Abstract
Depressive mothers often find mother-child interaction to be challenging. Maternal stress may further impair mother-child attachment, which may increase the risk of negative developmental consequences. We used rats with different vulnerability to depressive-like behavior (Wistar and Kyoto) to investigate the impact of stress (maternal separation-MS) on maternal behavior and adolescent offspring cognition. MS in Kyoto dams increased pup-contact, resulting in higher oxytocin levels and lower anxiety-like behavior after weaning, while worsening their adolescent offspring cognitive behavior. Whereas MS in Wistar dams elicited higher quality of pup-directed behavior, increasing brain-derived neurotrophic factor (BDNF) in the offspring, which seems to have prevented a negative impact on cognition. Hypothalamic oxytocin seems to affect the salience of the social environment cues (negatively for Kyoto) leading to different coping strategies. Our findings highlight the importance of contextual and individual factors in the understanding of the oxytocin role in modulating maternal behavior and stress regulatory processes.

Similar content being viewed by others
Introduction
Depressive mothers often display higher difficulty in interacting with their child, increasing the liability of child abuse and negligence [1]. In addition, maternal adverse events cause increased maternal stress, which may translate to a higher rate of depressive mothers, and rise of the worldwide number of children growing up under chronic stress [2]. Thus, maternal stress may have long-lasting effects on mothers, their children, and their families. Despite the importance of vulnerability to depression and its relationship with maternal stress, there is a lack of research combining the impact of such risk factors on mother’s behavior and its consequences for the offspring.
In rodents, maternal behavior allows a proper mother/pup attachment, adequate nutrition, and temperature regulation [3]. It includes a complex variety of behaviors and its quality/quantity have been linked to the development of emotional and cognitive processes in the offspring [4]. Maternal behavior is regulated by oxytocin [5], a neuropeptide released from the paraventricular nucleus and the supraoptic nucleus of the hypothalamus [6]. While oxytocin activates brain areas related to maternal behavior, its blockage leads to maternal care deficits [7, 8]. Furthermore, the developing oxytocinergic system is impacted by mother’s absence [9]. The maternal separation paradigm (MS), used as a maternal adverse event, is the most widely used animal model to study early-life stress and disruption of the infant-mother relationship [10]. In a recent systematic review, we found that MS induces changes in dam’s emotional behavior by increasing anxiety and depressive-like symptoms [4], aggression, and impacting on maternal care [11]. MS seems to increase the total time spent in maternal behavior [4, 12], which was suggested to likely represent an attempt to overcompensate for the time off-nest [12]. Under MS, pups increase solicitation calls [13], compelling mothers to increase their maternal behavior as a response to the pup needs [4], which has been associated with increased expression of oxytocin receptors (OXTR) [14].
The existing literature seems to indicate that MS induces changes in the hippocampus of the offspring [15, 16], leading to a maladaptive brain development and cognitive impairment in adulthood [17]. The impact of maternal separation on the hypothalamic-pituitary-adrenal (HPA) axis is well-recognized [18, 19]. However, other factors are increasingly recognized as also relevant in triggering MS-induced behavioral alterations, like anxious and depressive-like behaviors [20]. Early-stress life events, including MS, have been associated with changes in immune activation and seem to affect microglia proliferation, morphology and phagocytic activity [21], priming them to contribute to later neuropsychiatric disorders [22]. The potential interplay between the immune system and depression, however, was yet barely investigated in MS models.
Psychosocial stressors can lead to increased microglial reactivity in the hippocampus, which may also contribute to cognitive impairment with neuroinflammation as a crucial mediator [23, 24]. Neuroinflammation is also linked to depression [25], primarily by its association with pro-inflammatory cytokines increased expression, such as the tumor necrosis factor (TNF), interleukin (IL)-6 and IL-1β [26,27,28].
In the present study, we used a depressive-like animal model (Wistar-Kyoto rat strain) combined with the MS paradigm to investigate the interplay between the role of pre-existing vulnerability to depression and environmental/social risk factors. In the present study, we used a depressive-like animal model (Wistar-Kyoto rat strain) combined with the MS paradigm to investigate the interplay between the role of a pre-existing vulnerability to depression and environmental/social risk factors. The Wistar-Kyoto (from now on referred as Kyoto) rat strain is a validated animal model of endogenous depression, which allows studying vulnerability to genetic depression [29]. The Kyoto strain was obtained by successive selective breeding resulting in rats that present: (i) excessive immobility in the forced swim test (FST), (ii) reduced activity in the open field (OF), (iii) increased anxiety-like behavior, (iv) behavioral despair, (v) lower social interaction and exploration, and (vi) anhedonia [30, 31]. Upon these behavioral characteristics, Kyoto display alterations in the HPA axis, characterized by decreased corticosterone (CORT)-mediated responses to acute stress and deficits in the serotonergic, dopaminergic and neurotrophic systems, thus exhibiting a behavioral and neurobiological phenotype with well-accepted face validity in depression [30, 32]. Emerging studies suggest also the involvement of neuroinflammation [31]. Thus, we studied the MS impact on maternal care and mother’s anxiety/depressive-like behaviors focusing on the oxytocinergic system and neuroinflammatory processes interactions. We predicted that depressive-like mothers, under maternal stress, would worsen their maternal behavior linked to a reduction in oxytocin. Furthermore, we expected that the mother’s maternal-stress-response impact on the adolescent offspring cognition would be worse in the depressive-like group and that it would be related to the pro-inflammatory cytokine levels.
Methods
Animals
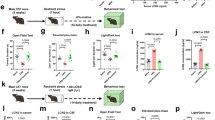
Wistar and Kyoto rats were obtained from Charles River (Barcelona/Spain). Rats were kept under standard conditions (21 ± 1 °C; 60 ± 5% humidity; 12h/12h light cycle – off at noon) with ad libitum food/water. Pregnant females were individually housed on pregnancy day 16. Animal behavior analyses were performed with the software Observer XT version10 (Noldus information technology, Netherlands) and Smart Video Tracking Software (Panlab) version 3.0, following the timeline in Fig. 1. Brain areas were collected 24 h after behavioral tests. All experiments involving animals were approved by the Portuguese regulatory agency Direcção Geral de Alimentação e Veterinária (DGAV) and the ethics committee of IBMC-i3S. The facility and people directly involved in animal experimentation were certified. All animal experiments followed the European guidelines for animal welfare (2010/63/ EU Directive).
Maternal separation
Each litter was sexed and adjusted to 8–10 pups in PND1 (PND0 = delivery day). Dams from each strain were randomly assigned to control (n = 8/strain) or MS group (n = 7/strain). Control group followed animal facility rearing (1 cage change/week). The MS dams were separated from their offspring at 9:00 am, 180 min/day, from PND2-14, following the MS protocol described by Alves et al. [33]. This study used 88 pups and the remaining were used in a different study [33]. Rats were weaned and co-housed (2-3/cage) at PND21 and 8 groups were established (n = 11/group): control Wistar male; control Wistar female; control Kyoto male; control Kyoto female; MS Wistar male; MS Wistar female; MS Kyoto male; and MS Kyoto female. Each group contained rats from all litters. Pup’s weight was measured at PND2, 12, 33.
