Abstract
Former prospective studies showed that the occurrence of relapse in Major Depressive Disorder (MDD) is associated with volume loss in the insula, hippocampus and dorsolateral prefrontal cortex (DLPFC). However, these studies were confounded by the patient’s lifetime disease history, as the number of previous episodes predict future recurrence. In order to analyze neural correlates of recurrence irrespective of prior disease course, this study prospectively examined changes in brain structure in patients with first-episode depression (FED) over 2 years. N = 63 FED patients and n = 63 healthy controls (HC) underwent structural magnetic resonance imaging at baseline and after 2 years. According to their disease course during the follow-up interval, patients were grouped into n = 21 FED patients with recurrence (FEDrec) during follow-up and n = 42 FED patients with stable remission (FEDrem). Gray matter volume changes were analysed using group by time interaction analyses of covariance for the DLPFC, hippocampus and insula. Significant group by time interactions in the DLPFC and insula emerged. Pairwise comparisons showed that FEDrec had greater volume decline in the DLPFC and insula from baseline to follow-up compared with FEDrem and HC. No group by time interactions in the hippocampus were found. Cross-sectional analyses at baseline and follow-up revealed no differences between groups. This longitudinal study provides evidence for neural alterations in the DLPFC and insula related to a detrimental course in MDD. These effects of recurrence are already detectable at initial stages of MDD and seem to occur without any prior disease history, emphasizing the importance of early interventions preventing depressive recurrence.
Similar content being viewed by others
Introduction
Following the first episode, about 15-35% of patients with Major Depressive Disorder (MDD) develop recurrent episodes within the first years [1]. The number of lifetime episodes, severity of preceding episode and presence of subclinical residual symptoms have been stated as risk factors to suffer from additional recurrent episodes [2,3,4]—resulting in cumulative illness burden and chronicity in the long-term [5]. The investigation of neural correlates of recurrence in MDD may help to advance our understanding of the underlying pathological mechanisms, which could in future support the early identification of patients at high risk for an unfavorable disease course, and potentially improve treatments. There is a high need for longitudinal studies investigating the directionality of the associations between brain structure and disease course to clarify the current uncertainty of whether reduced brain volumes represent a cause or a consequence of recurrence. In this context, individuals with first-episode depression (FED) constitute a promising, well-characterized subgroup in which potentially confounding factors of prior disease history and treatment may have less impact on the underlying neural mechanisms of recurrence.
While cross-sectional research in MDD repeatedly showed that decreased gray matter volumes (GMV) and (sub-)cortical thickness are associated with more severe lifetime disease trajectories [6,7,8,9,10], the direct interplay between neural changes and recurrence remains unclear. Previous longitudinal studies reported morphometric changes [11], for example, in the dorsolateral prefrontal cortex (DLPFC), insula, hippocampus, and anterior cingulate cortex, showing greater GMV decline in dependence of more detrimental disease courses [12,13,14,15,16]. In this context, the occurrence of depressive relapse as a clearly distinguishable marker of disease progression has been specifically linked to GMV decline in cortical thickness and surface area in the insula and DLPFC [13, 14, 17]. Moreover, reductions of hippocampal GMVs in relation to chronic disease courses or residual symptomatology have been reported [12, 15, 16]. These findings clearly point toward adverse effects of disease progression on the morphology of reported brain regions. Nonetheless, other longitudinal studies did not find brain structural changes being related to disease trajectories [11, 18, 19]. Patients from all these prospective studies had experienced multiple episodes before study assessment [11], exacerbating the challenge to disentangle the distinct neural mechanisms of disease history and future recurrence.
In an initial longitudinal study on early depressive recurrence [20], the authors neither found longitudinal changes in FED patients suffering recurrence compared with FED patients remaining in full remission during the study interval, nor significant cross-sectional GMV differences between FED patients and HC at baseline and follow-up, respectively [20]. Notably, only a small sample of 27 FED patients and 17 HC was investigated. The results of this study stand in contrast to former literature reporting GMV alterations in FED patients compared with HC [21,22,23,24], and GMV changes associated with disease progression [8,9,10,11,12,13,14,15,16].
To overcome some of the above-mentioned challenging aspects regarding the longitudinal investigations of brain structure and disease course, the aim of this study was to prospectively investigate a larger sample of FED patients over a two-year span in order to enhance our understanding of potential neural risk factors for and consequences of recurrence without bias of disease history. To this end, we utilized data of voxel-based morphometry (VBM), cortical thickness, and surface area (Freesurfer, https://surfer.nmr.mgh.harvard.edu/) as such have already been investigated in relation to depressive recurrence [12,13,14,15,16,17, 25]. Based on literature reporting that the insula, hippocampus, and DLPFC seem to be particularly affected by the incidence of recurrence or disease progression [13, 14, 17], a ROI-approach was utilized. We expected that (a) FED patients with recurrence would exhibit more volume/thickness/surface decline compared with FED patients without recurrence and HC, (b) all FED patients would show less volume/thickness/surface compared with HC at baseline and follow-up, and, (c) baseline volume/thickness/surface differences between FED patients with and without recurrence may reveal a potential neural risk factors precipitating early recurrence.
Materials and methods
Participants and study design
For this study, participants from the Marburg-Münster-Affective-Cohort-Study (MACS) were selected. Baseline data were acquired between 2014/09 and 2016/09. Between 2016/10 and 2018/08, all participants were re-assessed about 2 years after their first study participation (mean = 2.10 years, SD = 0.16 years). Recruitment was conducted via psychiatric hospitals in Münster and Marburg, newspaper advertisement and flyers. General exclusion criteria comprised any history of neurological, autoimmune or cardiovascular disease, cancer, current pregnancy, head trauma, psychotic, schizoaffective and/or bipolar disorder, substance and alcohol dependence, intelligence quotient below 80 or common MRI contraindications (e.g. pacemakers, metal implants). Inclusion criteria for this study were age between 18 and 65 years at baseline and complete data of structural magnetic resonance imaging (MRI) and clinical interviews at both time points. The present study was independent of a previous 2-year follow-up study of our working group with no overlap in participants, scanners and imaging methods [13].
