Abstract
Repeated hospitalizations are a characteristic of severe disease courses in patients with affective disorders (PAD). To elucidate how a hospitalization during a nine-year follow-up in PAD affects brain structure, a longitudinal case-control study (mean [SD] follow-up period 8.98 [2.20] years) was conducted using structural neuroimaging. We investigated PAD (N = 38) and healthy controls (N = 37) at two sites (University of Münster, Germany, Trinity College Dublin, Ireland). PAD were divided into two groups based on the experience of in-patient psychiatric treatment during follow-up. Since the Dublin-patients were outpatients at baseline, the re-hospitalization analysis was limited to the Münster site (N = 52). Voxel-based morphometry was employed to examine hippocampus, insula, dorsolateral prefrontal cortex and whole-brain gray matter in two models: (1) group (patients/controls)×time (baseline/follow-up) interaction; (2) group (hospitalized patients/not-hospitalized patients/controls)×time interaction. Patients lost significantly more whole-brain gray matter volume of superior temporal gyrus and temporal pole compared to HC (pFWE = 0.008). Patients hospitalized during follow-up lost significantly more insular volume than healthy controls (pFWE = 0.025) and more volume in their hippocampus compared to not-hospitalized patients (pFWE = 0.023), while patients without re-hospitalization did not differ from controls. These effects of hospitalization remained stable in a smaller sample excluding patients with bipolar disorder. PAD show gray matter volume decline in temporo-limbic regions over nine years. A hospitalization during follow-up comes with intensified gray matter volume decline in the insula and hippocampus. Since hospitalizations are a correlate of severity, this finding corroborates and extends the hypothesis that a severe course of disease has detrimental long-term effects on temporo-limbic brain structure in PAD.
Similar content being viewed by others
Introduction
A subgroup of patients with affective disorders (PAD) has a severe course of disease with repeated hospitalization: While approximately 20% of all patients with major depressive disorder (MDD) show a severe and recurrent course of disease, nearly 50% of patients with bipolar disorder (BD) show severe disease courses with recurrent episodes [1,2,3,4]. Longitudinal structural neuroimaging provides insights into the underlying neural mechanisms of severe courses of disease in PAD. In the long term, understanding these neural mechanisms could aid the prevention of severe disease courses and thus improve treatment of severe affective disorders.
Structural brain alterations in PAD have already been demonstrated by cross-sectional studies in frontal, temporal and limbic regions such as the dorsolateral prefrontal cortex (dlPFC), insula and hippocampus [5,6,7,8,9,10,11,12,13]. In particular, lower volume in the dlPFC and hippocampus has been linked to characteristics of the previous disease course such as the lifetime number of hospitalizations or the number of manic or depressive episodes [14,15,16,17].
However, only prospective studies allow to approximate causal conclusions about the relationship between structural brain changes and the course of disease. Unfortunately, only very few longitudinal studies focused on the interplay of gray matter changes and the course of disease [18,19,20]. These studies revealed greater brain volume loss in PAD compared to healthy control participants (HC), in particular in temporo-limbic areas such as the hippocampus and insula as well as parts of the frontal cortex (e.g., dlPFC) [21,22,23,24]. More recently, longitudinal studies have also established a link between these volume declines and recurrent manic [25] and depressive episodes [26].
In these few longitudinal studies, patients’ course of disease in concordance with gray matter alterations has only very rarely been investigated over time periods longer than 6 years [23, 27]. These studies also corroborate the idea that a detrimental disease course is associated with fronto-limbic volume decline. For instance, they have shown that the number of depressive relapses is associated with reduced insular volume [23]. However, most of the studies with longer follow-up intervals contained significant methodological limitations reducing their impact and generalizability, such as the use of different MRI scanners at baseline and follow-up [27], the investigation of an elderly sample [23], or the lack of a control group [25]. Still, conclusive neuroimaging studies with substantially longer follow-up intervals are needed to investigate how relevant features of a severe disease course affect gray matter volume.
Previous longitudinal structural MRI studies have mostly used the number of episodes as a correlate of severe disease courses. Number of episodes are usually based on retrospective patient reports. However, retrospective patient reports of depressed episodes may have questionable reliability and validity [28] specifically over longer time periods. For such longer follow-up periods, objective markers of a severe disease courses are needed. The number and duration of hospitalizations provide such criteria, since they are verifiable by medical reports.
