Abstract
Anaerobic ammonia-oxidizing (Anammox) bacteria (AnAOB) rely on nitrite supplied by ammonia-oxidizing bacteria (AOB) and archaea (AOA). Affinities for ammonia and oxygen play a crucial role in AOA/AOB competition and their association with AnAOB. In this work we measured the affinity constants for ammonia and oxygen (half-saturation; km) of two freshwater AOA enrichments, an AOA soil isolate (N. viennensis), and a freshwater AnAOB enrichment. The AOA enrichments had similar kinetics (μmax ≈ 0.36 d−1, km,NH4 ≈ 0.78 µM, and km,O2 ≈ 2.9 µM), whereas N. viennensis had similar km values but lower μmax (0.23 d−1). In agreement with the current paradigm, these AOA strains showed a higher affinity for ammonia (lower km,NH4; 0.34–1.27 µM) than published AOB measurements (>20 µM). The slower growing AnAOB (μmax ≈ 0.16 d−1) had much higher km values (km,NH4 ≈ 132 µM, km,NO2 ≈ 48 µM) and were inhibited by oxygen at low levels (half-oxygen inhibition; ki,O2 ≈ 0.092 µM). The higher affinity of AOA for ammonia relative to AnAOB, suggests AOA/AnAOB cooperation is only possible where AOA do not outcompete AnAOB for ammonia. Using a biofilm model, we show that environments of ammonia/oxygen counter diffusion, such as stratified lakes, favors this cooperation.
Similar content being viewed by others
Introduction
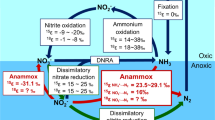
Anaerobic ammonia oxidizing (Anammox) bacteria (AnAOB) grow autotrophically on ammonia and nitrite under oxygen deplete conditions, which gives them a unique ecological niche. As a result, AnAOB are widely distributed in suboxic environments throughout the world, including marine, freshwater, terrestrial, and various extreme environments [1, 2]. In fact, it is estimated that Anammox is responsible for ~30% of global oceanic nitrogen loss [3]. However, the use of nitrite necessitates synergistic relationships between AnAOB and organisms that produce it, including ammonia oxidizing bacteria (AOB) and archaea (AOA) that aerobically oxidize ammonia to nitrite [4]. Since Anammox is inhibited by oxygen there is a delicate oxygen balance between the aerobic and anaerobic oxidation of ammonia. For example, in the oxic/anoxic boundary region of the stratified water column of the Black Sea, diffusion of nitrite produced by microaerophilic AOA and AOB growing in the upper low-oxygen region supports AnAOB in the adjacent lower suboxic region (<5 µM oxygen) [5]. Another example of their concomitant growth is in engineered biofilm systems, such as granular sludge wastewater treatment, where AOB reside in the aerobic surface layer of the spherical biofilm and AnAOB reside in an anoxic interior [6].
AOB are the dominant ammonia oxidizers in most wastewater treatment plants due to their copiotrophic growth characteristics [7, 8], whereas AOA dominate oligotrophic open oceans and soils where ammonia concentrations are significantly lower [9, 10]. AOB and AOA affinity, or the degree that these organisms can grow at low levels of ammonia and oxygen, therefore plays a crucial role in niche differentiation [11]. AOA also have a more efficient pathway for carbon fixation than do the AOB, contributing to better growth on limited substrate [12]. Similarly, affinity plays a role in niche differentiation of AnAOB species, where it has been suggested that members of the Brocadia clade are copiotrophic and dominate under nutrient replete conditions whereas members of the Kuenenia clade have more oligotrophic characteristics [1, 13]. Finally, affinity plays a role in cooperation (passing of nitrite) and competition (for ammonia) between AOA or AOB and AnAOB.