During face-to-face interviews, clinical diagnoses or the lack thereof were verified with the Structured Clinical Interview (SCID-I) according to DSM-IV-TR criteria [26] at both time points. These criteria resulted in a sample of n = 65 patients fulfilling criteria for a single (lifetime or current) episode of MDD at baseline. To enhance precision of each individual’s disease course, a life-chart method was utilized during follow-up interviews [27] to collect information about recurrence of depressive episodes, duration of depressive episodes and inpatient treatment during the study interval. Depending on the disease course during the interval, FED patients were divided into two subgroups: n = 21 patients experiencing at least one recurrent depressive episode (FEDrec) and n = 42 patients without subsequent episodes following the index episode and thus achieving or staying in remission (FEDrem). N = 2 FED patients experienced a chronic disease course during the follow-up interval and were therefore excluded as they could not be clearly assigned to one of the two patient groups. In order to fulfill criteria of discriminable recurrent illness episodes, patients had to remain symptom-free for at least two months before experiencing a new episode. In the case of recurrent depressive episodes, DSM-IV-TR criteria were applied to verify clinical pertinence. N = 63 healthy controls (HC), who did not fulfil any criteria of a lifetime or current psychiatric diagnosis were matched to the patients with respect to sex and age using the MatchIt package in R (Version 3.0.2, Table 1) [28].
At both time points, the presence and severity of depressive symptomatology was assessed with the Hamilton Depression Rating Scale (HDRS, [29]) and information on current psychopharmacological treatment was obtained. Psychopharmacological agents were combined into a medication load index, as previously utilized [30, 31]. To calculate the index, each active agent was coded according to the recommended average daily dose (0 = absent,1 = low/average dose, 2 = high dose) and summed up for each patient and each time point.
The study was conducted in accordance with the ethical guidelines and regulations of the Declaration of Helsinki and was approved by the Ethics Committees of the Medical Faculties of the Universities of Münster (AZ: 2014-422-b-S) and Marburg (AZ: 07/14). Prior to study participation, written informed consent was obtained and participants received financial compensation afterwards.
Image acquisition
At both time points, T1-weighted high-resolution anatomical data were acquired at 3 T MRI scanners using three-dimensional (3D) fast gradient echo sequences (MPRAGE). Detailed information on acquisition parameters is provided in Supplement 1. Between baseline and follow-up, the body-coil at the Marburg site was exchanged, resulting in two dummy-coded variables (body-coil change yes vs. no) accounting for the scanner settings with Münster as reference category. Details of the quality assurance protocol and scanner harmonization of the present study can be found elsewhere [32].
Pre-processing of VBM data
All T1-weighted images were pre-processed using the default settings of the longitudinal pipeline implemented in the CAT12-toolbox (http://www.neuro.uni-jena.de/cat,version r1742). This longitudinal VBM pipeline has been optimized to detect larger GMV changes over time intervals comprising several years. Pre-processing included the following steps using default parameters: Realignment, bias correction, tissue classification and spatial normalization to MNI-space were performed using the Geodesic Shooting algorithm. Additional modulation and warping steps were conducted as integrated into the longitudinal pre-processing pipeline of CAT12. Lastly, data were smoothed with an 8 mm full width at half-maximum Gaussian kernel. All images passed outlier detection of gray matter segmentation by using the check homogeneity function of CAT12 and visual inspections.
Pre-processing of Freesurfer Data
The longitudinal pipeline of Freesurfer (Version 5.3.) was employed to pre-process all T1-weighted structural images. Following the standard protocol for cortical parcellation and subcortical segmentation of the ENIGMA consortium (http://enigma.ini.usc.edu/protocols/imaging-protocols), we used default parameters. Quality of resulting segments was assured by visual inspections and statistical evaluation of possible outliers, not resulting in the exclusion of data. Parcellation of cortical thickness and surface area was done using the Desikan-Killiany atlas [33], and segmentation of hippocampal volumes with the Aseg atlas [34].
Statistical analyses
Descriptive and clinical data were analysed by using IBM SPSS Statistics 28 (SPSS Inc., Chicago, IL, USA). Statistical Parametric Mapping (SPM12, version 7771, Wellcome Department of Cognitive Neurology, London, UK) was used to analyze VBM data, while Freesurfer data were analysed using SPSS.
For all VBM second level analyses, the absolute threshold masking was set to 0.1 and threshold-free cluster enhancement (TFCE) implemented in the TFCE-toolbox (http://dbm.neuro.uni-jena.de/tfce,Version222) with 5000 permutations per test was applied. A statistical threshold using a family-wise-error (FWE) correction of p < 0.05 was employed. The DLPFC, hippocampus, and insula as regions of interest (ROI) were created as three separate bilateral masks. The DLPFC was defined according to Brodmann’s area 46 [35], and the insula and hippocampus according to the AAL-atlas definitions [36]. Both atlases are integrated into the WFU-pickatlas [37] in SPM12. All statistical analyses were performed separately for the three ROIs. For VBM analyses, extracted mean cluster values of significant clusters were used to calculate post-hoc pairwise comparisons in SPSS applying Bonferroni correction to account for multiple statistical tests (implying a threshold of p = 0.005 for 9 post-hoc t-tests: Three pairwise comparisons of groups at baseline, follow-up and within-group comparisons over time, respectively). Lastly, exploratory VBM whole-brain analyses were performed at a cluster threshold of k > 50 voxels, p < 0.001 uncorrected.
For Freesurfer analyses, right and left (sub-)cortical segments were used. The rostral middle frontal gyrus as a representative of the DLPFC [13], the insula and the hippocampus were chosen as ROIs. Again, all statistical analyses were performed for the ROIs separately and the Bonferroni method was applied for post-hoc t-tests.
Longitudinal analyses
For VBM data, F-tests on group by time interaction were analysed by calculating 3 × 2 analyses of covariance (ANCOVAs) including group (FEDrec, FEDrem, HC) as between-subjects factor and time (baseline, follow-up) as within-subjects factor using a second level flexible-factorial model in SPM12. A third factor “subject” was added to account for the individual constants of each participant. Age and scanner settings served as nuisance variables in the flexible-factorial models. Covariates, which do not change over time (such as sex) or underlie only subtle changes (such as total intracranial volume (TIV) [38]), are not recommended as control variables in flexible-factorial models by the CAT12 manual. The flexible-factorial model of SPM is particularly suitable for longitudinal analyses but does not allow meaningful cross-sectional contrasts. Therefore, the cross-sectional group analyses were performed for each time point in separate full-factorial ANCOVAs as described below.