The aim of this article is to elucidate the relationship between brain structural alterations and particularly severe disease courses in PAD in both frontal and temporo-limbic areas as well as on a whole-brain level. For this purpose, at least one hospitalization during follow-up was considered as a characteristic of a severe course of disease. To elucidate how these characteristics are associated with changes in brain structure, brain structural alterations were observed over nine years with a focus on the hippocampus, insula and dlPFC.
To tackle these research gaps, PAD were investigated at two study sites (Münster, Dublin). We hypothesize that (a) patients lose more volume over time than controls in the insula, hippocampus and dlPFC and (b) patients with a hospitalization until follow-up will lose more volume than HC and patients without re-hospitalization in these regions.
To allow for specific conclusions regarding the impact of hospitalization as a specific correlate of severe disease courses within affective disorders, we also explore the impact of important other correlates of severe disease courses, for instance, comorbid mental disorders, the number of depressive episodes during the study interval, the intake of psychotropic medication, baseline depressive symptom-severity, baseline number of hospitalizations on our effects, as well as the inclusion of patients with BD.
Materials and methods
Participants
Eighty-seven participants took part in our longitudinal structural MRI investigation across sites (Münster N = 61, Dublin N = 26). All participants gave written informed consent according to the Declaration of Helsinki. Inclusion criterion for PAD was a lifetime diagnosis of BD or MDD at follow-up. Patients with dysthymia were not included in the analysis. Next to the general exclusion criteria for MRI studies such as metal implants, we also excluded participants with severe cardiovascular diseases, unstable diabetes, life-time alcohol-dependence and substance dependence at follow-up, as well as severe neurological diseases such as Parkinson’s disease, stroke, and dementia. After quality control of the imaging data, N = 52 participants (28 PAD: BD = 6, MDD = 22; HC = 24) remained in the Münster sample as well as N = 23 participants (10 PAD: MDD = 10; HC = 13) in the Dublin sample (see Table 1 and Appendix A: Supplementary Table 1, Supplementary Table 2, Supplementary Table 3). The focus of this analysis were the effects of diagnosis and course of affective disorder on brain volume loss. Because of the unique follow-up length and to increase sensitivity for this analysis, patients with both, BD and MDD, were included. To also increase specificity of conclusions regarding MDD-patients, the analysis was repeated excluding patients with BD (total N = 69, N = 32 MDD-patients, N = 37 HC).
In Münster, all patients were recruited during in-patient treatment at the Department of Psychiatry at the University of Münster at baseline (BD = 8, MDD = 27, HC = 26). Diagnoses were made by a clinical psychologist or psychiatrist at baseline and reviewed by a second clinician at baseline and follow-up using the SCID-I interview according to DSM-IV criteria [29]. HC were recruited from the local community and by newspaper announcements in Münster. Inclusion criterion was the absence of any lifetime mental disorder at baseline or follow-up. A SCID-I interview verified the absence of lifetime diagnosis. All participants have been taking part in cross-sectional studies conducted by our lab between 2005–2008 and are part of the Münster Neuroimaging Cohort [30,31,32,33,34]. During follow-up, one MDD patient reported a hypomanic episode that led to a change in diagnosis from MDD to BD at follow-up. Two participants, initially healthy controls, were diagnosed with at least one depressive episode during the interval, leading to a change in diagnosis from HC to MDD. For the analysis participants were assigned to the group (patients vs. controls) using the diagnostic information of the follow-up. Descriptive data of the Münster sample can be found in Supplementary Table 2 (Appendix A).
In Dublin, 26 participants (MDD = 12, HC = 14) were investigated at follow-up, approximately six years after baseline measurements. At baseline, all patients were undergoing outpatient treatment at the mental health services of Tallaght Hospital, Dublin and St. James’s Hospital, Dublin, Ireland. MDD was clinically diagnosed by consultant psychiatrists based on DSM-IV criteria using the SCID-I. HCs were recruited from the local community in Dublin. Descriptive data of the Dublin sample can be found in Supplementary Table 3 (Appendix A).
Measures
Self-rated and observer-rated scales were completed for all participants at both times points. These included the Hamilton Depression Rating Scale (HAM-D) [35] and Beck’s Depression Inventory (Münster: BDI-I, Dublin: BDI-II) [36] At follow-up, full remission was defined according to DSM-IV criteria (two months without depressive symptoms) in combination with a HAM-D Score lower than 8 (see also Table 1).