Since affinity plays a large role in determining what environments AnAOB and AOA or AOB cooperate and compete, in this work we characterized the kinetic affinity constants (half-saturation concentrations; ks) of two freshwater AOA enrichment cultures, one AOA pure culture (N. viennensis), and an AnAOB enrichment. Those data were then used to model environments supporting the synergistic association of ecotypes having differing affinities. Those analyses suggest that AOA and AnAOB cooperation is enhanced by cross-diffusional conditions (as in the ocean), whereas AOB and AnAOB cooperative growth is more compatible with co-diffusional habitats (as in biofilm systems).
Materials and methods
AOA and AnAOB culturing
Three AOA strains were used in this study, enrichments from Lakes Delaware (DW) and Acton (AC2) in Ohio, and a pure culture Nitrososphaera viennensis isolated from soil, all belonging to the group I.1a of the Thaumarchaeota [14, 15]. On the basis of ammonia monooxygenase gene sequence comparisons, AC2 is 82% identical to DW, and N. viennensis is 70 and 71% identical to AC2 and DW, respectively [15]. AnAOB granular biomass collected from an Anammox reactor treating anaerobic digester effluent pretreated with a nitritation reactor was provided by the wastewater treatment plant in Rotterdam Sluisjesdijk, the Netherlands [16]. The dominant Anammox organism in this sludge was Brocadia anammoxidans [16]. All cultures were grown at 30 °C in Crenarchaeota Medium containing 1 mM or 2 mM NH4Cl. Per L the media contained: 53.5 or 107 mg NH4Cl, 476.8 mg HEPES, 48 mg NaOH, 585 mg NaCl, 75 mg KCl, 147 mg CaCl2·2H2O, and 49 mg MgSO4·7H2O, 168 mg NaCO3, 2.8 mg FeNaEDTA, 0.2 mg KH2PO4, and 1 ml trace minerals mixture (per L): 8 mL 37% HCl, 30 mg H3BO3, 100 mg MnCl2·4H2O, 190 mg CoCl2·6H2O, 24 mg NiCl2·6H2O, 2 mg CuCl2·2H2O, 144 mg ZnSO4·7H2O, and 36 mg Na2MoO4·2H2O. Sustained growth of N. viennensis required the addition of 1 mM pyruvate as previously reported [14]. AnAOB cultures were supplemented with 1.3 or 2.0 mM NaNO2. Except during AOA batch growth tests, nitrite, ammonia (total ammonia; ammonia and ammonium), and total oxidized nitrogen (TON; nitrite plus nitrate) were measured using a GalleryTM Automated Photometric Analyzer (ThermoFisher Scientific, Waltham, MA U.S.A.) with the respective reagents, and measurements were calibrated using NaNO2, NH4Cl, and NaNO3, respectively. During batch growth tests, nitrite was measured by hand colorimetrically using diazotized sulfanilamide and NED dihydrochloride [17].
Maximum specific growth rate for AOA
The maximum specific growth rate (µmax) of the four strains as well as the kinetics for oxygen (limitation of AOA or inhibition of AnAOB), ammonia limitation, and nitrite limitation of AnAOB were characterized for all cultures. For determination of the µmax of AOA, cultures were placed in 100 ml of media in an unmixed closed 250 ml glass media bottle. AOA activity was tracked by measuring nitrite production, which is presumed proportional to biomass production, and µmax was fit to the resultant exponential growth curve. These tests were conducted at both 1 mM and 2 mM NH4Cl.
Oxygen and ammonia affinities for AOA
Similar to studies reported by Martens-Habbena et al. [11], AOA nutrient limitation tests were conducted using a Unisense oxygen needle sensor OX-N 13621 (Unisense, Aarhus N, Denmark) to measure activity. 120 ml of culture was placed in a 120 ml serum bottle (no head space), sealed with a rubber stopper, and continuously mixed using a magnetic stir bar to ensure homogeneity. The oxygen sensor was calibrated using a two-point calibration with the zero taken as the flat line of the oxygen depletion experiments and a saturation point (0.238 mM at 30 °C) taken by bubbling air in deionized water for at least an hour. The sensor was inserted through a hole punched through the rubber stopper and calked around it to prevent gas exchange. Oxygen measurements were taken every minute and “smoothed” by taking a 30 min average, computing oxygen consumption as the change in oxygen divided by the time step (1 min). Oxygen affinity was determined by tracking oxygen consumption as oxygen was depleted, and ammonia affinity was determined by tracking oxygen consumption as ammonia was depleted.