For Freesurfer data, we performed 3 × 2 × 2-ANCOVAs for repeated measurements with group (FEDrec, FEDrem, HC) as between-subjects factor and time (baseline, follow-up) and hemisphere (right, left) as within-subjects factor in SPSS to calculate F-tests for the group by time interaction. Analyses of DLPFC and insula thickness included the covariates age, sex and scanner settings, and analyses of surface area were additionally controlled for TIV. For hippocampal volumes, the covariates were age, sex, scanner settings, and TIV.
Cross-sectional analyses
To investigate cross-sectional group effects and to identify potential neural risk factors for recurrence, we performed full-factorial ANCOVAs for VBM data with (FEDrec, FEDrem, HC) as between-subjects factor controlling for age, sex, TIV, and scanner settings at baseline and follow-up, separately.
For the cross-sectional analyses of Freesurfer data, we performed separate 3 × 2 ANCOVAs with a group (FEDre, FEDrem, HC) as between-subjects factor and hemisphere (right, left) as within-subjects-factor for baseline and follow-up, respectively. Included control variables were again age, sex, and scanner settings, and additionally TIV for surface area and hippocampal volumes.
Additional analyses
In case of the significant group by time effects in the main ANCOVAs of VBM and Freesurfer data, and in order to control for potentially confounding effects of pharmacotherapy, current symptomatology, and remissions status, we performed further 2 × 2 ANCOVAs for VBM and 2 × 2 × 2 ANCOVAs for Freesurfer data by (1) including only FED patients while additionally controlling for medication and HDRS scores, and (2) including only acutely depressed patients at baseline (FEDrec: n = 14, FEDrem: n = 19) while controlling for medication. All additional analyses included the previously described control variables (age, sex, scanner settings, and TIV in the case of Freesurfer surface area or hippocampal volumes).
Results
Descriptive and clinical characteristics
The groups did not differ significantly regarding age, sex, and site (Table 1). FED patient groups differed significantly in HDRS scores at baseline as well as HDRS scores, remission status and medication at follow-up. For details on medication and the presence of psychiatric comorbidities, see Supplement 2.
Longitudinal analyses
DLPFC
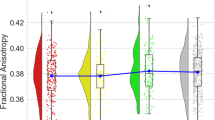
In the 3 × 2-ANCOVA of VBM data, a significant group by time interaction in the right DLPFC emerged (F(2,121) = 9.26, ptfce-FWE = 0.007, k = 230 voxels, partial η² = 0.107, Fig. 1A, B). No significant group by time interaction was found in the left DLPFC (ptfce-FWE = 0.053). Post-hoc t-tests revealed that FEDrec patients showed significant volume decline in the right DLPFC from baseline to follow-up (t(20) = 5.06, p < 0.001, 95%-CI: 0.550 to 1.642, Cohen’s d = 1.11) while no significant volume decline was found in FEDrem patients (p = 0.305) and HC (p = 0.881).
A Mean cluster values of the group by time interaction of the right dorsolateral prefrontal cortex for healthy controls, FED patients without recurrence and FED patients with recurrence. B Significant cluster of the group by time interaction in the right dorsolateral prefrontal cortex depicted at x = 46, y = 34, z = 14, with a threshold of p < 0.001, uncorrected. C Mean cluster values of the group by time interaction of the right insula for healthy controls, FED patients without recurrence and FED patients with recurrence. D Significant cluster of the group by time interaction in the right insula depicted at x = 39, y = −15, z = −6 with a threshold of p < 0.001, uncorrected. Error bars indicate one standard error.
The 3 × 2 × 2-ANCOVA of DLPFC thickness data revealed a significant group by time interaction (F(2,118) = 4.77, p = 0.010, partial η² = 0.075, Supplement 3). In line with the foregoing VBM results, FEDrec patients showed a significant decline in bilateral DLPFC thickness from baseline to follow-up (right: t(20) = 4.75, p < 0.001, Cohen’s d = 1.04, mean difference: 0.0365 mm, 95%-CI: 0.0205 to 0.0526 mm; left: t (20) = 2.73, p = 0.013, Cohen’s d = 0.59, mean difference: 0.0361 mm, 95%-CI: 0.0085 to 0.0636 mm). DLPFC volumes did not change over time in HC (right: p = 0.718; left: p = 0.631) and FEDrem patients (right: p = 0.977; left: p = 0.352). When repeating the analysis separately for both hemispheres, significant group by time interactions were found for right (F(2,118) = 3.46, p = 0.035, partial η² = 0.055) and left DLPFC thickness (F(2,118) = 3.61, p = 0.030, partial η² = 0.058).
The 3 × 2 × 2-ANCOVA of DLPFC surface area did not reveal a significant group by time interaction (p = 0.102).
Insula
The 3 × 2-ANCOVA of VBM data showed a significant group by time interaction in the right insula (F(2,121) = 11.84, ptfce-FWE = 0.036, k = 13 voxels, partial η² = 0.129, Fig. 1C, D). No significant group by time interaction effect was found in the left insula (ptfce-FWE = 0.204). Post-hoc t-tests indicated that right insula volumes decreased significantly from baseline to follow-up in FEDrec patients (t(20) = 6.77, p < 0.001, Cohen’s d = 1.47). In the FEDrem group, insula volume tended to decrease (p = 0.024), however, not statistically significant after Bonferroni correction. For HC, no significant insula volume decline from baseline to follow-up was found (p = 0.898).
The Freesurfer analyses neither revealed a significant group by time interaction in the 3 × 2 × 2-ANCOVA of insula thickness (p = 0.730) nor surface (p = 0.922).
Hippocampus
Neither the 3 × 2-ANCOVA of VBM data (ptfce-FWE = 0.309) nor the 3 × 2 × 2-ANCOVA of Freesurfer data (p = 0.391) showed a significant group by time interaction in hippocampal volumes.