Severity of the course of disease was determined by at least one hospitalization within the follow-up interval. Hospitalization during follow-up was determined by a list of patients’ lifetime hospitalizations with date, duration, diagnosis, and hospital based on their medical reports. Patients reported exact dates based on their medical reports or brought their medical reports for verification. Hence, hospitalization were objective and verifiable characteristics of a severe disease course. Reason for hospitalization was treatment of a mood episode during BD or MDD. PAD that had at least one psychiatric in-patient treatment during follow-up are referred to as “patients with hospitalization”, while those without in-patient treatment during follow-up were considered as “patients without hospitalization”. Since the Dublin patients were outpatients, the analysis regarding the effects of hospitalization in the interval was limited to the Münster sample (N = 52).
We collected a list of current medication intake at baseline and follow-up. Psychotropic medication was measured at follow-up using a medication load index [37]: Each psychotropic medication was coded as absent = 0, low = 1 (equal or lower average dose), or high = 2 (greater than average dose), relative to the midpoint of the daily dose range recommended by Physician’s-Desk-Reference [38]. To build the index, all medication codes per participant and time point were summed, which finally yielded a composite measure of total medication exposure for each subject at follow-up (medication load index).
Details of MRI data collection and preprocessing can be found in the supplements (Appendix B).
Statistical analysis
Longitudinal VBM analyses were conducted on gray matter segments in SPM12 using an absolute threshold masking of 0.1. We used a flexible-factorial design and conducted three 2×2/3 × 2 analyses of covariance (ANCOVA) with group as between-participant factor and time (baseline and follow-up) as within-participant factor controlling for age. In model 1, we investigated the effect of diagnosis on gray matter volume with a directed group (AD-patients, HC) × time contrast: We hypothesized that PAD lost more volume during the follow-up than HC. In model 2, limited to the Muenster sample only, we investigated another directed group (patients with hospitalization, patients without hospitalization, HC) × time interaction on gray matter volume to determine the effects of severe disease courses on gray matter volume loss over time according to our a priori hypothesis. In another supplemental model (model 3), we used a different definition of severe disease course and compared the brain structural development of remitted and unremitted patients at follow-up to HC (see Appendix C). Main effects of group were estimated using post hoc tests controlling for age, sex and site (see Appendix D).
We conducted three region of interest (ROI)-analysis on the bilateral hippocampus, insula and dlPFC, as well as an exploratory whole-brain analysis. ROIs were determined using the AAL-atlas implemented in the Wake Forest University Pickatlas (http://fmri.wfubmc.edu/software/PickAtlas). Anatomical labeling of significant VBM clusters in the whole-brain analysis was performed by the AAL toolbox (release 3v1 [39, 40]). We used a rigorous statistical threshold of p < 0.05 (one-tailed), familywise error (FWE) corrected on the voxel level. Exploratory results are reported at a statistical threshold of p < 0.001, uncorrected, with a cluster threshold of k = 300 voxels (see Appendix E, Supplementary Table S4).
We performed spearman correlation analyses to investigate potentially confounding effects of psychotropic medication using the medication load index at follow-up as well as baseline symptom severity and the number of hospitalizations before baseline, the number of episodes as well as lifetime comorbid mental disorders on gray matter volume differences in the patient subsamples (see Appendix F, Supplementary Table S5). Therefore, VBM gray matter volume differences were computed by subtracting contrast values extracted at baseline from those at follow-up.
Results
Model 1: volume decline in patients compared to healthy participants
Model 1 included 38 PAD (20 women and 18 men, mean [SD] age, 37.08 [10.19] years) and 37 HC (25 women and 12 men, mean [SD] age, 33.97 [12.71] years, see also Table 1, Supplementary Table 1 and Supplementary Table 2 in Appendix A). Main effects of time and group are reported in Appendix D.
The ROI analysis on hippocampal volume revealed a significant group × time interaction: Patients lost significantly more volume than controls bilaterally in the hippocampus (left: t(71) = 4.48, pFWE = 0.001, k = 183, x = −21, y = −9, z = −12; right: t(71) = 4.28, pFWE = 0.008, k = 57, x = 16, y = −4, z = −16). The ROI analysis on insular volume revealed a significant group × time interaction. Patients lost significantly more volume than controls in three clusters of the right insula (t(71) = 4.68, pFWE = 0.004, k = 85, x = 42, y = −15, z = −2; t(71) = 4.34, pFWE = 0.004; k = 54, x = 27, y = 12, z = −20; t(71) = 4.1, pFWE = 0.018, k = 33, x = 51, y = 2, z = −2).