All AOA nutrient limitation results followed Michaelis–Menten type kinetics:
where µ is the specific growth rate (mM O2 d−1 mM NO2−1), S is the limiting substrate concentration (oxygen or ammonia; mM), and km is the half-saturation constant (mM). We determined µ by taking the oxygen consumption (mM O2 d−1) normalized to nitrite concentration (mM NO2), which was assumed to be proportional to biomass production. The unit conversion between d−1 (reported for the exponential growth µmax determination) and mM O2 d−1 mM NO2−1 (reported µ for the nutrient limitation experiments) was assumed 1.5 (i.e., nitrite is produced in a 1:1.5 ratio of nitrite produced to oxygen consumed). To determine km, µ was plotted against oxygen or ammonia depending on which nutrient was depleted in that experiment to generate an affinity curve. For ammonia, the ammonia concentration was computed by assuming ammonia is consumed at a 1.5:1 ratio to oxygen and was depleted when oxygen consumption stopped. The µmax value was fixed to the average value determined for each organism, and the initial biomass concentration (as nitrite) and km were fit by minimizing the sum of squared difference between the measured and modeled µ using Microsoft® Excel 2016 and the Solver Add-in. Initial nitrite concentration (surrogate for biomass) was fit instead of using an initial measurement because unlike during µmax determination, the biomass had gone through periods of starvation, and, therefore, biomass had decayed when nitrite had not, and measured nitrite was not a reliable measure of initial biomass concentration.
Maximum specific growth rate for AnAOB
Similar to AOA, AnAOB µmax was determined by growing the AnAOB enrichment in a batch culture and measuring the removal of ammonia and nitrite. AnAOB granules were broken up using a tissue potter and grown in serum bottle batch cultures. Bottles were sparged with nitrogen gas to remove oxygen prior to initiating batch growth and mixed with a stir bar throughout the experiment. The µmax was determined by fitting to the exponential nitrite removal curve (taken as the nitrite measurement at each time point minus the initial nitrite). Thus, each batch culture yielded two µmax values (from ammonia and nitrite). However, µmax determined using ammonia was systematically higher than for nitrite. We speculated that there were other organisms in the AnAOB culture contributing to this consumption (e.g., due to ammonia assimilation) as indicated by the reported ratio of nitrite to ammonia consumed during these tests. Since ammonia µmax was not a reliable measure of Anammox activity, µmax was only determined using nitrite removal.
Oxygen and ammonia affinities for AnAOB
Nutrient limitation and oxygen inhibition were determined using septum bottles with OxiTop® anaerobic pressure sensing heads (Xylem Analytics Germany Sales GmbH & Co. KG, Oberbayern, Germany), which detects pressure difference caused by the dinitrogen gas produced during anaerobic ammonia oxidation. The OxiTop system recorded a pressure every 1–4 min depending on the duration of the experiment recorded (6–24 h). Because the OxiTop system reports the pressure in discrete increments of whole number hPa, the pressure data was “smoothed” by taking a 2 h average, as opposed to the 30 min average used with the oxygen sensor. Gas production was computed as the change in pressure divided by the time step between sampling points. Ammonia and nitrite were measured at the beginning and end of each OxiTop experiment. Comparing the change in pressure to the change in ammonia or nitrite gave an approximate ratio of ammonia or nitrite consumed to gas pressure produced, which was used to compute a curve of continuous nitrite and ammonia consumption at the resolution of the pressure readings. The nitrite consumption rate was used as the measured μ, which, along with the nitrite or ammonia concentrations, were used to determine km. Similar to the procedure for AOA, the µmax value was fixed to the average value determined in the exponential growth experiment, and the initial biomass concentration (as nitrite) and km were fit by minimizing the sum of squared difference between the measured and modeled µ using Excel Solver.
