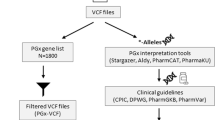
Abstract
Beyond the identification of causal genetic variants in the diagnosis of Mendelian disorders, exome sequencing can detect numerous variants with potential relevance for clinical care. Clinical interventions can thus be conducted to improve future health outcomes for patients and their at-risk relatives, such as predicting late-onset genetic disorders accessible to prevention, treatment or identifying differential drug efficacy and safety. To evaluate the interest of such pharmacogenetic information, we designed an “in house” pipeline to determine the status of 122 PharmGKB (Pharmacogenomics Knowledgebase) variant-drug combinations in 31 genes. This pipeline was applied to a cohort of 90 epileptic patients who had previously an exome sequencing (ES) analysis, to determine the frequency of pharmacogenetic variants. We performed a retrospective analysis of drug plasma concentrations and treatment efficacy in patients bearing at least one relevant PharmGKB variant. For PharmGKB level 1A variants, CYP2C9 status for phenytoin prescription was the only relevant information. Nineteen patients were treated with phenytoin, among phenytoin-treated patients, none were poor metabolizers and four were intermediate metabolizers. While being treated with a standard protocol (10–23 mg/kg/30 min loading dose followed by 5 mg/kg/8 h maintenance dose), all identified intermediate metabolizers had toxic plasma concentrations (20 mg/L). In epileptic patients, pangenomic sequencing can provide information about common pharmacogenetic variants likely to be useful to guide therapeutic drug monitoring, and in the case of phenytoin, to prevent clinical toxicity caused by high plasma levels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
For data sharing requests, please contact SV or YD at simon.verdez@chu-dijon.fr or yannis.duffourd@u-bourgogne.fr respectively.
Code availability
Code is available at the following link: http://gitlab.gad-bioinfo.org/gad-public/pharmAnnot/tree/public
References
Hesselink D. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Therapeutics. 2003;74:245–54.
Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature 2015;526:343–50.
Sim SC, Kacevska M, Ingelman-Sundberg M. Pharmacogenomics of drug-metabolizing enzymes: a recent update on clinical implications and endogenous effects. Pharmacogenomics J. 2013;13:1–11.
Relling MV, Klein TE, Gammal RS, Whirl-Carrillo M, Hoffman JM, Caudle KE. The clinical pharmacogenetics implementation consortium: 10 years later. Clin Pharmacol Ther. 2020;107:171–5.
Bank PCD, Caudle KE, Swen JJ, Gammal RS, Whirl‐Carrillo M, Klein TE, et al. Comparison of the guidelines of the clinical pharmacogenetics implementation Consortium and the Dutch pharmacogenetics Working group. Clin Pharm Ther. 2018;103:599–618.
Visscher H, Ross CJD, Rassekh SR, Barhdadi A, Dubé MP, Al-Saloos H, et al. Canadian Pharmacogenomics Network for Drug Safety Consortium Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012;30:1422–8.
Picard N, Boyer JC, Etienne‐Grimaldi MC, Barin‐Le GC, Thomas F, Loriot MA. Pharmacogenetics‐based personalized therapy: levels of evidence and recommendations from the French Network of Pharmacogenetics (RNPGx). Therapie. 2017;72:185–92.
Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, et al. Pharmacogenomics Knowledge for Personalized Medicine. Clin Pharm Ther. 2012;92:414–7.
Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393:689–701.
Orsini A, Zara F, Striano P. Recent advances in epilepsy genetics. Neurosci Lett. 2018;667:4–9.
Symonds JD, Zuberi SM, Johnson MR. Advances in epilepsy gene discovery and implications for epilepsy diagnosis and treatment. Curr Opin Neurol. 2017;30:193–9.
Bruel A-L, Levy J, Elenga N, Defo A, Favre A, Lucron H, et al. INTU -related oral-facial-digital syndrome type VI: A confirmatory report. Clin Genet. 2018;93:1205–9.
Thevenon J, Duffourd Y, Masurel-Paulet A, Lefebvre M, Feillet F, El Chehadeh-Djebbar S, et al. Diagnostic odyssey in severe neurodevelopmental disorders: toward clinical whole-exome sequencing as a first-line diagnostic test. Clin Genet. 2016;89:700–7.
Bruel A-L, Nambot S, Quéré V, Vitobello A, Thevenon J, et al. Increased diagnostic and new genes identification outcome using research reanalysis of singleton exome sequencing. Eur J Hum Genet. 2019;27:1519–31.
Cousin MA, Matey ET, Blackburn PR, Boczek NJ, McAllister TM, Kruisselbrink TM, et al. Pharmacogenomic findings from clinical whole exome sequencing of diagnostic odyssey patients. Mol Genet Genom Med. 2017;5:269–79.
Blom S. trigeminal neuralgia: its treatment with a new anticonvulsant drug (G-32883). Lancet. 1962;279:839–40.
Arif H, Buchsbaum R, Weintraub D, Koyfman S, Salas-Humara C, Bazil CW, et al. Comparison and predictors of rash associated with 15 antiepileptic drugs. Neurology. 2007;68:1701–9.
Karnes JH, Rettie AE, Somogyi AA, Huddart R, Fohner AE, Formea CM, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C9 and HLA‐B Genotypes and Phenytoin Dosing: 2020 Update. Clin. Pharmacol. Ther. 2020;96:542–8.
Locharernkul C, Loplumlert J, Limotai C, Korkij W, Desudchit T, Tongkobpetch S, et al. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia. 2008;49:2087–91.
Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. A marker for Stevens–Johnson syndrome. Nature. 2004;428:486.