The ROI analysis on the dlPFC revealed no significant results (pFWE = 0.552).
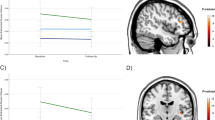
The whole-brain analysis revealed a significant group × time interaction in the right temporal lobe, showing that patients lost more volume than HC (temporal pole: t(71) = 5.25, pFWE = 0.018, k = 14, x = 24, y = 9, z = −21; superior temporal gyrus: t(71) = 5.05, pFWE = 0.042, k = 3, x = 44, y = −2, z = 15; see Fig. 1). An exploratory analysis (p < 0.001, k = 300) revealed that this cluster extended into the right insula and hippocampus, further corroborating our results from the ROI analysis on a whole-brain level. The results of this exploratory whole-brain analysis can be found in the supplements (Appendix E).
A Depicted is the whole-brain cluster of the groupxtime interaction, for display reasons, the statistical threshold was set to p < 0.001, uncorrected, color bar indicates t-values of the group by time contrast of the comparison patients vs. controls (model 1), extracted at the peak voxel: x = 24, y = 9, z = −21. B Contrast values were extracted at the peak voxel (hippocampus: x = −21, y = −9, z = −12, insula: x = 42, y = −15, z = −2, whole-brain analysis: x = 24, y = 9, z = −21) and depicted for patients and healthy participants: patients show a steeper reduction of gray matter volume of the whole-brain cluster and the regions of interest (ROI) hippocampus and insula. Graph shows mean gray matter values (mean and individual data points) extracted from the flexible-factorial model at baseline and follow-up with a boxplot (median, first and third quartile) to illustrate the distribution of the data.
Model 2: effects of hospitalization as a correlate of severe disease course
Model 2 contained the Münster sample: 13 patients with hospitalization during follow-up (7 women and 6 men; mean [SD] age, 47.31 [10.98] years) and 15 patients without hospitalization (6 women and 9 men-, mean [SD] age, 42.47 [8.68] years) as well as 24 HC (16 women and 8 men; mean [SD] age, 40.21 [10.47] years, see Supplementary Table 1 in Appendix A).
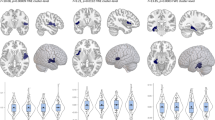
While patients with hospitalization lost more volume in the ROI analysis of the right insula (t(49) = 3.92, pFWE = 0.025, k = 29, x = 34, y = 12, z = −16) in comparison to HC, patients without hospitalization did not differ significantly from HC (all pFWE > 0.26) or hospitalized patients in their insula volume loss (all pFWE > 0.32, see Fig. 2). The ROI analysis of the hippocampus indicated that patients with hospitalization lost more volume than HC and patients without hospitalization (t(49) = 4.03, pFWE = 0.023, k = 25, x = −23, y = −10, z = −21). The ROI analysis of the dlPFC yielded no significant results (all pFWE > 0.96). The whole-brain analysis revealed no significant results (all pFWE > 0.53).
Differences in brain volume decline between the patient groups according to their course of disease and healthy participants: We extracted the contrast values of the significant region of interest (ROI) analysis of the hippocampus and insula. Here, the hippocampus was yielding a significant group x time interaction for the patients with hospitalization in comparison to the patients without hospitalization and for the insula a significant group x time interaction for the patients with hospitalization in comparison to the controls. Both contrasts were extracted at the peak voxel (hippocampus: x = −23, y = −10, z = −21, insula: x = 34, y = 12, z = −16). Graph shows mean gray matter values (mean and individual data points) extracted from the flexible-factorial model at baseline and follow-up with a boxplot (median, first and third quartile) to illustrate the distribution of the data.
Effects of confounders
Gray matter changes significantly correlated with the number of depressive episodes in the interval. This is an effect that can be expected since both high recurrence and repeated hospitalization are both correlates of a severe disease course. Gray matter changes were not significantly correlated with previous course of disease or initial symptom severity at baseline. Gray matter changes were also not correlated with the intake of psychotropic medication or did not differ between patients with BD and MDD in both models (see Appendix F, Supplementary Table S5), as well as patients reporting comorbidities or not. To also test the specificity of our results to patients with depression, we excluded patients with BD in a secondary analysis (see Appendix G).