Whole-brain
The exploratory VBM whole-brain analysis of the group by time interaction showed decreased GMVs in the insula, precuneus, (orbito-)frontal and temporal regions in FEDrec patients compared with HC and in temporal and frontal regions in FEDrec compared with FEDrem patients over the two year follow-up period (Supplement 4).
Cross-sectional analyses
DLPFC
In the cross-sectional analyses of DLPFC GMVs, neither a main effect of group at baseline (ptfce-FWE = 0.164) nor at follow-up (ptfce-FWE = 0.354) was found. Further, the analyses of DLPFC thickness and surface data revealed no main effects of group at baseline (thickness: p = 0.400; surface: p = 0.185) and follow-up (thickness: p = 0.998; surface: p = 0.115).
Insula
No significant group effects at baseline (ptfce-FWE = 0.784) and follow-up (ptfce-FWE = 0.336) were found for insula GMVs in VBM data. The analyses of insula thickness and surface area also revealed no main effect of group at baseline (thickness: p = 0.749; surface: p = 0.341) nor at follow-up (thickness: p = 0.945; surface: p = 0.209).
Hippocampus
The cross-sectional analyses of hippocampal volumes neither revealed a group effect at baseline (ptfce-FWE = 0.999) nor at follow-up (ptfce-FWE = 0.999) in VBM data. Freesurfer data also showed no group differences in hippocampal volumes at baseline (p = 0.068) and follow-up (p = 0.085).
Whole-brain
The exploratory VBM whole-brain analysis at baseline revealed lower GMV in temporal regions in FEDrec patients compared with HC, parietal and temporal regions in FEDrem patients compared with HC and frontal, parietal, and temporal regions in FEDrem compared with FEDrec patients (Supplement 5). At follow-up, the analysis showed decreased GMV in temporal regions and caudate nucleus in FEDrec patients compared with HC, parietal and temporal regions in FEDrem patients compared with HC and frontal, temporal, and parietal regions in FEDrem compared with FEDrec patients (Supplement 6).
Additional analyses
DLPFC
In the 2 × 2-ANCOVAs, the group by time interaction remained bilaterally significant when (1) additionally controlling for psychiatric medication and HDRS scores (right: F(1,57) = 14.81, ptfce-FWE = 0.019, k = 160 voxels, partial η2 = 0.132; left: F(1,57) = 11.14, ptfce-FWE = 0.039, k = 6 voxels, partial η2 = 0.094), and (2) only including acutely depressed patients (right: F(1,28) = 15.55, ptfce-FWE = 0.037, k = 72 voxels, partial η2 = 0.277; left: F(1,28) = 15.20, ptfce-FWE = 0.029, k = 25 voxels, partial η2 = 0.175).
Repeating the 2 × 2 × 2-ANCOVA with DLPFC thickness, the group by time interaction remained significant when (1) additionally controlling for medication and HDRS scores (F(1,51) = 7.69, p = 0.008, partial η2 = 0.131). Looking at lateralization effects, significant group by time interactions were found for right (F(1,52) = 5.75, p = 0.020, partial η2 = 0.100) and left DLPFC thickness (F(1,52) = 4.55, p = 0.038, partial η2 = 0.081), after repeating the analyses for both hemispheres separately. For the 2 × 2 × 2 ANCOVA including (2) only acutely depressed FED patients, the group by time interaction was also found (F(1,24) = 4.79, p = 0.039, partial η2 = 0.167). This effect was located in right (F(1,25) = 4.96, p = 0.035, partial η2 = 0.166), but not detected in left DLPFC thickness (p = 0.111).
Insula
Neither the 2 × 2-ANCOVA of insula GMVs including only FED patients under control of medication and HDRS scores (ptfce-FWE = 0.117), nor the analyses including only acutely depressed patients (ptfce-FWE = 0.053) reached statistical significance.
The 2 × 2 × 2-ANCOVA of insula thickness neither revealed a significant group by time interaction between FED groups (p = 0.521) controlling for HDRs scores and medication, nor in the analyses including acutely depressed FED patients only (p = 0.162).
Discussion
This study investigated longitudinal changes as well as cross-sectional differences regarding brain structure in patients with first-episode depression in a prospective design over 2 years. Our results show that FED patients with recurrence following the index episode have more volume decline in DLPFC volumes and thickness and insula volumes than HC and FED patients in remission. The brain structural changes in the DLPFC were not affected by pharmacotherapy, current symptomatology and patient’s remission status at baseline and follow-up. In the cross-sectional analyses, we neither found significant differences between groups in DLPFC and insula GMV, thickness or surface at baseline nor at follow-up. For hippocampal volumes, the results showed no significant group by time interaction over the 2 years, and no significant cross-sectional group differences.
Our results of volume and thickness decline of the DLPFC as a consequence of recurrence are in line with a previous study [13]. Further, the additional analyses controlling for pharmacological treatment and current depression severity suggest that volume/thickness decline of the DLPFC is rather associated with recurrent disease course than other clinical characteristics. The DLPFC has been repeatedly implicated in the pathophysiology of MDD [39] and involved in executive operations such as decision making, goal-directed behavior and emotion regulation [40, 41]. Animal and (postmortem) human studies suggest stress-induced neurotoxic effects in the prefrontal brain of MDD patients [42] and that these alterations aggravate with disease progression [13], as also in line with our findings. Moreover, treatment studies imply involvement of the DLPFC in the recovery of MDD, as repetitive transcranial magnetic stimulation of the DLPFC can lead to increased volumes of the insula, anterior cingulate cortex, temporal and angular gyrus [43]. Together with previous literature, our results underline the importance of the DLPFC in the progression of MDD—already being critically involved at an early stage.
Furthermore, we found insula volume decline in dependence of recurrence during the study interval, but no significant effects for insula thickness. The insula has also been implicated in the pathophysiology of MDD [44] and has a broad range of functions including the integration of interoceptive information about the person’s own emotional state, salience detection and empathic experience and is reciprocally connected with the DLPFC [45]. The finding, that insula volumes are affected by recurrence is in line with prior longitudinal studies reporting lower insula GMV and thickness being associated with depressive recurrences [13, 14]. However, after the additional control of medication, current symptomatology and remission states, the effect was no longer significant. Furthermore, insula thickness and surface measures were not associated with depressive recurrence in our study, which is contrary to a former study reporting insula thickness decline following depressive relapse in MDD [13]. Together, these findings cannot provide a clear role of the insula in MDD disease course and effects could possibly be attributed to time effects rather than specific disease trajectories, especially in the early phase of disease. This could explain why patients without recurrence also showed slight decline of insula GMVs, although this result did not survive Bonferroni correction.