Mothers evaluation
Maternal behavior
Maternal behavior toward offspring was scored immediately after pup reunion, at PND 2, 6, 10, 14, for 10 min. Animals were not disturbed during behavioral observations. The following behavior categories were observed: total maternal behavior (affiliative plus non-affiliative behavior); affiliative behavior (grooming/licking and nursing time); non-affiliative behavior (dam in contact with the offspring but not in grooming/licking and nursing).
Pup retrieval test (PRT)
At PND4, pups were returned to the original cage after MS away from the nest, followed by the return of the mother. Control group mothers were removed for 3 min and returned, while their pups were moved to the opposite-nest side. The observation ended when mothers retrieved all pups, or the 10 min observation limit was achieved [34]. First-contact latency; first-pup-retrieval latency; and full litter retrieval time were quantified.
Elevated plus maze (EPM)
Mother anxiety-like behavior was measured during the MS period (PND 7). The apparatus consisted of four connected arms (44 cm × 14 cm) in a plus-shaped structure and elevated 72 cm above the floor. Two arms were an open platform (no sidewalls) and two were enclosed within 22 cm-high sidewalls. Arms were positioned so opposing arms were of the same type. Each mother was placed in the central platform (the intersection of the four arms - 14 × 14 cm area) facing an open arm and left undisturbed for 5 min. Testing was conducted in the dark phase of the light/dark cycle. The apparatus was cleaned between animals with neutral, odor free soap [35]. The following behaviors were measured: (a) open-arms time; (b) open-arm entries; (c) center-area time; (d) immobility time; (e) total arm entries; (f) rearing; and (g) head dipping.
Open-field (OF)
On weaning day (PND21), mothers were tested in the OF to measure anxiety-like symptoms. The apparatus consisted of an empty rectangular arena (80 × 60 cm). Mothers were placed in the corner of the apparatus and left to explore the arena for 10 min. The following behaviors were recorded: (a) center time; (b) center entries; (c) center latency; (d) total distance traveled; and (e) rearing.
Forced swimming test (FST)
Mother passive coping behavior was assessed as a depressive-like behavior indicator with the FST at PND22 according to the protocol described by Aguggia et al. [34]. Dams were placed in a transparent container filled with water (25 ± 2 °C) for 10 min (pre-test session to reduce acute stress) and tested 24 h later for 5 min in the same container. Swimming, climbing, and immobility duration were recorded.
Offspring evaluation
Morris water maze (MWM)
The MWM test was used to evaluate the impact of MS on the cognitive performance of the adolescent offspring. The apparatus consisted of a water-filled (24 °C) circular pool (111 cm diameter). The procedure was adapted from Vorhees and Williams [36]. In preparation for the memory task tests, cued learning was conducted at PND37-38. Four trials were performed during which both, start quadrant and platform locations were changed. If a rat did not locate the platform before 60 s elapsed, it was guided to the platform and could stay there for 15 s.
Spatial learning and reference memory
After cued learning, rats were subjected to the spatial learning task (PND39–42, four trials/day). Extra maze cues were placed in the room and the platform was submerged in a fixed location. Latency to reach the platform was recorded. On probe day (PND43), the reference memory was evaluated (1 trial). The platform was removed, and rats were released in the quadrant opposite the platform’s previous location. Rats could swim freely during 30 s. The following measures were quantified: (a) target mean distance; (b) total distance traveled; (c) target quadrant time percentage; (d) target quadrant latency; (e) target crossings.
Spatial working memory
At PND44–47, the platform and rat starting positions were the same in each day but changed every day (four trials/day, adapted from Tata et. al. [37]). Maze cues were kept as in the spatial learning task. Latency to reach the platform was recorded. Only latency to reach the platform within the day was expected to decrease, but not between days.
Mothers and offspring biomarkers
Protein extract preparation
Dams were sacrificed at PND24, 24 h after the FST. The adolescent offspring were sacrificed at PND48, 24 h after the spatial working memory test.
For dams, the amygdala (Bregma −1.60 to −2.30) [38], the prefrontal cortex (PFC, Bregma 2.70 to 5.20) [38], and the whole hippocampus were homogenized in a buffer containing 50 mM Tris-Base, 10 mM EDTA, 150 mM NaCl, 0.1% Tween 20 and protease inhibitors [39]. The offspring’s hippocampus was homogenized using the same buffer. Samples were sonicated and centrifuged for 15 min at 2271 × g and posteriorly, the supernatant was centrifuged for 5 min at 13500 g. The protein concentration was estimated using Pierce BCA Protein Assay kit (Thermo Scientific), following the manufacturer’s instructions. After centrifugation, total protein concentration was determined in the supernatant using a BCA kit (23227, ThermoFisher Scientific).
Enzyme-linked immunosorbent assays (ELISA)
Oxytocin was measured by ELISA (ADI-900-153A, Enzo Life Sciences – Detection range: 15.6 to 1.000 pg/ml) in mother’s hypothalamus protein homogenates obtained by dissecting the hypothalamic region between Bregma −0.26 and −1.40 [38], which encompass the medial preoptic area (MPOA), the paraventricular and supraoptic (suprachiasmatic) nuclei. BDNF (mediator of neuronal plasticity [40]) levels were measured in offspring’s hippocampus homogenates by ELISA (CYT306, Millipore). The quantification of IL‐1β, IL-6, and TNF in the mother’s hippocampus and of IL-1β and IL-6 in the offspring’s hippocampus were also performed by ELISA (900-M91, 900-M86, 900-M73, PeproTech).
Western blot
We determined the expression of (i) OXTR on mother’s PFC, hippocampus (100 µg protein), and amygdala (70 µg protein); (ii) postsynaptic density protein 95 (PSD95, abundant scaffold protein present in the glutamatergic postsynaptic terminal [41]) and vesicular glutamate transporter 1 (vGlut1, a presynaptic transporter protein that mediates glutamate uptake into synaptic vesicles, linked to the efficacy of glutamatergic neurotransmission [42]) on offspring’s hippocampus (using 30 µg protein). Samples were denatured at 95 °C for 5 min and loaded into a 10% SDS-page gel, transferred to a nitrocellulose membrane, processed with blocking solution, and incubated with primary antibody overnight, at 4 °C (OXTR, 1:400 (Thermo Fisher Scientific); PSD95, 1:2000 (ThermoFisher Scientific); vGlut1, 1:1000 (Synaptic Systems); GAPDH, 1:100,000 (HyTest). Primary antibodies were visualized with HRP-conjugated anti-rabbit or HRP-conjugated anti-mouse (1:10,000, Jackson ImmunoResearch). Signals were enhanced using SuperSignal™ West Pico PLUS Chemiluminescent Substrate (ThermoFisher Scientific). Data was quantified using ImageJ software [43].
Statistical analyses
Sample size was determined based on literature and previous experience that used the same strain and behavioral tests used in this study. Mother rats and their offspring were allocated to experimental groups randomly. Since treatment (maternal separation vs control) had to be applied to whole offspring, litter effect was considered random effect during analysis. Animals from the same litter were later divided into different tests. The investigator was not blinded to the group allocation but had not control over the animal testing order.