Discussion
This study investigated the association of hospitalization as a feature of severe disease courses of PAD with brain structural changes over nine years. In our study, PAD lost more volume in a cluster in the temporal lobe extending into the hippocampus and insula in comparison to HC. Most importantly, patients with hospitalization until follow-up lost more volume in the insula and hippocampus in comparison to HC. In contrast, patients without further hospitalization did not differ from HC in their brain volume decline of insula and hippocampus. Thus, we were able to confirm and extend our earlier findings that volume decline merely appeared in patients with a worse disease outcome [21, 26]. We carefully controlled our findings for associations with putative confounders: Since there was no correlation between the number of hospitalizations that happened before baseline measurements and volume decline between baseline and follow-up, we could rule out that the brain structural alterations result from the disease course before the patients took part in our study. The effects of hospitalization on brain structure were also replicated after excluding BD-patients from the analysis (see Appendix G). While MDD-patients and HC did not differ in their brain structural changes, hospitalized MDD-patients lost significantly more hippocampal and insular volume to follow-up than HC. Moreover, MDD-patients with hospitalization also lost more hippocampal volume than MDD-patients without hospitalization during follow-up, corroborating the stability of our findings in MDD-patients. We also replicated our results by using remission at follow-up to group patients in the entire sample (n = 75) and showed that the number of episodes in the interval was correlated with insular volume loss as another marker for a severe disease course. These additional analyses indicate that brain volume loss is linked to a severe course of affective disorders independent of the correlate that is used (follow-up remission, re-hospitalization or number of depressive episodes).
While all participants lost volume over time, surprisingly, PAD did not show lower gray matter volume than healthy controls across time points. This fits well with findings indicating that differences in gray matter volume between patients with depression and HC are rather small [12, 41] and that extremely large neuroimaging samples are needed to detect these subtle gray matter volume differences in cross-sectional analyses. At this point, our results further highlight the importance of longitudinal studies in structural neuroimaging of affective disorders. Our results show that cross-sectional quantitative differences in gray matter volume cannot be demonstrated between patients and controls in our sample, but that development over time between both groups can very well be quantitatively delineated. Moreover, our results associate progressive gray matter decline with hospitalization as a feature of a severe disease course rather than with the diagnosis itself.
The observed gray matter loss in temporo-limbic areas extend previous cross-sectional observations indicating a negative association between the lifetime number and duration of hospitalization, the lifetime number and duration of depressive episodes and hippocampal volume [17, 42] as well as the association between relapse and volume decreases of the insula, hippocampus and temporal lobe [21, 23, 26].
We investigated gray matter volume loss using voxel-based morphometry, which among other techniques enables us to detect gray matter changes of the brain that could result from changes in neural organization (e.g., glial cell density, neuron size or dendritic branching) [43,44,45]. These changes may in turn affect the functional neuronal circuitry of regions showing volume loss. Although we cannot form any firm conclusions regarding the functional role of this gray matter decline, gray matter loss in the hippocampus and insula might predispose or result from mood-congruent biased emotion processing in PAD and may intensify suppressive emotion regulation. Further speculating on the functional nature of this volume decline, it has been revealed that more and longer hospitalizations are also associated with lower activity in the insula and hippocampus during facial emotion processing [46], suggesting a decreased metabolic activity in emotion-processing areas as a result of severe disease course. Volume loss may predispose or result from a reduced propensity to process and regulate emotional experiences ultimately reinforcing disturbed affective states, that is depression or mania. This is also reflected by functional neuroimaging studies in PAD indicating a reduced capacity to inhibit and regulate limbic activity resulting in a hyperreactivity to mood-congruent stimuli during depression and (hypo-)mania [47,48,49,50,51,52,53].
Next to only little evidence on the impact of adverse disease courses characterized by multiple hospitalizations on brain structure over time, neurobiological processes in the brain during recovery from affective disorder remain unclear. Our results point towards the idea that a course of affective disorders without recurrent hospitalization may ultimately result in a normalized brain structural development, specifically in emotion-processing regions such as the insula and hippocampus. Here, future studies should focus on defining and monitoring recovery in PAD also on a brain structural level and elucidate if recovery is associated with brain structural normalization.
To the best of our knowledge, this is the first study investigating the effects of hospitalization as a feature of severe disease course on brain volume with a follow-up interval of almost a decade and thus the first study allowing for conclusions regarding the long-term effects of severity in affective disorders on brain structure.