Anzenbacher P, Anzenbacherová E. Cytochromes P450 and metabolism of xenobiotics: CMLS. Cell Mol Life Sci. 2001;58:737–47.
Kidd RS, Curry TB, Gallagher S, Edeki T, Blaisdell J, Goldstein JA. Identification of a null allele of CYP2C9 in an African–American exhibiting toxicity to phenytoin. Pharmacogenetics Genomics. 2001;11:803–8.
Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005;5:6–13.
Karnes JH, Rettie AE, Somogyi AA, Huddart R, Fohner AE, Formea CM, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C9 and HLA-B Genotypes and Phenytoin Dosing: 2020 Update. Clin Pharmacol Therap. 2020;109:302–9.
Thauvin-Robinet C, Thevenon J, Nambot S, Delanne J, Kuentz P, Bruel AL, et al. Secondary actionable findings identified by exome sequencing: expected impact on the organisation of care from the study of 700 consecutive tests. Eur J Hum Genet. 2019;27:1197–214.
Ruden DM, Cingolani P, Patel VM, Coon M, Nguyen T, Land SJ, et al. Using Drosophila melanogaster as a Model for Genotoxic Chemical Mutational Studies with a New Program, SnpSift. Front Gene. 2012;3:35.
Ka S, Lee S, Hong J, Cho Y, Sung J, Kim HN, et al. HLAscan: genotyping of the HLA region using next-generation sequencing data. BMC Bioinforma. 2017;18:1–11.
Giancarlo GM, Venkatakrishnan K, Granda BW, von Moltke LL, Greenblatt DJ. Relative contributions of CYP2C9 and 2C19 to phenytoin 4-hydroxylation in vitro: inhibition by sulfaphenazole, omeprazole, and ticlopidine. Eur J Clin Pharm. 2001;57:31–36.
Bajpai M, Roskos LK, Shen DD, Levy RH. Roles of cytochrome P4502C9 and cytochrome P4502C19 in the stereoselective metabolism of phenytoin to its major metabolite. Drug Metab Dispos. 1996;24:1401–3.
van der Weide J, Steijns LS, van Weelden MJ, de Haan K. The effect of genetic polymorphism of cytochrome P450 CYP2C9 on phenytoin dose requirement. Pharmacogenetics. 2001;11:87–91.
Kudo T, Endo Y, Taguchi R, Yatsu M, Ito K. Metronidazole reduces the expression of cytochrome P450 enzymes in HepaRG cells and cryopreserved human hepatocytes. Xenobiotica. 2015;45:413–9.
Fohner AE, Ranatunga DK, Thai KK, Lawson BL, Rischx N, Oni-Orisan A, et al. Assessing the clinical impact of CYP2C9 pharmacogenetic variation on phenytoin prescribing practice and patient response in an integrated health system. Pharmacogenet Genomics. 2019;29:192–9.
Dorado P, López-Torres E, Peñas-Lledó EM, Martínez-Antón J, Llerena A. Neurological toxicity after phenytoin infusion in a pediatric patient with epilepsy: influence of CYP2C9, CYP2C19 and ABCB1 genetic polymorphisms. Pharmacogenomics J. 2013;13:359–61.
Cresteil T, Beaune P, Kremers P, Celier C, Guengerich FP, Leroux J-P. Immunoquantification of epoxide hydrolase and cytochrome P-450 isozymes in fetal and adult human liver microsomes. Eur J Biochem. 1985;151:345–50.
Koukouritaki SB, Manro JR, Marsh SA, Stevens JC, Rettie AE, McCarver DG. et al. Developmental Expression of Human Hepatic CYP2C9 and CYP2C19. J Pharm Exp Ther. 2004;308:965–74.
McCormack M, Alfirevic A, Bourgeois S, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N. Engl J Med. 2011;364:1134–43.
Yip VL, Marson AG, Jorgensen AL, Pirmohamed M, Alfirevic A. HLA genotype and carbamazepine-induced cutaneous adverse drug reactions: a systematic review. Clin Pharm Ther. 2012;92:757–65.
Zhou Y, Krebs K, Milani L, Lauschke VM. Global Frequencies of Clinically Important HLA Alleles and Their Implications For the Cost‐Effectiveness of Preemptive Pharmacogenetic Testing. Clin Pharmacol Ther. 2021;109:160–74.
Xue Y, Ankala A, Wilcox WR, Hegde MR. Solving the molecular diagnostic testing conundrum for Mendelian disorders in the era of next-generation sequencing: single-gene, gene panel, or exome/genome sequencing. Genet Med. 2015;17:444–51.
Acknowledgements
We thank the University of Burgundy Centre de Calcul (CCuB) for providing technical support and management of the informatics platform. This work was supported by grants from Dijon University Hospital, the ISITE-BFC (PIA ANR) and the European Union through the FEDER programs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors thank Suzanne Rankin from the Dijon University Hospital, Nicole Dorssers from simon fraser university and Cynthia Reichling for proofreading the manuscript.
Author information
Authors and Affiliations
Contributions
SV, PG, LF, NP and YD designed the study. SV, QT performed the clinical analysis. AV, FTMT, CP, interpreted exome data. FTMT, AV and CP performed the molecular laboratory work. SV, YD and ET performed the bioinformatics analysis. All the authors contributed to the writing and review of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Verdez, S., Thomas, Q., Garret, P. et al. Exome sequencing allows detection of relevant pharmacogenetic variants in epileptic patients. Pharmacogenomics J 22, 258–263 (2022). https://doi.org/10.1038/s41397-022-00280-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-022-00280-w