Normality and homoscedasticity were verified (Shapiro–Wilk or Kolmogorov–Smirnov test and Levene’s test respectively). Treatment effects on dams were determined with two-way ANOVA (Strain: Wistar/Kyoto, and MS: control/MS). Generalized linear models with 3 factors (Strain: Wistar/Kyoto MS: control/MS, and Sex: male/female) and their interactions were used for the offspring analyses with normal or Poisson distributions to obtain normal distribution of the model residuals and homoscedasticity. Time was added as a fourth factor for learning and working memory results. A Linear Mixed Model was also used to verify litter random effect, but significance was not found (except for pup weight). Multiple comparisons were Bonferroni adjusted (dams) or Tukey-Kramer adjusted (offspring). Data transformation, when needed due to heteroscedasticity, was: arcsine of percentages; square-root of zero containing data, and log transformation. IBM SPSS Statistics 23 software was used and SAS OnDemand for Academics software. Copyright © 2021 SAS Institute Inc, Cary, NC, USA. α = 0.05.
Results
Mothers
Wistar dams presented higher-quality maternal behavior than Kyoto dams in response to MS
MS impact on mothers and their offspring occurs due to the separation period but more importantly, due to changes in their interaction afterward. Thus, we started by investigating the MS effect on maternal behavior. Total maternal behavior was higher in both MS groups (F(1,26) = 15.7, p ≤ 0.001). MS dams spent more time in maternal behavior than control dams of the same strain (Wistar: p < 0.01; Kyoto: p < 0.05) (Fig. 2A). Within Wistar dams, affiliative behavior was higher in MS group (p < 0.01), but not for Kyoto (Fig. 2B). In contrast, MS Kyoto dams had more non-affiliative contact than their control (p < 0.001) and MS Wistar (p < 0.001) (Fig. 2C). The MS impact on non-affiliative contact was strain-dependent (Strain X MS: F(1,26) = 14.0, p ≤ 0.001; MS: F(1,26) = 4.95, p < 0.05). Pup weight was only affected by strain (p < 0.001) (Fig. 2D). We also performed the Pup Retrieval Test (PRT), revealing that Kyoto dams present a higher latency to the first mother/pup contact (Strain: F(1,25) = 4.55, p < 0.05) (Fig. 2E). However, the first retrieval latency and the total retrieval time had no differences (Fig. 2F, G). Taken together, control Wistar and Kyoto dams spent similar time in maternal behavior. However, under MS conditions, the strains increased differently the time spent in maternal behavior, with higher affiliative behavior for Wistar and higher non-affiliative contact for Kyoto.
A MS increased maternal behavior in both strains. However, Wistar MS dams increased B affiliative behavior (high-quality behaviors) and Kyoto MS dams increased C non-affiliative contact time. D Wistar showed higher weight gain than Kyoto strain, but it was not impacted by MS. Data were analyzed by two-way ANOVA (2 × 2 factorial design) with Bonferroni comparisons, except for D which was analyzed with a linear mixed model (strain and MS as fixed effects and litter as random effect). E Latency to first contact was higher in the Kyoto strain. A, B, and C were data arcsine transformed. E, F, and G data were log-transformed. n = 8 (controls Wistar and Kyoto); n = 7 (MS Wistar and Kyoto, except for PRT: n = 6 for MS Wistar). Results are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. MS = maternal separation; PND = postnatal day.
Increased exploration in Wistar dams during the MS period and decreased anxiety-like behavior in Kyoto dams after the weaning period
We hypothesize that an animal that experienced maternal-parental stress is expected to alter its emotional state, and thus, mothers’ exploratory and anxiety-like behaviors were also assessed. The EPM was conducted during the MS protocol period while the OF was conducted after weaning. In the EPM, no strain or MS differences were found for the time or entries in the open arms (Fig. 3A, B). However, Kyoto dams spent more time in the EPM center than Wistar, independently of MS (Strain: Wald χ2 = 16.6, p < 0.001) (Fig. 3C) and that the Kyoto increased the total arm entries due to MS (MS: F(1,26) = 7.55, p < 0.05) (Fig. 3D). In contrast, Wistar dams displayed more rearing (Strain: F(1,26) = 50.4, p ≤ 0.001, MS: F(1,26) = 4.22, p ≤ 0.05), with MS further increasing the Wistar rearing (p < 0.05) (Fig. 3E). Head dipping followed the same pattern, with Wistar dams performing more head dipping (Strain X MS: Wald χ2 = 7.32, p < 0.01; Strain: Wald χ2 = 10.2, p ≤ 0.001; MS: Wald χ2 = 6.08, p < 0.05) and MS impacting head dipping only in Wistar (p < 0.001) (Fig. 3F).
During MS period (using EPM), A, B no differences were found regarding anxiety. MS increased D Kyoto total arm entries and increased Wistar E number of rearing and F number of head dipping. G example of Wistar and Kyoto control groups tracking in OF (Smart v3.0, Panlab, Barcelona, Spain; https://www.panlab.com/en/). After weaning (using OF), MS increased Kyoto H time in center area and J number of rearing, indicating decreased anxiety levels. FST confirmed Kyoto depressive profile since Kyoto strain showed higher time K immobility and lower L climbing comparing with Wistar. MS did not impact depression-like behaviors. Data were analyzed by two-way ANOVA (2 × 2 factorial design) with Bonferroni comparations. A, C, H, K, L Data were arcsine transformed. B, D, E, F Data were log-transformed. I, J Data were square-root transformed. n = 8 (control and MS Wistar); n = 7 (control and MS Kyoto). Results are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. MS = maternal separation.
After weaning, exploratory and anxiety-like behaviors were studied using the OF (Fig. 3G). Kyoto dams spent more time in the center (Strain X MS: F(1,26) = 5.07, p < 0.05; Strain: F(1,26) = 12.1, p < 0.01), with MS Kyoto dams spending more time in the center area than the control Kyoto (p < 0.05) and MS Wistar dams (p ≤ 0.001) (Fig. 3H). Total distance was similar in all groups (Fig. 3I) and the exploratory behavior, assessed as rearing occurrence, was different between the two strains (Strain: F(1,26) = 11.4, p < 0.01). Kyoto dams displayed fewer rearing, yet MS Kyoto dams performed more rearing than their control (p < 0.05) (Fig. 3J).
Altogether, the results obtained in the EPM suggest that anxiety-like behavior was not affected during the MS protocol. However, Wistar dams displayed more exploratory behaviors than Kyoto dams and MS further increased exploration in Wistar. The OF results suggest that Kyoto dams present less anxiety-like behavior after weaning than the Wistar and MS resulted in less anxiety-like behavior in the Kyoto dams. Although MS increased exploration only in Kyoto, Wistar dams displayed more exploratory behavior.