Limitations
Data on patients with BD were only available for the Münster site. To date, there is no study comparing structural brain alterations over time in patients with BD and MDD. Hence, we do not know whether the effects of severe course of disease on brain structure are a transdiagnostic phenomenon.
Unlike previous studies [17, 23, 46], the report of depressive and manic episodes in the interval was not used to measure the severity of disease course. Retrospectively reported mood episodes can be biased by the current mood state [28] and have a recall-rate lower than 50% over longer periods of time [54]. To increase reliability of these retrospective reports, electronic diaries of mood and life-charting have been suggested. Since patients with a very severe course of disease and significant impairment would probably not continue a mood diary for nine years, this approach would not have been feasible and suitable for the aim of our study. Since hospitalization data is accessible, verifiable and does not rely on patient effort and motivation, hospitalization during follow-up was used as a marker of severe disease course.
Given our recent findings [55] the sample size is too small for meaningful cross-sectional findings. However, our longitudinal within-subject analysis using a flexible-factorial model has an increased sensitivity compared to the cross-sectional analysis and thus allows viable conclusions. In comparison to previous longitudinal neuroimaging studies with a comparable follow-up length [23, 27] our sample size (N = 75) is the biggest so far.
Conclusions
The present study revealed detrimental effects of hospitalization as a marker of a severe disease courses on brain structure in PAD using a longitudinal design. Observed volume decline in patients from baseline to follow-up affected brain structures involved in emotion processing and regulation. Our results highlight the association between hospitalization as a feature of long-term disease progression and morphologic brain alterations in PAD. This study demands a more fine-grained analysis of long-term disease progression and its interaction with brain structure.
References
Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800.
Schauenburg H, Clarkin J. Relapse in depressive disorders - is there a need for maintenance psychotherapy? Z für Psychosom Med Psychother. 2003;49:377–90.
Paykel ES. Cognitive therapy in relapse prevention in depression. Int J Neuropsychopharmacol. 2007;10:131–6.
Gitlin MJ, Swendsen J, Heller TL, Hammen CL. Relapse and impairment in bipolar disorder. Am J Psychiatry. 1995;152:1635–40.
Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9–18.
Cao B, Passos IC, Mwangi B, Bauer IE, Zunta-Soares GB, Kapczinski F, et al. Hippocampal volume and verbal memory performance in late-stage bipolar disorder. J Psychiatr Res. 2016;73:102–7.
Ching CRK, Agartz I, Hibar DP, Thomopoulos SI, Abé C, Brouwer RM et al. What we learn about bipolar disorder from large-scale neuroimaging: Findings and future directions from the ENIGMA Bipolar Disorder Working Group. Hum Brain Mapp 2020;43:56–82.
Hajek T, Kopecek M, Höschl C, Alda M. Smaller hippocampal volumes in patients with bipolar disorder are masked by exposure to lithium: a meta-analysis. J Psychiatry Neurosci. 2012;37:333–43.
Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23:932–42.
Koolschijn MP, van Haren NEM, Lensvelt-Mulders GJLM, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–35.
Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900–9.
Schmaal L, Veltman DJ, Van Erp TGM, Smann PG, Frodl T, Jahanshad N, et al. Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2016;21:806–12.
Wang X, Luo Q, Tian F, Cheng B, Qiu L, Wang S, et al. Brain grey-matter volume alteration in adult patients with bipolar disorder under different conditions: a voxel-based meta-analysis. J Psychiatry Neurosci. 2019;44:89–101.
Ekman CJ, Lind J, Rydén E, Ingvar M, Landén M. Manic episodes are associated with grey matter volume reduction - a voxel-based morphometry brain analysis. Acta Psychiatr Scand. 2010;122:507–15.
Sheline YI. Depression and the hippocampus: cause or effect? Biol Psychiatry. 2011;70:308–9.
Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–66.
Zaremba D, Enneking V, Meinert S, Förster K, Bürger C, Dohm K, et al. Effects of cumulative illness severity on hippocampal gray matter volume in major depression: a voxel-based morphometry study. Psychol Med. 2018;48:2391–8.
Abé C, Ching CRK, Liberg B, Lebedev AV, Agartz I, Akudjedu TN, et al. Longitudinal structural brain changes in bipolar disorder: A multicenter neuroimaging study of 1232 individuals by the ENIGMA Bipolar Disorder Working Group. Biol Psychiatry. 2021;91:582–92.