Oxygen inhibition for AnAOB
For oxygen inhibition of Anammox, the OxiTop system was again used to measure activity. In addition, a Unisense oxygen needle sensor OX-N 13621 (same as was used for the AOA nutrient limitation tests) was inserted into the OxiTop serum bottle to track oxygen. At the beginning of the experiment a small volume of pure oxygen was injected into the bottle (0.8–1.2 µM O2 as measured by the oxygen sensor). The microbial community in the AnAOB enrichment slowly consumed the oxygen until it was depleted. The resulting pressure reading showed a slow decline in pressure as the oxygen was consumed and began to increase once Anammox activity was resumed (presented in Supplemental Information Figure S13). Similar to the km values, the half-maximum inhibition concentration of oxygen (ki; mM) was determined using μ (computed from pressure change) and oxygen concentration, and ki and initial biomass concentration (as nitrite) were fit using Excel Solver.
A typical inhibition function was used to describe oxygen inhibition:
where S is the oxygen concentration (mM).
Mathematical model
A one-dimensional biofilm model was used to demonstrate spatial cooperation/competition between AOA, AnAOB, and AOB. The model was implemented using Aquasim software [18], model structure, and diffusion properties from Volcke et al. [19], and typical AOB kinetic parameters from Rittmann and McCarty [20] extrapolated to 30 °C (µmax = 1.36 d−1, km,NH4 = 0.16 mM, km,O2 = 0.016 mM, and b (decay) = 0.19 d−1). The AOA and AnAOB kinetic parameters determined as described above were used, except the above determined µmax was a net growth and therefore, considered µmax −b in the model. For the model, b was assumed 0.14 * µmax(measured) and µmax = µmax(measured) + b. Yield was set to 0.1337 gCOD gN−1 for AOA and 0.0905 gCOD gN−1 for AOB assuming 1.44 gCOD gVSS−1 [12]. For ammonia/oxygen co-diffusion, bulk oxygen was fixed to 0.3 mg/L, and for ammonia/oxygen counter-diffusion oxygen diffused from the biofilm substratum set at 300 mg/L (approximate air). The models were run for 300 days with a hydraulic retention time of 0.4 d and influent ammonia of 7.0 mM. The complete equations and variables are displayed in Supplementary Information Tables S1 and S2.
Results
AOA and AnAOB kinetic parameters
Representative plots used for calculating μmax, km,NH4, and km,O2 for the DW AOA enrichment culture are displayed in Fig. 1, and a summary of results for AOA enrichments DW and AC2 and pure culture N. viennensis are present in Table 1. All parameter curve fittings are present in Supplemental Information Figures S1–S9.
Representative plots used to determine the maximum specific growth rate (μmax; d−1; plot a) half-saturation constant for ammonia (km,NH4; mM; plot b) and half-saturation constant for oxygen (km,O2; mM; plot c) for DW ammonia oxidizing archaea enrichment culture. The exponential production of nitrite was used to determine μmax in plot A (μmax = 0.41 d−1). Half-saturation constants were determined using the rate of oxygen consumption in plots B and C (km,NH4 = 0.77 µM; km,O2 = 3.6 µM)
Representative plots used for calculating μmax and ki,O2 for the AnAOB enrichment are shown in Fig. 2. Determinations of km,NH4 and km,O2 were done as for AOA (Fig. 1), except activity was determined with pressure change with the OxiTop system. The values for μmax, ratio of nitrite to ammonia consumed, ki,O2, km,NH4, and, km,NO2 determined for an AnAOB enrichment are present in Table 2. All parameter curve fittings are present in Supplemental Information Figures S10–S14.
Representative plots used to determine the maximum specific growth rate (μmax; d−1; plot a) and half-maximum inhibition concentration of oxygen (ki,O2; µM; plot b) for AnAOB. The exponential consumption of nitrite was used to determine μmax in plot A (μmax = 0.15 d−1). Gas production as pressure change was used to determine ki,O2 (ki,O2 = 0.12 µM)
AnAOB competition/cooperation with AOA/AOB and mathematical modeling
For cooperation between AOA and AnAOB to occur there needs to be a balance with oxygen (promotion of AOA/AOB and inhibition of AnAOB) and ammonia (supplied to both). We address two possible scenarios for this cooperation to occur: microaerophilic conditions, where oxygen is at a level that AOA activity is promoted to the same level as AnAOB is inhibited, and spatial separation, where nitrite produced by AOA/AOB diffuses from aerobic to anoxic environments for AnAOB to consume.