MS did not influence depressive-like behavior in dams
As expected due to their strain characteristics, Kyoto dams spent more time immobile in comparison with Wistar dams in the FST (F(1,26) = 36.3, p < 0.001) (Fig. 3K). Consistently, Kyoto dams also spent less time climbing (F(1,26) = 43.5, p < 0.001) (Fig. 3L). Kyoto dams presented more depressive-like behaviors than Wistar, with no observed effect from MS, which may be due to a ceiling effect since the Kyoto control already spent most of the test immobile.
MS increased oxytocin expression in the hypothalamus of Kyoto dams
Since maternal behavior is strongly influenced by oxytocin, we analyzed the expression of oxytocin in the hypothalamus of dams. Surprisingly, MS Kyoto dams presented higher oxytocin expression (Strain X MS: F(1,16) = 6.60, p < 0.05) (Fig. 4A). Because altered levels of oxytocin may result in altered expression of OXTR in oxytocin projection regions, we evaluated the expression of this receptor in oxytocin target regions that are recognized as relevant for regulation of maternal behavior, such as the PFC, the hippocampus, and the amygdala. No differences were observed in the OXTR expression (Fig. 4B–D).
After weaning, MS increased Kyoto A oxytocin levels, in agreement with the decrease of anxiety observed. E Kyoto showed high levels of TNF than Wistar strain. Quantification of A oxytocin (pg/ml) levels in hypothalamus by ELISA; OXTR in B PFC, C hippocampus, and D amygdala by Western blotting; E TNF (pg/ml), F IL-6 (pg/ml), and (G) IL-1β (pg/ml) levels in hippocampus by ELISA. Data were analyzed by two-way ANOVA (2 × 2 factorial design) with Bonferroni comparisons. A–C, F, G data were log-transformed. n = 5, except for B MS Kyoto, and C, D for control Wistar: n = 4. Results are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. MS = maternal separation.
Kyoto dams present higher TNF in the hippocampus than Wistar dams
As pro-inflammatory cytokines can influence anxiety- and depressive-like behaviors, we analyzed the expression of TNF, IL-6, and IL-1β in the hippocampus. Kyoto dams presented higher expression of TNF (Wald χ2 = 18.3, p < 0.001) (Fig. 4E). Concerning IL-6 (Fig. 4F) and IL-1β (Fig. 4G) no significant differences were observed.
Offspring
MS worsened the spatial learning and the reference memory in Kyoto adolescents
We evaluated the offspring cognitive ability using the MWM. Kyoto adolescents displayed reduced spatial learning when compared to the Wistar group (represented by a reduction in latency over the learning days) (Fig. 5A). MS Kyoto performed poorly than the Kyoto, with MS Kyoto female adolescents presenting longer latency to the target (Strain: Chi-Square(1) = 157.26, p < 0.0001, MS:Chi-Square(1) = 10.12, p < 0.01, Strain X MS:Chi-Square(1) =11.59, p < 0.001, Sex: Chi-Square(1) = 6.11, p < 0.05, Strain X Sex: Chi-Square(1) = 5.28, p < 0.05, Strain X Days:Chi-Square(1) = 57.78, p < 0.0001) (Fig. 5A). During the probe test Kyoto performed poorly than Wistar, and a main sex effect was only observed for the total traveled distance (Fig. 5C–G). The mean distance to the target was higher for the Kyoto strain and MS worsened both strains (Strain, Chi-Square(1) = 60.85, p < 0.0001; MS, Chi-Square(1) = 7.74, p < 0.01) (Fig. 5C). Kyoto adolescents also spent less time in the target quadrant (strain, Chi-Square(1) = 246.00, p < 0.0001), with the MS Kyoto group spending even less time in that quadrant (Strain X MS Chi-Square(1) =20.64, p < 0.0001; z value = 4.19, p < 0.0001) (Fig. 5D). Regarding the latency to enter the target quadrant, Kyoto presented higher latency to enter it (Chi-Square(1) = 387.79, p < 0.0001), with the MS Kyoto showing even higher latency (Strain X MS Chi-Square(1) =7.81, p < 0.01; z value = −4.16, p < 0.0001) (Fig. 5E). A strain effect was also observed for target crossings, which were decreased in Kyoto adolescents (Chi-Square(1) =38.72, p < 0.0001) (Fig. 5F). For total traveled distance we found main effects of strain, MS and sex, plus interactions between them (Strain: Chi-Square(1) =6772.47, p < 0.0001, MS:Chi-Square(1) =458.24, p < 0.0001, Strain X MS: Chi-Square(1) = 204.40, p< 0.0001, Sex: Chi-Square(1) =105.69, p < 0.0001, Strain X Sex: Chi-Square(1) =6.64, p < 0.01, MS X Sex: Chi-Square(1) = 5.55, p < 0.05; Strain X MS X Sex Chi-Square(1) = 135.54, p < 0.0001), with the Kyoto strain traveling a shorter distance, and with MS groups traveling less then their respective controls (Wistar vs MS Wistar, z value = 6.41, p < 0.0001; Kyoto vs MS Kyoto, z value = 21,26, p < 0.0001). Upon that, we also found sex differences within both strains (Male Wistar vs Female Wistar, z value = −11,69, p < 0.0001; Male Kyoto vs Female Kyoto, zvalue = −4.60, p < 0.0001), and between MS within each strain, with males traveling less than females (Control Male Wistar vs Control Female Wistar, z value = −14,48, p < 0.0001; MS Male Wistar vs MS Female Wistar, z value = −2,26, p = 0.3138; Control Male Kyoto vs Control Female Kyoto, z value = −2.95, p = 0.0634; MS Male Kyoto vs MS Female Kyoto, z value = −8.42, p < 0.0001) (Fig. 5G).
MS increased Kyoto A latency to reach the platform, showing learning impairment. Regarding probe day (B–G) MS slightly affected both strains. H MS-induced poor performance in working memory. J VGlut expression was higher in Kyoto strain. K MS led to an increase of BDNF level on Wistar, which may be related to the increase of affiliative behavior received by their mothers. M MS altered IL1-β levels on Kyoto that can be associated with cognitive impairments presented. Quantification of I PSD95 and J VGlut in hippocampus by Western blotting; K BDNF (pg/ml), L IL-6 (pg/ml), and M IL-1β (pg/ml) levels in hippocampus by ELISA. Generalized linear models with 3 factors (strain: Wistar/Kyoto, MS: control/MS, and sex: male/female) and their interactions were used. Time was added as a fourth factor for learning and working memory results. A, F–M used models with normal distribution and link function = identity, C–E used Poisson distribution with link function = log. A, C–G n = 22. I–M n = 16, except for I n = 13 for control Wistar and Kyoto, n = 11 for MS Wistar and Kyoto; K n = 12 for control Wistar and MS Kyoto, L n = 15 for control Wistar and n = 14 for MS Kyoto, M n = 15 for MS Kyoto. Results are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. MS = maternal separation; PND = postnatal day.