Dohm K, Redlich R, Zwitserlood P, Dannlowski U. Trajectories of major depression disorders: a systematic review of longitudinal neuroimaging findings. Aust NZ J Psychiatry. 2017;51:441–54.
Förster K, Horstmann RH, Dannlowski U, Houenou J, Kanske P Progressive grey matter alterations in bipolar disorder across the life span – a systematic review. Bipolar Disorders 2023; n/a. https://doi.org/10.1111/bdi.13318.
Frodl TS, Koutsouleris N, Bottlender R, Born C, Jäger M, Scupin I, et al. Depression-related variation in brain morphology over 3 years: effects of stress? Arch Gen Psychiatry. 2008;65:1156–65.
Moorhead TWJ, McKirdy J, Sussmann JED, Hall J, Lawrie SM, Johnstone EC, et al. Progressive gray matter loss in patients with bipolar disorder. Biol Psychiatry. 2007;62:894–900.
Soriano-Mas C, Hernndez-Ribas R, Pujol J, Urretavizcaya M, Deus J, Harrison BJ, et al. Cross-sectional and longitudinal assessment of structural brain alterations in melancholic depression. Biol Psychiatry. 2011;69:318–25.
Steffens DC, McQuoid DR, Payne ME, Potter GG. Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry. 2011;19:4–12.
Abé C, Ekman CJ, Sellgren C, Petrovic P, Ingvar M, Landén M. Manic episodes are related to changes in frontal cortex: a longitudinal neuroimaging study of bipolar disorder 1. Brain. 2015;138:3440–8.
Zaremba D, Dohm K, Redlich R, Grotegerd D, Strojny R, Meinert S, et al. Association of brain cortical changes with relapse in patients with major depressive disorder. JAMA Psychiatry. 2018;75:484–92.
Ahdidan J, Hviid LB, Chakravarty MM, Ravnkilde B, Rosenberg R, Rodell A, et al. Longitudinal MR study of brain structure and hippocampus volume in major depressive disorder. Acta Psychiatr Scand. 2011;123:211–9.
Schraedley PK, Turner RJ, Gotlib IH. Stability of retrospective reports in depression: Traumatic events, past depressive episodes, and parental psychopathology. J Health Soc Behav. 2002;43:307–16.
Wittchen H-U, Wunderlich U, Gruschwitz S, Zaudig M SKID I. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Interviewheft und Beurteilungsheft. Eine deutschsprachige, erweiterte Bearb. d. amerikanischen Originalversion des SKID I. 1997. http://hdl.handle.net/11858/00-001M-0000-000E-AAB1-4 (accessed 14 Sep 2021).
Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, et al. Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatry Res. 2007;154:13–20.
Reker M, Ohrmann P, Rauch AV, Kugel H, Bauer J, Dannlowski U, et al. Individual differences in alexithymia and brain response to masked emotion faces. Cortex. 2010;46:658–67.
Schöning S, Engelien A, Bauer C, Kugel H, Kersting A, Roestel C, et al. Original research—intersex and gender identity disorders: neuroimaging differences in spatial cognition between men and male-to-female transsexuals before and during hormone therapy. J Sex Med. 2010;7:1858–67.
Schöning S, Zwitserlood P, Engelien A, Behnken A, Kugel H, Schiffbauer H, et al. Working-memory fMRI reveals cingulate hyperactivation in euthymic major depression. Hum Brain Mapp. 2009;30:2746–56.
Sehlmeyer C, Konrad C, Zwitserlood P, Arolt V, Falkenstein M, Beste C. ERP indices for response inhibition are related to anxiety-related personality traits. Neuropsychologia. 2010;48:2488–95.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and-II in psychiatric outpatients. J Pers Assess. 1996;67:588–97.
Hassel S, Almeida JRC, Kerr N, Nau S, Ladouceur CD, Fissel K, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008;10:916–27.
Reynolds C Physician’s Desk Reference. 2008. https://doi.org/10.1002/9780470373699.speced1606.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–9.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89.
Winter NR, Leenings R, Ernsting J, Sarink K, Fisch L, Emden D et al. More alike than different: Quantifying deviations of brain structure and function in major depressive disorder across neuroimaging modalities. http://arxiv.org/abs/2112.10730 (accessed 12 Jan 2022).
