y, Tai FD, Zeng SY, et al. Strain and sex differences in anxiety-like and social behaviors in C57BL/6J and BALB/cJ mice. Exp Anim. 2011;60:111–23.
Hougaard KS, Andersen MB, Kjaer SL, Hansen AM, Werge T, Lund SP. Prenatal stress may increase vulnerability to life events: comparison with the effects of prenatal dexamethasone. Brain Res Dev Brain Res. 2005;159:55–63.
Griffin WC, Skinner HD, Salm AK, Birkle DL. Mild prenatal stress in rats is associated with enhanced conditioned fear. Physiol Behav. 2003;79:209–15.
Smythe JW, McCormick CM, Meaney MJ. Median eminence corticotrophin-releasing hormone content following prenatal stress and neonatal handling. Brain Res Bull. 1996;40:195–9.
Francis DD, Meaney MJ. Maternal care and the development of stress responses. Curr Opin Neurobiol. 1999;9:128–34.
Wakshlak A, Weinstock M. Neonatal handling reverses behavioral abnormalities induced in rats by prenatal stress. Physiol Behav. 1990;48:289–92.
Hartman S, Freeman SM, Bales KL, Belsky J. Prenatal stress as a risk-and an opportunity-factor. Psychol Sci. 2018;29:572–80.
Sharp H, Hill J, Hellier J, Pickles A. Maternal antenatal anxiety, postnatal stroking and emotional problems in children: outcomes predicted from pre- and postnatal programming hypotheses. Psychol Med. 2015;45:269–83.
Grant KA, McMahon C, Austin MP, Reilly N, Leader L, Ali S. Maternal prenatal anxiety, postnatal caregiving and infants’ cortisol responses to the still-face procedure. Dev Psychobiol. 2009;51:625–37.
Austin MP, Christl B, McMahon C, Kildea S, Reilly N, Yin C, et al. Moderating effects of maternal emotional availability on language and cognitive development in toddlers of mothers exposed to a natural disaster in pregnancy: the QF2011 Queensland Flood Study. Infant Behav Dev. 2017;49:296–309.
Shapiro GD, Fraser WD, Frasch MG, Séguin JR. Psychosocial stress in pregnancy and preterm birth: associations and mechanisms. J Perinat Med. 2013;41:631–45.
Ding X, Liang M, Wu Y, Zhao T, Qu G, Zhang J, et al. The impact of prenatal stressful life events on adverse birth outcomes: a systematic review and meta-analysis. J Affect Disord. 2021;287:406–16.
Lilliecreutz C, Larén J, Sydsjö G, Josefsson A. Effect of maternal stress during pregnancy on the risk for preterm birth. BMC Pregnancy Childbirth. 2016;16:5.
Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am J Obstet Gynecol. 1993;169:858–65.
Gueron-Sela N, Atzaba-Poria N, Meiri G, Marks K. The caregiving environment and developmental outcomes of preterm infants: diathesis stress or differential susceptibility effects? Child Dev. 2015;86:1014–30.
Landry SH, Smith KE, Swank PR, Assel MA, Vellet S. Does early responsive parenting have a special importance for children’s development or is consistency across early childhood necessary? Dev Psychol. 2001;37:387–403.
Landry SH, Smith KE, Swank PR. Responsive parenting: establishing early foundations for social, communication, and independent problem-solving skills. Dev Psychol. 2006;42:627–42.
Nichols T, Jaekel J, Bartmann P, Wolke D. Differential susceptibility effects of maternal sensitivity in childhood on small for gestational age adults’ wealth. Dev Psychopathol. 2020;32:197–203.
Jaekel J, Pluess M, Belsky J, Wolke D. Effects of maternal sensitivity on low birth weight children’s academic achievement: a test of differential susceptibility versus diathesis stress. J Child Psychol Psychiatry. 2015;56:693–701.
Thayer ZM, Wilson MA, Kim AW, Jaeggi AV. Impact of prenatal stress on offspring glucocorticoid levels: a phylogenetic meta-analysis across 14 vertebrate species. Sci Rep. 2018;8:4942.