Under the work memory protocol, the Kyoto presented a worse performance, with MS-induced changes resulting in an even poor performance (Strain: Chi-Square(1) = 357.68, p < 0.0001, MS: Chi-Square(1) = 21.47, p < 0.0001, Trial Chi-Square(1) = 146.64, p < 0.0001; Strain X MS: Chi-Square(1) = 11.81, p < 0.001; Strain X Trial: Chi-Square(1) = 68.71, p < 0.0001; Strain X MS X Trial: Chi-Square(1) = 8.72, p < 0.05) (Fig. 5H). For this test we also found sex differences for the Kyoto strain, where MS males performed better then MS females, independently of the trial (Sex, Chi-Square(1) = 14.93, p < 0.0001, Strain X Sex: Chi-Square(1) = 8.40, p < 0.01, MS X Sex; Chi-Square(1) = 9.39, p < 0.01; Strain X MS X Sex, Chi-Square(1) = 20.36, p < 0.0001; MS Male Kyoto vs MS Female Kyoto, z value = 7.24, p < 0.0001) (Fig. 5H).
Altogether, we observed that Kyoto adolescents presented reduced spatial learning, reference and working memory in comparison with age-matched Wistar, with MS worsening their performance, particularly in females.
Kyoto adolescents presented higher levels of vGlut1 expression
To better understand the observed learning and memory deficits, we analyzed the expression of two different glutamatergic synaptic markers the postsynaptic density protein 95 (PSD95), which is a well-characterized and abundant scaffold protein present in the glutamatergic postsynaptic terminal, and the vesicular glutamate transporter 1 (vGlut1) that is a presynaptic transporter protein, mediating glutamate uptake into synaptic vesicles, the expression levels of these transporter determines the vesicle load in glutamate, shaping the efficacy of glutamatergic neurotransmission.
For PSD95 hippocampal expression levels, no differences between strains, or resulting from MS were observed (Fig. 5I). Regarding the expression levels of vGlut1, we found only strain-related differences, with Kyoto adolescents presenting increased vGlut1 levels (Strain, Chi-Square(1) = 4.23, p < 0.05) (Fig. 5J). These changes in vGlut1 may be associated with strain differences reported for the MWM assessments.
MS increased brain-derived neurotrophic factor (BDNF) expression in Wistar but not in Kyoto adolescents
BDNF is a crucial mediator of neuronal plasticity and a key factor for synapse formation/maintenance during development, as well as for memory processes. BDNF expression was impacted by MS (Chi-Square(1) = 16.26, p < 0.0001) and an interaction between Strain and MS (Chi-Square(1) = 14.37, p < 0.001), which translates in MS-induced increase in BDNF in Wistar adolescents when compared with their control (z-value = −5.93, p < 0.001) (Fig. 5K). This BDNF increase was previously linked to early aversive experiences that may trigger adaptive processes, allowing better adaptation to later events [44]. However, it is also possible that the increase in BDNF may result from improved maternal care (Fig. 2).
MS decreased IL-1β expression in Kyoto adolescents
Because BDNF levels may associate with altered inflammatory markers, and pro-inflammatory cytokines can influence learning, memory and shape the neuronal circuit, we decided to evaluate IL-6 and IL-1β expression. Kyoto adolescents presented lower IL-6 levels (strain: Chi-Square(1) = 19.44, p < 0.0001), which were further decreased by MS in females (MS X Sex: Chi-Square(1) = 4.65, p < 0.05, z-value = 2.88, p < 0.05) (Fig. 5L). For IL-1β, we found differences due to strain, MS and sex M (Strain, Chi-Square(1) = 28.35, p < 0.0001; MS, Chi-Square(1) = 278.01, p < 0.0001; strain X MS, Chi-Square(1) = 751.09, p < 0.0001; Sex, Chi-Square(1) = 546.10, p < 0.0001; Strain X Sex, Chi-Square(1) = 671.26, p < 0.0001, MS X Sex, Chi-Square(1) = 8.59, p < 0.01; Strain X MS X Sex, Chi-Square(1) = 39.76, p < 0.0001) (Fig. 5M), with MS having opposite effects in Wistar, where MS increases IL-1β, and Kyoto, where MS decreases it. In addition, when considering sex differences, MS male Wistar presented higher IL-1β than females (MS Male Wistar vs MS Female Wistar, z value = 3.52, p < 0.05) (Fig. 5M). Likewise, in the Kyoto group, males also presented higher IL-1β with or without MS (Control Male Kyoto vs Control Female Kyoto, z value = 28.36, p < 0.0001; MS Male Kyoto vs MS Female Kyoto, z value = 20,47, p < 0.0001) (Fig. 5M). These suggest that a worse cognitive performance may associated with lower levels of pro-inflammatory markers. Previous studies have determined the association between hippocampal-dependent learning deficits and reduced IL-1β [45].
Discussion and conclusion
This study explored the impact of MS on the maternal behavior of dams with different vulnerabilities to depressive-like behavior and explored its consequences on adolescent cognitive performance. Considering the high comorbidity between mothers´ depression and low maternal care [1], one could predicted that MS would reduce maternal behavior in depressive-like Kyoto mothers. However, confirming the results reported in publications that reviewed this issue [4, 46], we observed an increase in maternal behavior, in both strains, in response to MS. Interestingly, each strain displayed different strategies for handling maternal stress: depressive-like mothers (Kyoto) spent more time in contact (simple physical contact) with pups, whereas non-depressive mothers (Wistar) spent more time directing their behavior towards pups, performing more affiliative behaviors (licking, grooming and nursing). These affiliative behaviors are the most common pup-oriented maternal behaviors [12] and they crucial for mother-pup attachment [47]. In contrast, during simple physical contact (skin contact), mothers lie over their pups, but without offspring-oriented behavior. The increased contact of Kyoto dams with offspring suggests that the mother keeps the offspring close, while keeping a watch on the environment around her, likely because she does not know when the pups will be taken from her again. This behavior in depressive-like rat dams seems to be a form of maternal defense to protect pups from unpredictable stress (MS). In fact, contact behavior was previously reported as an alternative maternal defense behavior towards pups when a threat was present [48]. These differences in maternal behavior seem to be due to distinct maternal experiences and/or distinct internal states of the dams. Our results showed that maternal depressive-like traits played an important role regarding how each dam adjusted its maternal behavior in response to maternal stress. In line with these results, a recent study, in humans, showed that mothers with personality disordered traits do not perceive themselves as having bonding impairments with their infants, although they are less sensitive during interactions [49]. This highlights the relevance of genetic components in the expression of maternal behavior, but further studies are needed to elucidate gene-environment interactions in depression/maternal behavior.
Of note, MS-related differences in maternal behavior did not impact pups’ weight gain (a measure of growth efficiency), which further supports a previous study that did not find differences between Wistar and Kyoto offspring’s physical development after MS [50]. It was also reported that offspring of low-licking mothers do not differ in weaning weight and survival rate [51]. Thus, even though the Kyoto maternal behavior appears to lack quality, these mothers exhibit awareness towards their pups and do not compromise the offspring’s physical health.