Regarding hippocampal structure, we did not find longitudinal effects of recurrence, standing in contrast to previous prospective MRI studies [16, 46,47,48]. One difference to our study and potentially explaining the disparity is that MDD patients from previous reports were diagnosed with MDD several years before baseline assessment and most of them had already suffered from multiple lifetime episodes. Thus, morphometric alterations in the hippocampus may be more likely observable in individuals after prolonged illness. In line, two meta-analyses did not report alterations in hippocampal GMVs in FED patients compared with HC, and the authors pointed towards accumulating neurotoxic effects on hippocampal structure in prolonged but not early disease course [49, 50]. In corroboration, a recent review suggests an essential role of chronic stress in the development of hippocampal pathology [51]. Concluding, hippocampal pathology may only develop over disease progression and may not be detectable at the beginning of disease.
The exploratory GMV whole-brain results suggest that furthermore frontal and temporal brain regions and the putamen show GMV reductions in dependence of recurrence. In conjunction with previous longitudinal studies [12,13,14,15,16, 52], our findings point towards more widespread effects of long-term disease course on brain structure. Nonetheless, our exploratory whole-brain results need to be interpreted with caution due to the uncorrected threshold.
In the cross-sectional analyses, we neither detected volume, thickness nor surface alterations in the insula, hippocampus and DLPFC as neural markers for depression at baseline and follow-up. These results stand in contrast to former meta-analyses which reported brain structural differences in several regions including the prefrontal brain, hippocampus and insula in MDD patients compared with HC [21, 22, 53,54,55,56]. Thus, it seems surprising that we did not find cross-sectional group differences in the ROI analyses in our study. However, former brain structural effects were more consistently found in patients with recurrent than in first-episode MDD [21]. Moreover, previous meta-analyses present small to moderate effects (d = 0.12 to d = 0.57 [21, 53, 54, 56]), which could not be detected in our comparably small sample primarily designed to detect longitudinal effects and not to replicate cross-sectional differences. In line with our results, another study also did not report cross-sectional group differences between HC and FED patients in a small study sample [20]. G*Power (version 3.1) calculates n > 60 participants per group to detect moderate effect sizes of d ≥ 0.025 [57], emphasizing the need for larger sample sizes in follow-up studies. Nevertheless, our exploratory whole-brain analyses point towards neural alterations more likely located in temporal and parietal regions in early disease patients.
The cross-sectional results also did not point towards the DLPFC, hippocampus and insula as putative neural risk markers predicting later recurrence. While attention has been paid to fronto-limbic regions as potential biomarkers being involved in the progression of MDD [58], consistent results are still missing, possibly owing to small sample sizes and distinct operationalization of disease trajectories [59]. Presuming high clinical relevance, the contribution of neurobiological markers supporting the early identification of patients at high risk for recurrence need further investigation. In this context, the employment of multivariate machine-learning techniques may be more promising approaches [59,60,61].
The major strength of our study is that FED patients comprise a well-characterized subgroup of MDD patients. The longitudinal investigation of patient’s disease course following the initial illness episode may help to extend current knowledge on neural mechanisms related to recurrence in MDD, simultaneously minimizing confounding effects of prior disease and treatment history. Another strength is that we performed additional analyses by 1) controlling for pharmacotherapy and symptom severity at both time points to investigate the potential influence of treatment and disease related characteristics, and, 2) repeated the analyses by only including acutely depressed patients to investigate whether recurrence effects remain stable when patient groups have equal remission states at baseline. Third, we utilized two different techniques to analyze measures of brain structure and could partly replicate the GMV results in thickness values supporting the robustness of our findings.
Some limitations need to be addressed. First, FED patients were either currently experiencing their first episode or had already experienced their first episode in the past and were therefore already remitted at baseline. This circumstance differs from former longitudinal studies which mainly included homogeneous patient groups with HDRS cut-offs or equal affective states at baseline [13, 16]. To account for this sample heterogeneity, we performed additional analyses for remission status. Second, FEDrec patients showed more severe depressive symptoms at baseline, reflected by higher HDRS scores. To account for this difference, we included HDRS scores as control variable in the additional analyses. Noteworthy, this incident may contribute to the naturalistic classification of our sample based on disease course, as one important predictor for recurrence is the severity of the preceding episode [2, 4, 5]. Third, at baseline a higher proportion of FEDrec patients were under pharmacotherapy compared to FEDrem patients (FEDrec:71%; FEDrem:42%; Supplement 2). While we controlled for psychopharmacological intake to control for potential effects on brain structure, this might have further subtracted out some variance underlying our classification of patients groups according to naturalistic disease course. Fourth, although our sample size was larger compared to the initial study investigating brain structural correlates of recurrence in FED patients [20], we strongly encourage future studies to validate these findings in larger datasets. Lastly, we only gathered information about psychiatric agents at both time points, but did not collect data on psychiatric agents during follow-up. Therefore, our recurrence-associated effects could have been affected by adjustment of medication during follow-up.
In conclusion, this study provides preliminary evidence that recurrence at an early stage of MDD can lead to detrimental effects in brain regions that are involved in emotion perception and regulation [40, 44, 45, 62, 63]. Although alterations of the DLPFC, hippocampus and insula were not identified as neural precursors of recurrence at this initial illness stage, the DLPFC and insula seem particularly sensitive to neurotoxic effects of recurrence over time and may therefore already play a crucial role in the early progression of MDD. To further elucidate consequential versus precipitating neural effects of depressive recurrence, larger samples of FED patients combining multivariate approaches and allowing sub-analyses considering remission states, symptom severity or comparing drug-naïve with psychopharmacologically treated individuals are encouraged.
References
Bukh JD, Andersen PK, Kessing LV. Rates and predictors of remission, recurrence and conversion to bipolar disorder after the first lifetime episode of depression—a prospective 5-year follow-up study. Psychol Med. 2016;46:1151–61.