Zijlmans MA, Riksen-Walraven JM, de Weerth C. Associations between maternal prenatal cortisol concentrations and child outcomes: a systematic review. Neurosci Biobehav Rev. 2015;53:1–24.
Pearson J, Tarabulsy GM, Bussières EL. Foetal programming and cortisol secretion in early childhood: A meta-analysis of different programming variables. Infant Behav Dev. 2015;40:204–15.
Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal, et al. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci. 1995;15:110–6.
Maccari S, Krugers HJ, Morley-Fletcher S, Szyf M, Brunton PJ. The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations. J Neuroendocrinol. 2014;26:707–23.
Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–65.
Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45.
Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J Clin Endocrinol Metab. 2005;90:4115–8.
Laplante DP, Brunet A, King S. The effects of maternal stress and illness during pregnancy on infant temperament: Project Ice Storm. Pediatr Res. 2016;79:107–13.
Spry EA, Aarsman SR, Youssef GJ, Patton GC, Macdonald JA, Sanson A. et al. Maternal and paternal depression and anxiety and offspring infant negative affectivity: a systematic review and meta-analysis. Dev Rev. 2020;58:100934.
Lautarescu A, Craig MC, Glover V. Prenatal stress: effects on fetal and child brain development. Int Rev Neurobiol. 2020;150:17–40.
Davis EP, Hankin BL, Glynn LM, Head K, Kim DJ, Sandman CA. Prenatal maternal stress, child cortical thickness, and adolescent depressive symptoms. Child Dev. 2020;91:e432–50.
Acosta H, Tuulari JJ, Scheinin NM, Hashempour N, Rajasilta O, Lavonius TI, et al. Maternal pregnancy-related anxiety is associated with sexually dimorphic alterations in amygdala volume in 4-year-old children. Front Behav Neurosci. 2019;13:175.
Qiu A, Rifkin-Graboi A, Chen H, Chong YS, Kwek K, Gluckman PD, et al. Maternal anxiety and infants’ hippocampal development: timing matters. Transl Psychiatry. 2013;3:e306.
Posner J, Cha J, Roy AK, Peterson BS, Bansal R, Gustafsson HC, et al. Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl Psychiatry. 2016;6:e935.
Smeeth D, Beck S, Karam EG, Pluess M. The role of epigenetics in psychological resilience. Lancet Psychiatry. 2021;8:620–29.
McGowan PO, Suderman M, Sasaki A, Huang TC, Hallett M, Meaney MJ, et al. Broad epigenetic signature of maternal care in the brain of adult rats. PLoS ONE. 2011;6:e14739.
Provenzi L, Brambilla M, Scotto di Minico G, Montirosso R, Borgatti R. Maternal caregiving and DNA methylation in human infants and children: systematic review. Genes Brain Behav. 2020;19:e12616.
Hompes T, Izzi B, Gellens E, Morreels M, Fieuws S, Pexsters A, et al. Investigating the influence of maternal cortisol and emotional state during pregnancy on the DNA methylation status of the glucocorticoid receptor gene (NR3C1) promoter region in cord blood. J Psychiatr Res. 2013;47:880–91.
Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, et al. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl Psychiatry. 2011;1:e21.
Conradt E, Lester BM, Appleton AA, Armstrong DA, Marsit CJ. The roles of DNA methylation of NR3C1 and 11β-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics. 2013;8:1321–9.
Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106.
Kertes DA, Kamin HS, Hughes DA, Rodney NC, Bhatt S, Mulligan CJ. Prenatal maternal stress predicts methylation of genes regulating the hypothalamic-pituitary-adrenocortical system in mothers and newborns in the Democratic Republic of Congo. Child Dev. 2016;87:61–72.
Jensen Peña C, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS ONE. 2012;7:e39791.
O’Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, et al. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology. 2012;37:818–26.
Monk C, Feng T, Lee S, Krupska I, Champagne FA, Tycko B. Distress during pregnancy: epigenetic regulation of placenta glucocorticoid-related genes and fetal neurobehavior. Am J Psychiatry. 2016;173:705–13.