Maternal care variations are associated with differences in oxytocin and OXTR expression in the brain [52]. However, we did not find changes in OXTR in the analyzed regions, but we showed, for the first time, that when a depressive-like dam is exposed to maternal stress (MS), the hypothalamic expression of oxytocin is augmented. In accordance, previous findings reported that skin contact between mother and pups, but not maternal licking or grooming, was positively related to pups’ hypothalamic oxytocin [53, 54]. A similar mechanism can be happening in mothers and help explaining our observations. Oxytocin release seems to promote social cohesion in the mother-offspring dyad, serving as a defensive behavior toward potential and unpredictable threats. Considering the social salience hypothesis of oxytocin [55], the oxytocin increase in depressive mothers could enhance social sensibility [56] to negative social cues (offspring absence), eliciting a more protective behavior from depressive mothers, as a defense against a potentially dangerous intruder. The mechanism by which oxytocin affects social behavior depends both on contextual and individual factors [57], which seems to influence the sensitivity and interpretation of the emotional meaning or relevance of an event.
In the present study, MS Kyoto dams, in combination with higher levels of hypothalamic oxytocin, displayed decreased anxiety-like behavior decrease in the OF and increased arm entries in the EPM, suggesting exploratory behavior disinhibition, likely because of lower anxiety. This outcome supports studies reporting an oxytocin anxiolytic effect [58, 59] and an oxytocin increase in response to conditioned fear and restrain [60, 61]. In agreement, Light and colleagues (2004) [62] suggested that oxytocin release during stressful situations alleviates physiological stress. The decreased anxiety-like behavior observed in depressive-like mothers can be a result of increased oxytocin. Of note, according to a recent publication, that reported that more resilient rats (as the Wistar strain) no longer display FST effects in corticosterone, BDNF or IL-1β levels, 24 h after the test, while more susceptible rats (such as the Kyoto strain) still display increased corticosterone and IL-1β at the same time point, is also possible that the FST performed before dams’ sacrifice, may somehow contribute to differences in oxytocin [63].
Data from human studies support that, under great psychosocial stress, mothers with higher oxytocin levels present fewer anxiety symptoms, suggesting that oxytocin may protect women in stressful situations from developing major depression [64, 65]. Moreover, human studies share a common assumption that mothers with higher oxytocin levels present fewer depressive symptoms, compared to mothers with lower oxytocin levels, and this supports a model where low oxytocin levels represent a causal effect of Postpartum Depression (PPD) rather than a consequence [66]. However, there are also inconsistent findings in this issue, that may be due to discrepancies in sample size, differences in assessment timepoints and in the methodologies used to measure both oxytocin and depressive symptoms. Our and other studies [67] are beginning to shed light on the complex nature of oxytocin’s effect in depression, bringing a new perspective in which the oxytocin’s role is dependent on the individual experience, such as lack of social support and teenage parenting, or single parenthood. In this context, the role of oxytocin on PPD needs further investigation.
Wistar mothers increased licking, grooming, and nursing behaviors due to MS, with no impact on the oxytocinergic system. These results suggest that Wistar dams increased pro-social behavior to counteract MS effects, exhibiting a more positive emotional regulation in coping with maternal stress. These dams did not reveal changes in anxiety-like behavior due to MS, but interestingly increased exploration (represented by rearing and head dipping). Moreover, the lack of increased oxytocin expression in the hypothalamus of MS Wistar mothers corroborates the idea that Wistar mothers were more emotionally regulated, given that increased oxytocin levels in the hypothalamus are part of the physiological response to social stress [62]. Of note, attenuation of the anxiety response by oxytocin occurs in individuals with poor coping, but not in individuals with adequate coping [68, 69], which is in accordance with our observations for the Kyoto and Wistar strains.
In the absence of other comorbidities, depressive disorders are related with increased levels of in central and peripheral pro-inflammatory cytokines, particularly with TNF [26]. In agreement, TNF was higher in Kyoto dams than in Wistar. Studies report that oxytocin reduces TNF production, inducing an anti-inflammatory state, however, the mechanisms involved are elusive and in vitro results seem to indicate that oxytocin does not directly regulate TNF [70].
Maternal care critically affects offspring’s brain maturation and cognitive and emotional behaviors development in mammalian species [71]. We observed that depressive-like adolescents (Kyoto) had significant learning and memory deficits. MS had higher impact in the depressive-like offspring, which suggests that MS effects on cognition depend on the interaction of both genetic (depressive trait) and environmental factors (maternal care quality and early-life stress, here represented by MS), which may explain the smaller MS impact on cognitive performance of non-depressive-like offspring raised with more licking and grooming. It is also important to note that the learning deficits observed in Kyoto exposed to MS were more pronounced in females, while working memory deficits were more pronounced in males. Considering the major role of synaptic proteins in learning and memory, the observed higher vGlut1 expression in Kyoto adolescents, seems to be consistent with the memory deficits shown in the behavioral test. Our data highlight the relevance of vGlut1 in memory and reinforces previous studies [72]. On the other hand, Wistar adolescents presented increased BDNF in response to MS, which could had a protective role, preventing possible MS-induced learning deficits. Besides, high levels of licking and grooming have been associated with higher offspring BDNF levels [73], as such, the lack of this affiliative maternal behavior may contribute to explain the worse cognition observed in MS Kyoto adolescents.
Pro-inflammatory cytokines are critical factors for memory and cognition. Notably, IL-6 is important for spatial learning and reference memory formation [74, 75]. In agreement, the worse cognitive performance of the Kyoto adolescents was associated with reduced IL-6 expression. In agreement, the observed sex difference in learning in the Kyoto strain (MS Kyoto females presented a worse learning performance) was also associated with a decrease in IL-6. Further studies will be necessary to determine the nature of such effect. Adequate levels of IL-1β are required for learning and memory processes [76]. IL-1β expression decreased in the hippocampus of MS Kyoto adolescents, particularly in females, which performed poorly in the MWM. This is in accordance with previous studies, showing poorer performances in mice lacking the IL-1 receptor [45] or after the IL-1 receptor antagonist (IL-1ra) administration [77].
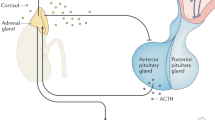
In summary, our study shows that beyond pro-social behaviors, oxytocin is involved in stress and mood disorder regulation (Fig. 6). We demonstrated that when a depressive mother is exposed to maternal stress, the release of endogenous hypothalamic oxytocin is triggered and leads to an altered perception of the environment (as insecure). Our study, in combination with previous studies [53, 54, 78], suggests that oxytocin not only increases sensitivity to environmental cues, but it may also amplify the social stress communication contributing to a social synchrony bidirectional intensification. This social synchrony in an adverse situation may contribute to better interactions, enabling the refinement of social communication and consequently promoting social cohesion as a defensive behavior toward potential threats. Finally, the different maternal stress-coping styles affect the offspring’s behavior, providing important clues about how early-life stress increases the risk for poor cognitive performances and how maternal care is a possible target for intervention.