Kennedy N, Abbott R, Paykel ES. Remission and recurrence of depression in the maintenance era: long-term outcome in a Cambridge cohort. Psychol Med. 2003;33:827–38.
Nelson J, Klumparendt A, Doebler P, Ehring T. Childhood maltreatment and characteristics of adult depression: Meta-analysis. Br J Psychiatry. 2017;210:96–104.
Pettit JW, Lewinsohn PM, Joiner TE. Propagation of major depressive disorder: relationship between first episode symptoms and recurrence. Psychiatry Res. 2006;141:271–8.
Hardeveld F, Spijker J, De Graaf R, Nolen WA, Beekman ATFF. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2009;122:184–91.
Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900–9.
McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54.
Stratmann M, Konrad C, Kugel H, Krug A, Schöning S, Ohrmann P, et al. Insular and hippocampal gray matter volume reductions in patients with major depressive disorder. PLoS ONE. 2014;9:e102692.
Lemke H, Romankiewicz L, Förster K, Meinert S, Waltemate L, Fingas SM, et al. Association of disease course and brain structural alterations in major depressive disorder. Depress Anxiety. 2022;39:441–51.
Zaremba D, Enneking V, Meinert S, Förster K, Bürger C, Dohm K, et al. Effects of cumulative illness severity on hippocampal gray matter volume in major depression: a voxel-based morphometry study. Psychol Med. 2018;48:2391–8.
Dohm K, Redlich R, Zwitserlood P, Dannlowski U. Trajectories of major depression disorders: a systematic review of longitudinal neuroimaging findings. Aust N Zeal J Psychiatry. 2017;51:441–54.
Phillips JL, Batten LA, Tremblay P, Aldosary F, Blier P. A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment-resistant depression. Int J Neuropsychopharmacol. 2015;18:pyv037–pyv037.
Zaremba D, Dohm K, Redlich R, Grotegerd D, Strojny R, Meinert S, et al. Association of brain cortical changes with relapse in patients with major depressive disorder. JAMA Psychiatry. 2018;75:484.
Soriano-Mas C, Hernández-Ribas R, Pujol J, Urretavizcaya M, Deus J, Harrison BJ, et al. Cross-sectional and longitudinal assessment of structural brain alterations in melancholic depression. Biol Psychiatry. 2011;69:318–25.
Frodl T, Jäger M, Smajstrlova I, Born C, Bottlender R, Palladino T, et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:423–30.
Frodl T, Koutsouleris N, Bottlender R, Born C, Jäger M, Scupin I, et al. Depression-related variation in brain morphology over 3 years. Arch Gen Psychiatry. 2008;65:1156.
Opel N, Redlich R, Dohm K, Zaremba D, Goltermann J, Repple J, et al. Mediation of the influence of childhood maltreatment on depression relapse by cortical structure: a 2-year longitudinal observational study. Lancet Psychiatry. 2019;6:318–26.
Weber K, Giannakopoulos P, Delaloye C, de Bilbao F, Moy G, Ebbing K, et al. Personality traits, cognition and volumetric MRI changes in elderly patients with early-onset depression: a 2-year follow-up study. Psychiatry Res. 2012;198:47–52.
Isıklı S, Ugurlu O, Durmusoglu E, Kizilates G, Kitis O, Ozan E, et al. Altered hippocampal formation shape in first-episode depressed patients at 5-year follow-up. J Psychiatr Res. 2013;47:50–55.
Carceller-Sindreu M, Serra-Blasco M, de Diego-Adeliño J, Vives-Gilabert Y, Vicent-Gil M, Via E, et al. Altered white matter volumes in first-episode depression: evidence from cross-sectional and longitudinal voxel-based analyses. J Affect Disord. 2019;245:971–7.
Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9–18.
Peng W, Chen Z, Yin L, Jia Z, Gong Q. Essential brain structural alterations in major depressive disorder: a voxel-wise meta-analysis on first episode, medication-naive patients. J Affect Disord. 2016;199:114–23.
Lai C-H, Wu Y-T. Frontal-insula gray matter deficits in first-episode medication-naïve patients with major depressive disorder. J Affect Disord. 2014;160:74–79.
Zhang H, Li L, Wu M, Chen Z, Hu X, Chen Y, et al. Brain gray matter alterations in first episodes of depression: a meta-analysis of whole-brain studies. Neurosci Biobehav Rev. 2016;60:43–50.
Voineskos AN, Mulsant BH, Dickie EW, Neufeld NH, Rothschild AJ, Whyte EM, et al. Effects of antipsychotic medication on brain structure in patients with major depressive disorder and psychotic features. JAMA Psychiatry. 2020;77:674.
Wittchen H-U, Wunderlich U, Gruschwitz S, Zaudig M. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Interviewheft und Beurteilungsheft. Eine deutschsprachige, erweiterte Bearbeitung der amerikanischen Originalversion des SKID I. Hogrefe: Goettingen, 1997.
Post RM, Roy- Byrne PP, Uhde TW. Graphic representation of the life course of illness in patients with affective disorder. Am J Psychiatry. 1988;145:844–8.
Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:1–28.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Hassel S, Almeida JRC, Kerr N, Nau S, Ladouceur CD, Fissell K, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008;10:916–27.
Lemke H, Probst S, Warneke A, Waltemate L, Winter A, Thiel K, et al. The course of disease in major depressive disorder is associated with altered activity of the limbic system during negative emotion processing. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7:323–32.
Vogelbacher C, Möbius TWD, Sommer J, Schuster V, Dannlowski U, Kircher T, et al. The Marburg-Münster Affective Disorders Cohort Study (MACS): a quality assurance protocol for MR neuroimaging data. Neuroimage. 2018;172:450–60.
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80.
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55.
Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: ii. variability in locations of areas 9 and 46 and relationship to the Talairach coordinate system. Cereb Cortex. 1995;5:323–37.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9.
Caspi Y, Brouwer RM, Schnack HG, van de Nieuwenhuijzen ME, Cahn W, Kahn RS, et al. Changes in the intracranial volume from early adulthood to the sixth decade of life: a longitudinal study. Neuroimage. 2020;220:116842.
Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207.
Dixon ML, Thiruchselvam R, Todd R, Christoff K. Emotion and the prefrontal cortex: an integrative review. Psychol Bull. 2017;143:1033–81.
Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202.
Liu W, Ge T, Leng Y, Pan Z, Fan J, Yang W, et al. The role of neural plasticity in depression: from hippocampus to prefrontal cortex. Neural Plast. 2017;2017:1–11.
Lan MJ, Chhetry BT, Liston C, Mann JJ, Dubin M. Transcranial magnetic stimulation of left dorsolateral prefrontal cortex induces brain morphological changes in regions associated with a treatment resistant major depressive episode: an exploratory analysis. Brain Stimul. 2016;9:577–83.
Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70.
Namkung H, Kim S-H, Sawa A. The Insula: an underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends Neurosci. 2017;40:200–7.
Frodl T, Meisenzahl EM, Zetzsche T, Höhne T, Banac S, Schorr C, et al. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. J Clin Psychiatry. 2004;65:492–9.
Taylor WD, McQuoid DR, Payne ME, Zannas AS, MacFall JR, Steffens DC. Hippocampus atrophy and the longitudinal course of late-life depression. Am J Geriatr Psychiatry. 2014;22:1504–12.
Phillips JL, Batten LA, Tremblay P, Aldosary F, Blier P. A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment-resistant depression. Int J Neuropsychopharmacol. 2015;18:pyv037–pyv037.
Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9–18.
Zhang H, Li L, Wu M, Chen Z, Hu X, Chen Y, et al. Brain gray matter alterations in first episodes of depression: a meta-analysis of whole-brain studies. Neurosci Biobehav Rev. 2016;60:43–50.
Belleau EL, Treadway MT, Pizzagalli DA. The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol Psychiatry. 2019;85:443–53.
Yüksel D, Engelen J, Schuster V, Dietsche B, Konrad C, Jansen A, et al. Longitudinal brain volume changes in major depressive disorder. J Neural Transm. 2018;125:1433–47.
Wise T, Radua J, Via E, Cardoner N, Abe O, Adams TM, et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol Psychiatry. 2017;22:1455–63.
Arnone D, McIntosh AM, Ebmeier KP, Munafò MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: Systematic review and meta-regression analyses. Eur Neuropsychopharmacol. 2012;22:1–16.
Du M-Y, Wu Q-Z, Yue Q, Li J, Liao Y, Kuang W-H, et al. Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;36:11–16.
Koolschijn PCMP, van Haren NEM, Lensvelt-Mulders GJLM, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–35.
Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–60.
MacQueen GM. Magnetic resonance imaging and prediction of outcome in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:343–9.
Schmaal L, Marquand AF, Rhebergen D, van Tol M-J, Ruhé HG, van der Wee NJA, et al. Predicting the naturalistic course of major depressive disorder using clinical and multimodal neuroimaging information: a multivariate pattern recognition study. Biol Psychiatry. 2015;78:278–86.
Dinga R, Marquand AF, Veltman DJ, Beekman ATF, Schoevers RA, van Hemert AM, et al. Predicting the naturalistic course of depression from a wide range of clinical, psychological, and biological data: a machine learning approach. Transl Psychiatry. 2018;8:241.
Frässle S, Marquand AF, Schmaal L, Dinga R, Veltman DJ, van der Wee NJA, et al. Predicting individual clinical trajectories of depression with generative embedding. NeuroImage Clin. 2020;26:102213.
Enneking V, Klug M, Borgers T, Dohm K, Grotegerd D, Frankenberger LM, et al. Changes in brain function during negative emotion processing in the long-term course of depression. Br J Psychiatry. 2022;221:476–84.
Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–43.
Acknowledgements
This work is part of the German multicenter consortium “Neurobiology of Affective Disorders. A translational perspective on brain structure and function“, funded by the German Research Foundation (Deutsche Forschungsgemeinschaft DFG; Forschungsgruppe/Research Unit FOR2107). Principal investigators (PIs) with respective areas of responsibility in the FOR2107 consortium are: Work Package WP1, FOR2107/MACS cohort and brainimaging: Tilo Kircher (speaker FOR2107; DFG grant numbers KI 588/14-1, KI 588/14-2), Udo Dannlowski (co-speaker FOR2107; DA 1151/5-1, DA 1151/5-2), Axel Krug (KR 3822/5-1, KR 3822/7-2), Igor Nenadić (NE 2254/1-2), Carsten Konrad (KO 4291/3-1). WP2, animal phenotyping: Markus Wöhr (WO 1732/4-1, WO 1732/4-2), Rainer Schwarting (SCHW 559/14-1, SCHW 559/14-2). WP3, miRNA: Gerhard Schratt (SCHR 1136/3-1, 1136/3-2). WP4, immunology, mitochondriae: Judith Alferink (AL 1145/5-2), Carsten Culmsee (CU 43/9-1, CU 43/9-2), Holger Garn (GA 545/5-1, GA 545/7-2). WP5, genetics: Marcella Rietschel (RI 908/11-1, RI 908/11-2), Markus Nöthen (NO 246/10-1, NO 246/10-2), Stephanie Witt (WI 3439/3-1, WI 3439/3-2). WP6, multi-method data analytics: Andreas Jansen (JA 1890/7-1, JA 1890/7-2), Tim Hahn (HA 7070/2-2), Bertram Müller-Myhsok (MU1315/8-2), Astrid Dempfle (DE 1614/3-1, DE 1614/3-2). CP1, biobank: Petra Pfefferle (PF 784/1-1, PF 784/1-2), Harald Renz (RE 737/20-1, 737/20-2). CP2, administration. Tilo Kircher (KI 588/15-1, KI 588/17-1), Udo Dannlowski (DA 1151/6-1), Carsten Konrad (KO 4291/4-1). Data access and responsibility: All PIs take responsibility for the integrity of the respective study data and their components. All authors and coauthors had full access to all study data. Acknowledgements and members by Work Package (WP): WP1: Henrike Bröhl, Katharina Brosch, Bruno Dietsche, Rozbeh Elahi, Jennifer Engelen, Sabine Fischer, Jessica Heinen, Svenja Klingel, Felicitas Meier, Tina