Börzsönyi B, Demendi C, Pajor A, Rigó J, Marosi K, Agota A, et al. Gene expression patterns of the 11β-hydroxysteroid dehydrogenase 2 enzyme in human placenta from intrauterine growth restriction: the role of impaired feto-maternal glucocorticoid metabolism. Eur J Obstet Gynecol Reprod Biol. 2012;161:12–7.
Demendi C, Börzsönyi B, Pajor A, Rigó J, Nagy ZB, Szentpéteri I, et al. Abnormal fetomaternal glucocorticoid metabolism in the background of premature delivery: placental expression patterns of the 11β-hydroxysteroid dehydrogenase 2 gene. Eur J Obstet Gynecol Reprod Biol. 2012;165:210–4.
Cottrell EC, Seckl JR, Holmes MC, Wyrwoll CS. Foetal and placental 11β-HSD2: a hub for developmental programming. Acta Physiol. 2014;210:288–95.
Sherwin E, Rea K, Dinan TG, Cryan JF. A gut (microbiome) feeling about the brain. Curr Opin Gastroenterol. 2016;32:96–102.
Hartman S, Sayler K, Belsky J. Prenatal stress enhances postnatal plasticity: the role of microbiota. Dev Psychobiol. 2019;61:729–38.
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971–5.
Walker RW, Clemente JC, Peter I, Loos RJF. The prenatal gut microbiome: are we colonized with bacteria in utero? Pediatr Obes. 2017;12 Suppl 1:3–17.
Jašarević E, Howerton CL, Howard CD, Bale TL. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology. 2015;156:3265–76.
Barrett E, Kerr C, Murphy K, O’Sullivan O, Ryan CA, Dempsey EM, et al. The individual-specific and diverse nature of the preterm infant microbiota. Arch Dis Child Fetal Neonatal Ed. 2013;98:F334–40.
Bailey MT, Lubach GR, Coe CL. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J Pediatr Gastroenterol Nutr. 2004;38:414–21.
Hiltunen H, Collado MC, Ollila H, Kolari T, Tölkkö S, Isolauri E, et al. Spontaneous preterm delivery is reflected in both early neonatal and maternal gut microbiota. Pediatr Res. 2022;91:1804–11.
Hu J, Ly J, Zhang W, Huang Y, Glover V, Peter I, et al. Microbiota of newborn meconium is associated with maternal anxiety experienced during pregnancy. Dev Psychobiol. 2019;61:640–49.
Zijlmans MA, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015;53:233–45.
Abbott PW, Gumusoglu SB, Bittle J, Beversdorf DQ, Stevens HE. Prenatal stress and genetic risk: How prenatal stress interacts with genetics to alter risk for psychiatric illness. Psychoneuroendocrinology. 2018;90:9–21.
Babineau V, Green CG, Jolicoeur-Martineau A, Bouvette-Turcot AA, Minde K, Sassi R, et al. Prenatal depression and 5-HTTLPR interact to predict dysregulation from 3 to 36 months-a differential susceptibility model. J Child Psychol Psychiatry. 2015;56:21–9.
Belsky J, Pokhvisneva I, Rema ASS, Broekman BFP, Pluess M, O’Donnell KJ, et al. Polygenic differential susceptibility to prenatal adversity. Dev Psychopathol. 2019;31:439–41.
Silveira PP, Pokhvisneva I, Parent C, Cai S, Rema ASS, Broekman BFP, et al. Cumulative prenatal exposure to adversity reveals associations with a broad range of neurodevelopmental outcomes that are moderated by a novel, biologically informed polygenetic score based on the serotonin transporter solute carrier family C6, member 4 (SLC6A4) gene expression. Dev Psychopathol. 2017;29:1601–17.
Belsky J, Beaver KM. Cumulative-genetic plasticity, parenting and adolescent self-regulation. J Child Psychol Psychiatry. 2011;52:619–26.
Green CG, Babineau V, Jolicoeur-Martineau A, Bouvette-Turcot AA, Minde K, Sassi R, et al. Prenatal maternal depression and child serotonin transporter linked polymorphic region (5-HTTLPR) and dopamine receptor D4 (DRD4) genotype predict negative emotionality from 3 to 36 months. Dev Psychopathol. 2017;29:901–17.