Our results showed that mothers with different depression-like vulnerabilities facing maternal stress (induced by MS) adopted different strategies. Although both strains increased the maternal behavior in response to MS, non-depressive-like mothers (Wistar) exhibited a higher quality maternal behavior (affiliative), showing a more adaptive response and more pro-social behavior (more active strategy). On the other hand, depressive-like mothers (Kyoto) displayed a more passive/defensive strategy (non-adaptive response) in response to MS. The higher levels of oxytocin observed in Kyoto mothers that experienced MS seem to be part of the stress-response mechanism, which amplifies the negative perception of the environment (as insecure), leading to a more defensive behavior by keeping the pups very close to them and away from outsiders (out-group anti-social behavior). This increase in oxytocin expression seems to decrease the anxiety-like state in Kyoto dams in response to MS. Furthermore, the quality of maternal behavior observed in non-depressive-like mothers (Wistar) after MS seems to protect the cognitive performance of adolescent rats from the negative effects of MS, leading to an increase of BDNF expression in the offspring hippocampus. On the other hand, depressive-like adolescents showed lower resilience to MS effects, exhibiting worst performance in the cognitive tests and alteration in the IL-1β levels.
References
Righetti-Veltema M, Conne-Perreard E, Bousquet A, Manzano J. Postpartum depression and mother-infant relationship at 3 months old. J Affect Disord. 2002;70:291–306.
Vedam S, Stoll K, Rubashkin N, Martin K, Miller-Vedam Z, Hayes-Klein H, et al. The Mothers on Respect (MOR) index: measuring quality, safety, and human rights in childbirth. SSM - Popul Health. 2017;3:201–10.
Fleming AS, O’Day DH, Kraemer GW. Neurobiology of mother–infant interactions: experience and central nervous system plasticity across development and generations. Neurosci Biobehav Rev. 1999;23:673–85.
Alves RL, Portugal CC, Summavielle T, Barbosa F, Magalhaes A. Maternal separation effects on mother rodents’ behaviour: a systematic review. Neurosci Biobehav Rev. 2020;117:98–109.
Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–36.
Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–38.
Pedersen CA, Boccia ML. Oxytocin antagonism alters rat dams’ oral grooming and upright posturing over pups. Physiol Behav. 2003;80:233–41.
Ferris CF. Functional magnetic resonance imaging and the neurobiology of vasopressin and oxytocin. In: Neumann ID, Landgraf R, editors. Progress in Brain Research, Vol. 170. Elsevier; 2008, p. 305–20.
Amini-Khoei H, Mohammadi-Asl A, Amiri S, Hosseini M-J, Momeny M, Hassanipour M, et al. Oxytocin mitigated the depressive-like behaviors of maternal separation stress through modulating mitochondrial function and neuroinflammation. Prog Neuro-Psychopharmacol Biol Psychiatry. 2017;76:169–78.
Newport DJ, Stowe ZN, Nemeroff CB. Parental depression: animal models of an adverse life event. Am J Psychiatry. 2002;159:1265–83.
Mundorf A, Bölükbas I, Freund N. Maternal separation: does it hold the potential to model consequences of postpartum depression? Dev Psychobiol. 2022;64:e22219.
Orso R, Creutzberg KC, Wearick-Silva LE, Wendt Viola T, Tractenberg SG, Benetti F, et al. How early life stress impact maternal care: a systematic review of rodent studies. Front Behavl Neurosci. 2019;13:197.
Kaidbey JH, Ranger M, Myers MM, Anwar M, Ludwig RJ, Schulz AM, et al. Early life maternal separation and maternal behaviour modulate acoustic characteristics of rat pup ultrasonic vocalizations. Sci Rep. 2019;9:19012.
Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci USA. 2001;98:12736.
Holubová A, Lukášková I, Tomášová N, Šuhajdová M, Šlamberová R. Early postnatal stress impairs cognitive functions of male rats persisting until adulthood. Front Behavl Neurosci. 2018;12:176.
Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63.
Aisa TorderaR, Lasheras B, Del Rio J, Ramirez MJ. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 2008;154:1218–26.
Caldji C, Liu D, Sharma S, Diorio J, Francis D, Meaney MJ, et al. Development of individual differences in behavioral and endocrine responses to stress: role of the postnatal environment. 2011. In Comprehensive Physiology (ed. R.Terjung), pp. 271–292. Hoboken, NJ: John Wiley & Sons, Inc
Gehrand AL, Hoeynck B, Jablonski M, Leonovicz C, Cullinan WE, Raff H. Programming of the adult HPA axis after neonatal separation and environmental stress in male and female rats. Endocrinology. 2018;159:2777–89.
Roque S, Mesquita AR, Palha JA, Sousa N, Correia-Neves M. The behavioral and immunological impact of maternal separation: a matter of timing. Front Behavl Neurosci. 2014;8:192.
Johnson FK, Kaffman A. Early life stress perturbs the function of microglia in the developing rodent brain: New insights and future challenges. Brain Behav Immun. 2018;69:18–27.
Catale C, Gironda S, Lo Iacono L, Carola V. Microglial function in the effects of early-life stress on brain and behavioral development. J Clin Med. 2020;9:468.
Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology. 2016;233:1637–50.
Kumar A. Editorial: Neuroinflammation and cognition. Front Aging Neurosci. 2018;10:413–413.
Hurley LL, Tizabi Y. Neuroinflammation, neurodegeneration, and depression. Neurotox Res. 2013;23:131–44.
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57.
Himmerich H, Fulda S, Linseisen J, Seiler H, Wolfram G, Himmerich S, et al. Depression, comorbidities and the TNF-alpha system. Eur Psychiatry. 2008;23:421–9.
Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF, et al. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS ONE. 2013;8:e58488–e58488.
Aleksandrova LR, Wang YT, Phillips AG. Evaluation of the Wistar-Kyoto rat model of depression and the role of synaptic plasticity in depression and antidepressant response. Neurosci Biobehav Rev. 2019;105:1–23.
Will CC, Aird F, Redei EE. Selectively bred Wistar–Kyoto rats: an animal model of depression and hyper-responsiveness to antidepressants. Mol Psychiatry. 2003;8:925–32.
Millard SJ, Weston-Green K, Newell KA. The Wistar-Kyoto rat model of endogenous depression: A tool for exploring treatment resistance with an urgent need to focus on sex differences. Prog Neuro-Psychopharmacol Biol Psychiatry. 2020;101:109908.
Gentsch C, Lichtsteiner M, Feer H. Genetic and environmental influences on behavioral and neurochemical aspects of emotionality in rats. Experientia. 1988;44:482–90.
Alves RL, Oliveira P, Lopes IM, Portugal CC, Alves CJ, Barbosa F, et al. Early-life stress affects drug abuse susceptibility in adolescent rat model independently of depression vulnerability. Sci Rep. 2020;10:13326.
Aguggia JP, Suarez MM, Rivarola MA. Early maternal separation: neurobehavioral consequences in mother rats. Behav Brain Res. 2013;248:25–31.
Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–8.
Vorhees W. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58.
Tata. Maternal separation as a model of early stress: effects on aspects of emotional behavior and neuroendocrine function. Hellenic J Psychol. 2012;9:84–101.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1997.
Clarke M, Pearl CA. Alterations in the estrogen environment of the testis contribute to declining sperm production in aging rats. Syst Biol Reprod Med. 2014;60:89–97.
Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharm Sci. 2003;91:267–70.
Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–81.
Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, et al. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci USA. 2004;101:7158.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82.
Récamier-Carballo S, Estrada-Camarena E, López-Rubalcava C. Maternal separation induces long-term effects on monoamines and brain-derived neurotrophic factor levels on the frontal cortex, amygdala, and hippocampus: differential effects after a stress challenge. Behav Pharm. 2017;28:545–57.
Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, et al. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–34.
Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17.
Keverne Curley. Vasopressin, oxytocin and social behaviour. Curr Opin Neurobiol. 2004;14:777–83.
Rickenbacher E, Perry RE, Sullivan RM, Moita MA. Freezing suppression by oxytocin in central amygdala allows alternate defensive behaviours and mother-pup interactions. Elife. 2017;6:e24080.
Nath S, Pearson RM, Moran P, Pawlby S, Molyneaux E, Howard LM. Maternal personality traits, antenatal depressive symptoms and the postpartum mother-infant relationship: a prospective observational study. Soc Psychiatry Psychiatr Epidemiol. 2020;55:621–34.
Rana S, Pugh PC, Jackson N, Clinton SM, Kerman IA. Inborn stress reactivity shapes adult behavioral consequences of early-life maternal separation stress. Neurosci Lett. 2015;584:146–50.
Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–71.
Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–6.
Kojima S, Alberts JR. Maternal care can rapidly induce an odor-guided huddling preference in rat pups. Developmental Psychobiol. 2009;51:95–105.
Kojima S, Stewart RA, Demas GE, Alberts JR. Maternal contact differentially modulates central and peripheral oxytocin in rat pups during a brief regime of mother-pup interaction that induces a filial huddling preference. J Neuroendocrinol. 2012;24:831–40.
Shamay-Tsoory SG, Abu-Akel A. The social salience hypothesis of oxytocin. Biol Psychiatry. 2016;79:194–202.
Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, et al. The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38:1883–94.
Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–9.
Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–52.
Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, et al. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology. 2006;185:218–25.
Onaka T. Neural pathways controlling central and peripheral oxytocin release during stress. J Neuroendocrinol. 2004;16:308–12.
Neumann ID, Krömer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept. 2000;96:31–38.
Light KC, Grewen KM, Amico JA, Boccia M, Brownley KA, Johns JM. Deficits in plasma oxytocin responses and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addict Behav. 2004;29:1541–64.
Ruiz-Sanchez E, Lopez-Ramirez AM, Ruiz-Chow A, Calvillo M, Resendiz-Albor AA, Anguiano B, et al. Variability in behavioral phenotypes after forced swimming-induced stress in rats is associated with expression of the glucocorticoid receptor, Nurr1, and IL-1beta in the hippocampus. Int J Mol Sci. 2021;22:12700.
Garfield L, Giurgescu C, Carter CS, Holditch-Davis D, McFarlin BL, Schwertz D, et al. Depressive symptoms in the second trimester relate to low oxytocin levels in African-American women: a pilot study. Arch Women’s Ment Health. 2015;18:123–9.
Zelkowitz P, Gold I, Feeley N, Hayton B, Carter CS, Tulandi T, et al. Psychosocial stress moderates the relationships between oxytocin, perinatal depression, and maternal behavior. Horm Behav. 2014;66:351–60.
Moura D, Canavarro MC, Figueiredo-Braga M. Oxytocin and depression in the perinatal period-a systematic review. Arch Women’s Ment Health. 2016;19:561–70.
Wong SF, Cardoso C, Orlando MA, Brown CA, Ellenbogen MA. Depressive symptoms and social context modulate oxytocin’s effect on negative memory recall. Soc Cogn Affect Neurosci. 2021;16:1234–43.
Cardoso C, Linnen AM, Joober R, Ellenbogen MA. Coping style moderates the effect of intranasal oxytocin on the mood response to interpersonal stress. Exp Clin Psychopharmacol. 2012;20:84–91.
Quirin M, Kuhl J, Düsing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology. 2011;36:898–904.
Panaro MA, Benameur T, Porro C. Hypothalamic neuropeptide brain protection: focus on oxytocin. J Clin Med. 2020; 19;9:1534.
Bath K, Manzano-Nieves G, Goodwill H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Hormones Behav. 2016;82:64–71.
Ménard C, Quirion R, Vigneault E, Bouchard S, Ferland G, El Mestikawy S, et al. Glutamate presynaptic vesicular transporter and postsynaptic receptor levels correlate with spatial memory status in aging rat models. Neurobiol Aging. 2015;36:1471–82.
Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806.
Baier PC, May U, Scheller J, Rose-John S, Schiffelholz T. Impaired hippocampus-dependent and -independent learning in IL-6 deficient mice. Behav Brain Res. 2009;200:192–6.
Bialuk I, Taranta A, Winnicka MM. IL-6 deficiency alters spatial memory in 4- and 24-month-old mice. Neurobiol Learn Mem. 2018;155:21–29.
Brennan FX, Beck KD, Servatius RJ. Low doses of interleukin-1β improve the leverpress avoidance performance of Sprague–Dawley rats. Neurobiol Learn Mem. 2003;80:168–71.
Yirmiya R, Winocur G, Goshen I. Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiol Learn Mem. 2002;78:379–89.
Kojima S, Alberts JR. Warmth from skin-to-skin contact with mother is essential for the acquisition of filial huddling preference in preweanling rats. Dev Psychobiol. 2011;53:813–27.
Funding
This work was financed through FCT—Fundação para a Ciência e a Tecnologia, I.P., Project UIDB/04293/2020; and the FEDER—Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020—Operational Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and FCT - Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior in the framework of the project POCI-01-0145-FEDER-032231 (PTDC/SAU-TOX/32231/2017); and FCT and Orçamento do Estado in the framework of the project EXPL-AMAGALHÃES - IF/00753/2014/CP1241/CT0005. RLA was supported by an FCT grant (PD/BD/114266/2016). CCP holds an employment contract financed by national funds through FCT - Fundação para a Ciência e a Tecnologia, I.P., in the context of the program-contract described in paragraphs 4, 5 and 6 of art. 23 of Law no. 57/2016, of August 29, as amended by Law no. 57/2017 of July 19. AM was supported by FCT (IF/00753/2014).
Author information
Authors and Affiliations
Contributions
RLA and AM conceived the study and edited the paper. RLA, AM, and CCP conceived the experimental approach. RLA, CCP, CJA, and AM conducted the experiments and RLA, PO, and IML analyzed the results. RLA, CCP, IML, FB, TS, and AM co-wrote the manuscript. All authors critically discussed the results and reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors report no conflicts of interest. The results and discussion here presented were previously posted as a preprint at bioRxiv on February 24, 2021 (https://doi.org/10.1101/2021.02.23.432580).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alves, R.L., Portugal, C.C., Lopes, I.M. et al. Maternal stress and vulnerability to depression: coping and maternal care strategies and its consequences on adolescent offspring. Transl Psychiatry 12, 463 (2022). https://doi.org/10.1038/s41398-022-02220-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-022-02220-5