Meller, Julia-Katharina Pfarr, Kai Ringwald, Torsten Sauder, Simon Schmitt, Frederike Stein, Annette Tittmar, Dilara Yüksel (Dept. of Psychiatry, Marburg University). Mechthild Wallnig, Rita Werner (Core-Facility Brainimaging, Marburg University). Carmen Schade-Brittinger, Maik Hahmann (Coordinating Centre for Clinical Trials, Marburg). Michael Putzke (Psychiatric Hospital, Friedberg). Rolf Speier, Lutz Lenhard (Psychiatric Hospital, Haina). Birgit Köhnlein (Psychiatric Practice, Marburg). Peter Wulf, Jürgen Kleebach, Achim Becker (Psychiatric Hospital Hephata, Schwalmstadt-Treysa). Ruth Bär (Care facility Bischoff, Neukirchen). Matthias Müller, Michael Franz, Siegfried Scharmann, Anja Haag, Kristina Spenner, Ulrich Ohlenschläger (Psychiatric Hospital Vitos, Marburg). Matthias Müller, Michael Franz, Bernd Kundermann (Psychiatric Hospital Vitos, Gießen). Christian Bürger, Katharina Dohm, Fanni Dzvonyar, Verena Enneking, Stella Fingas, Katharina Förster, Janik Goltermann, Dominik Grotegerd, Hannah Lemke, Susanne Meinert, Nils Opel, Ronny Redlich, Jonathan Repple, Katharina Thiel, Kordula Vorspohl, Bettina Walden, Lena Waltemate, Alexandra Winter, Dario Zaremba (Dept. of Psychiatry, University of Münster). Harald Kugel, Jochen Bauer, Walter Heindel, Birgit Vahrenkamp (Dept. of Clinical Radiology, University of Münster). Gereon Heuft, Gudrun Schneider (Dept. of Psychosomatics and Psychotherapy, University of Münster). Thomas Reker (LWL-Hospital Münster). Gisela Bartling (IPP Münster). Ulrike Buhlmann (Dept. of Clinical Psychology, University of Münster). WP2: Marco Bartz, Miriam Becker, Christine Blöcher, Annuska Berz, Moria Braun, Ingmar Conell, Debora dalla Vecchia, Darius Dietrich, Ezgi Esen, Sophia Estel, Jens Hensen, Ruhkshona Kayumova, Theresa Kisko, Rebekka Obermeier, Anika Pützer, Nivethini Sangarapillai, Özge Sungur, Clara Raithel, Tobias Redecker, Vanessa Sandermann, Finnja Schramm, Linda Tempel, Natalie Vermehren, Jakob Vörckel, Stephan Weingarten, Maria Willadsen, Cüneyt Yildiz (Faculty of Psychology, Marburg University). WP4: Jana Freff (Dept. of Psychiatry, University of Münster). Susanne Michels, Goutham Ganjam, Katharina Elsässer (Faculty of Pharmacy, Marburg University). Felix Ruben Picard, Nicole Löwer, Thomas Ruppersberg (Institute of Laboratory Medicine and Pathobiochemistry, Marburg University). WP5: Helene Dukal, Christine Hohmeyer, Lennard Stütz, Viola Lahr, Fabian Streit, Josef Frank, Lea Sirignano (Dept. of Genetic Epidemiology, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University). Stefanie Heilmann-Heimbach, Stefan Herms, Per Hoffmann (Institute of Human Genetics, University of Bonn, School of Medicine & University Hospital Bonn). Andreas J. Forstner (Institute of Human Genetics, University of Bonn, School of Medicine & University Hospital Bonn; Centre for Human Genetics, Marburg University). WP6: Anastasia Benedyk, Miriam Bopp, Roman Keßler, Maximilian Lückel, Verena Schuster, Christoph Vogelbacher (Dept. of Psychiatry, Marburg University). Jens Sommer, Olaf Steinsträter (Core-Facility Brainimaging, Marburg University). Thomas W.D. Möbius (Institute of Medical Informatics and Statistics, Kiel University). CP1: Julian Glandorf, Fabian Kormann, Arif Alkan, Fatana Wedi, Lea Henning, Alena Renker, Karina Schneider, Elisabeth Folwarczny, Dana Stenzel, Kai Wenk, Felix Picard, Alexandra Fischer, Sandra Blumenau, Beate Kleb, Doris Finholdt, Elisabeth Kinder, Tamara Wüst, Elvira Przypadlo, Corinna Brehm (Comprehensive Biomaterial Bank Marburg, Marburg University). The FOR2107 cohort project (WP1) was approved by the Ethics Committees of the Medical Faculties, University of Marburg (AZ: 07/14) and University of Münster (AZ: 2014-422-b-S). We are deeply indebted to all participants of this study, the recruitment sites and their staff.
Funding
This work was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) (TK, grant numbers KI 588/14-1, KI 588/14-2, KI 588/15-1, KI 588/17-1), (UD, grant numbers DA 1151/5-1, DA 1151/5-2, DA 1151/6-1, SFB-TRR58, Projects C09 and Z02), (AK, grant numbers KR 3822/5-1, KR 3822/7-2), (IN, grant numbers NE 2254/1-2), (AJ, grant numbers JA 1890/7-1, JA 1890/7-2), (TH, grant number HA 7070/2-2); the Interdisciplinary Center for Clinical Research (IZKF) of the medical faculty of Münster (UD, grant number Dan3/012/17), (NO, grant number SEED 11/18); “Innovative Medizinische Forschung” (IMF) of the medical faculty of Münster (JR, grant number RE221707), (EJL, grant number LE121703), (KD, grant number KO121806), and the Deanery of the Medical Faculty of the University of Münster. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published: HL, UD, HK, JS, KT, LW, AW, FB, SM, MK, VE, NW, DG, EJL, JR, KD, NO, FS, TM, KB, KGR, JKP, FTO, TH, AK, AJ, WH, IN, and TK.
Corresponding author
Ethics declarations
Competing interests
TK received unrestricted educational grants from Servier, Janssen, Recordati, Aristo, Otsuka, neuraxpharm. This funding is not associated with the current work. On behalf of all other authors, the corresponding author states that there is no conflict of interest and nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lemke, H., Klute, H., Skupski, J. et al. Brain structural correlates of recurrence following the first episode in patients with major depressive disorder. Transl Psychiatry 12, 349 (2022). https://doi.org/10.1038/s41398-022-02113-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-022-02113-7