Lemke H, Romankiewicz L, Förster K, Meinert S, Waltemate L, Fingas SM, et al. Association of disease course and brain structural alterations in major depressive disorder. Depress Anxiety. 2022;39:441–51.
Anderson BJ. Plasticity of gray matter volume: the cellular and synaptic plasticity that underlies volumetric change. Dev Psychobiol. 2011;53:456–65.
Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–77.
Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–98.
Lemke H, Probst S, Warneke A, Waltemate L, Winter A, Thiel K, et al. The course of disease in major depressive disorder is associated with altered activity of the limbic system during negative emotion processing. Biol Psychiatry. 2021. https://doi.org/10.1016/J.BPSC.2021.05.008.
García-Blanco AC, Perea M, Salmerón L. Attention orienting and inhibitory control across the different mood states in bipolar disorder: An emotional antisaccade task. Biol Psychol. 2013;94:556–61.
Grotegerd D, Stuhrmann A, Kugel H, Schmidt S, Redlich R, Zwanzger P, et al. Amygdala excitability to subliminally presented emotional faces distinguishes unipolar and bipolar depression: An fMRI and pattern classification study. Hum Brain Mapp. 2014;35:2995.
Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Nature Publishing Group, 2005. https://doi.org/10.1038/sj.mp.4001585.
Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord. 2011;1:1–17.
Wessa M, Linke J. Emotional processing in bipolar disorder: behavioural and neuroimaging findings. Int Rev Psychiatry. 2009;21:357–67.
Kurtz M, Mohring P, Förster K, Bauer M, Kanske P. Deficits in explicit emotion regulation in bipolar disorder: a systematic review. Int J Bipolar Disord. 2021;9:15.
Förster K, Kurtz M, Konrad A, Kanske P, Förster K, Kurtz M, et al. Emotional reactivity, emotion regulation and social emotions in affective disorders: Neural models informing treatment approaches. Z für klin Psychol Psychother. 2022;51:11–25.
Wells EJ, Horwood LJ. How accurate is recall of key symptoms of depression? A comparison of recall and longitudinal reports. Psychol Med. 2004;34:1001–11.
Winter NR, Leenings R, Ernsting J, Sarink K, Fisch L, Emden D, et al. Quantifying deviations of brain structure and function in major depressive disorder across neuroimaging modalities. JAMA Psychiatry. 2022;79:879–88.
Acknowledgements
This work was funded by the German Research Foundation (DFG, grant FOR2107 DA1151/5-1 and DA1151/5-2 to UD; SFB-TRR58, Projects C09 and Z02 to UD) and the Interdisciplinary Center for Clinical Research (IZKF) of the medical faculty of Münster (grant Dan3/012/17 to UD), the “Innovative Medizinische Forschung“ (IMF) of the medical faculty Münster (grant ME122205 to SM) and the Else Kröner-Fresenius-Stiftung (grant 2022_EKEA.102 to KF) as well as the Graduate Academy of the TU Dresden with funds of the Federal Ministry of Education and Research (BMBF) and the Freestate of Saxony under the Excellence Strategy of the Federal Government and the Länder (to KF). We also thank Elisabeth Eisele for her contribution to the graphical work of this article.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: KF, DG, UD, TF. Acquisition of data: KF, DG, KaD, HL, VE, SM, RR, WH, JB, HK, TS, PO, AC, VO, AF, KeD, HM, TF, UD. Analysis of data: KF, DG, KaD, HL, VE, SM, RR, WH, JB, HK, TS, PO, AC, VO, AF, KeD, HM, TF, UD. Interpretation of data: KF, DG, PK, TF, UD. Drafting of the article: KF, DG. Critical revision of the article: KaD, HL, VE, SM, RR, WH, JB, HK, TS, PO, AC, VO, AF, KeD, HM, TF, UD. Final approval of the article: KF, DG, KaD, HL, VE, SM, RR, WH, JB, HK, TS, PO, AC, VO, AF, KeD, HM, TF, UD.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Förster, K., Grotegerd, D., Dohm, K. et al. Association of hospitalization with structural brain alterations in patients with affective disorders over nine years. Transl Psychiatry 13, 170 (2023). https://doi.org/10.1038/s41398-023-02452-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02452-z
This article is cited by
-
Genetic and symptomatic risks associated with longitudinal brain morphometry in bipolar disorder
Nature Mental Health (2024)