As opposed to ammonia, which promotes growth of AOA and AnAOB, oxygen inhibits AnAOB and promotes growth of AOA. Therefore, there is a concentration of oxygen that inhibits AnAOB activity to the same level as AOA growth promotion. This is shown in Fig. 3, using the oxygen affinity curve for AOA (generalized to be the average μmax and km,O2 of DW and AC2) and inhibition curve for AnAOB. Because our “activity” was normalized to measured nitrite production or consumption, the point where the curves cross is where the AOA are producing nitrite at the same rate AnAOB are consuming it; 0.32 µM or 10 µg/L.
Alternatively, spatial distributions could achieve cooperation if nitrite produced by AOA diffuses from aerobic to anoxic environments. To illustrate the separation of aerobic ammonia oxidation and Anammox, Fig. 4 shows modeled results of a biofilm with oxygen and ammonia supplied from the same side (co-diffusion) and from opposite sides (counter-diffusion).
Discussion
AOA kinetics
All three strains of AOA had approximately the same km,NH4 and km,O2. The km,NH4 was also similar to the value previously reported for an AOA enrichment from agricultural soil (km,NH4 = 0.69 ± 0.04 µM), where the dominant AOA was Candidatus Nirosoarchaeum koreensis, MY1 (99.8% identical to AC2 and 81% similar to DW [15]) [21]. The km,O2 of the strains from this study (km,O2 ≈ 2.9 µM), however, showed higher affinity for oxygen than that determined for MY1 (km,O2 = 10.38 ± 1.08 µM). These results further confirm the previous observations that AOA have a higher affinity for ammonia than AOB (km,NH4 ranging from ~20 to 2000 µM) [8]. The km,NH4 of these AOA is also comparable to the reported affinity of the complete ammonia oxidizing (comammox) bacteria Nitrospira inopinata (0.65 (0.12) µM), however in the same study they reported a substantially higher km,NH4 for N. viennensis (5.4 (0.6) µM) than we report here [22]. From a kinetic standpoint comammox and AOA should compete for ammonium and future studies are required to enhance our understanding of their ecological niche differentiation.
The measured μmax for the AOA enrichment cultures is similar to that measured by French et al. (≈ 0.38 d−1) [15]. While the measured μmax is similar for the two enrichment cultures, the maximum growth rate is markedly slower for N. viennensis (≈33% lower). A possible reason is that N. viennensis has been shown to have an optimal temperature of 35–37 °C whereas these characterizations were done at 30 °C [14]. Tourna et al. (2011) also reported that enrichment cultures of N. viennensis grew very well, but pure cultures only grew when they added pyruvate [14]. Although, pyruvate was added to the N. viennensis culture experiments reported here, it is possible that they are still limited (compared to the two enrichment cultures) due to factors consumed or supplied when growing with other organisms in enrichment culture. Thus, in consideration of high similarity of the other measured parameters for all three AOA, the μmax of N. viennensis may be closer to that of the DW and AC2 strains characterized in this study.
While affinity characterization can be very useful in determining niche differentiation, it cannot be taken as absolute determinant. As we have shown, there is variability in parameter determination, and other environmental conditions such as temperature, pH, and community factors can be important in apparent kinetic parameters. The extremely high affinity of AOA for ammonia, and a capacity for some to grow at an acidic pH where ammonium is the major species, has served for speculation that ammonium rather than ammonia is their substrate. Although recent modeling [23] indicates that a charged S-layer typical of most AOA could act to concentrate ammonium near the active site of ammonia oxidation, as yet there is no direct evidence for either species being the substrate.