Qiu A, Shen M, Buss C, Chong YS, Kwek K, Saw SM, et al. Effects of antenatal maternal depressive symptoms and socio-economic status on neonatal brain development are modulated by genetic risk. Cereb Cortex. 2017;27:3080–3092.
Simcock G, Elgbeili G, Laplante DP, Kildea S, Cobham V, Stapleton H, et al. The effects of prenatal maternal stress on early temperament: the 2011 Queensland Flood Study. J Dev Behav Pediatr. 2017;38:310–21.
Li J, Olsen J, Vestergaard M, Obel C. Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: a nationwide follow-up study in Denmark. Eur Child Adolesc Psychiatry. 2010;19:747–53.
Quarini C, Pearson RM, Stein A, Ramchandani PG, Lewis G, Evans J. Are female children more vulnerable to the long-term effects of maternal depression during pregnancy? J Affect Disord. 2016;189:329–35.
Davis EP, Pfaff D. Sexually dimorphic responses to early adversity: implications for affective problems and autism spectrum disorder. Psychoneuroendocrinology. 2014;49:11–25.
O’Connor TG, Heron J, Golding J, Glover V, Team AS. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry. 2003;44:1025–36.
Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33:536–45.
Huizink AC, de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Psychological measures of prenatal stress as predictors of infant temperament. J Am Acad Child Adolesc Psychiatry. 2002;41:1078–85.
Laplante DP, Brunet A, Schmitz N, Ciampi A, King S. Project Ice Storm: prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J Am Acad Child Adolesc Psychiatry. 2008;47:1063–72.
Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol. 1994;6:341–5.
Takahashi LK, Kalin NH. Early developmental and temporal characteristics of stress-induced secretion of pituitary-adrenal hormones in prenatally stressed rat pups. Brain Res. 1991;558:75–8.
Brunton PJ, Russell JA. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J Neuroendocrinol. 2010;22:258–71.
St-Cyr S, McGowan PO. Adaptation or pathology? The role of prenatal stressor type and intensity in the developmental programing of adult phenotype. Neurotoxicol Teratol. 2018;66:113–24.
Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiol (Bethesda). 2005;20:180–93.
Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–48.
O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–8.
Räikkönen K, Gissler M, Kajantie E. Associations between maternal antenatal corticosteroid treatment and mental and behavioral disorders in children. JAMA. 2020;323:1924–33.
Hartman S, Eilertsen EM, Ystrom E, Belsky J, Gjerde LC. Does prenatal stress amplify effects of postnatal maternal depressive and anxiety symptoms on child problem behavior? Dev Psychol. 2020;56:128–137.
Mychasiuk R, Ilnytskyy S, Kovalchuk O, Kolb B, Gibb R. Intensity matters: brain, behaviour and the epigenome of prenatally stressed rats. Neuroscience. 2011;180:105–10.
Rice F, Harold GT, Boivin J, Hay DF, van den Bree M, Thapar A. Disentangling prenatal and inherited influences in humans with an experimental design. Proc Natl Acad Sci USA. 2009;106:2464–7.
Rice F, Harold GT, Boivin J, van den Bree M, Hay DF, Thapar A. The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychol Med. 2010;40:335–45.
Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neurosci Biobehav Rev. 2011;35:1562–92.
Keers R, Pluess M. Childhood quality influences genetic sensitivity to environmental influences across adulthood: A life-course Gene × Environment interaction study. Dev Psychopathol. 2017;29:1921–33.
Li Z, Sturge-Apple ML, Davies PT. Family context in association with the development of child sensory processing sensitivity. Dev Psychol. 2021;57:2165–78.
Selye H. Stress and distress. Compr Ther. 1975;1:9–13.
Author information
Authors and Affiliations
Contributions
SH and MP conceptualized and wrote the first draft of the paper. SH, MP and JB commented and revised further drafts of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hartman, S., Belsky, J. & Pluess, M. Prenatal programming of environmental sensitivity. Transl Psychiatry 13, 161 (2023). https://doi.org/10.1038/s41398-023-02461-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02461-y