AnAOB kinetics
The variability among replicates of the measured parameters for AnAOB was generally higher than for AOA, which is likely due to a combination of: (i) less precise activity measurements (OxiTop pressure sensor for AnAOB verses Unisense oxygen sensor for AOA), (ii) added variability due to clustered growth of AnAOB (despite dispersal of the culture), and (iii) higher degree of community complexity. Although AnAOB granular biomass was disintegrated, the resulting cells tended to flocculate/cluster again and settle faster, which could have resulted in diffusion limitations in the culture. The measured affinities were similar to some reported values [13], and other studies report much higher affinities for ammonia and nitrite (values for km,NH4 and km,NO2 that are an order of magnitude lower; down to 5 and 2.5 µM, respectively) [24,25,26]. The likely explanation is niche differentiation associated with affinity differences among AnAOB strains. This enrichment came from a wastewater treatment plant receiving very high concentrations of ammonia and nitrite [16], which likely selected for an R-strategist population. In contrast, AnAOB that are active in oligotrophic environments, such as found in oxygen minimum zones of the ocean, would be expected to have higher affinities typical of k-strategists. Aerobic nitrite oxidizers show a similar range of km,NO2 values: 9–1400 µM [27]. The ki,O2, determined for AnAOB on the other hand, is on the order of the value used by Strous et al. [26] (313 µM) and is quite low. Still, it does show some limited tolerance to oxygen suggesting the potential for activity in microaerophilic conditions. The μmax reported in literature ranges from 0.0038 d−1 to 0.33 d−1 at 30 °C [28], and therefore, the value we report is within this range. Similar to affinity, the μmax of different strains would be expected to differ based on their environment.
AnAOB competition/cooperation with AOA/AOB and mathematical modeling
When assessing competition between AnAOB and AOA for ammonia, it is clear that the AOA in this study have a higher affinity. The AOA km,NH4 is an order of magnitude lower than the lowest reported KNH4 for AnAOB (km,NH4 = 5 µM [26]). Thus, in oligotrophic environments with ammonia as the growth limiting substrate, AOA would easily outcompete AnAOB for ammonia. Cooperation between these AOA and AnAOB, on the other hand, depends on both nitrite, from AOA, and ammonia, not consumed by AOA, supplied to AnAOB. Therefore, the oxidation of ammonia by AOA needs to be partial, generating nitrite but not consuming all ammonia. This cooperation can be encouraged by limiting oxygen available to the system (microaerophilic; displayed in Fig. 3) or by spatial separation of the AnAOB and AOA activities (Fig. 4). Under oxygen saturated conditions nitrite oxidizing bacteria (NOB) would outcompete Anammox for nitrite, however this study sets its focus on environments that are oxygen limited—such as oxygen minimum zones in the oceans—and therefore NOB were not integrated into the mathematical models.
Spatial separation can occur in biofilm environments, such as in wastewater treatment where AOB consume oxygen and ammonia at the surface while excess ammonia and nitrite from the AOB diffuse into the lower anoxic areas of the biofilm for AnAOB to consume. However, this depends on ammonia being supplied in excess of oxygen so there is not an over-abundance of nitrite produced. In more oligotrophic environments these biofilms would favor AOA (or N. inopinata showing a similarly high affinity) over AOB, but oxygen would need to be significantly restricted to allow ammonia to diffuse to AnAOB. On the other hand, if ammonia was supplied in the anaerobic/anoxic side of a biofilm, AnAOB would have preferential access to ammonia. This leads to a situation where AnAOB are supplied with higher ammonia, shielded from oxygen, and limited by nitrite, and AOA have higher oxygen and limited ammonia, ideal for outcompeting AOB. In a conventional biofilm, AOB/AOA are oxygen limited. This “cross-diffusional” biofilm could form a more natural synergism between AOA and Anammox than through a microaerophilic environment analogous to the cohabitation situation illustrated by Fig. 3.
From the modeled results, AOB dominate in the co-diffusion biofilm, whereas the higher affinity AOA dominate in the counter-diffusion biofilm due to the ammonia limitation created by AnAOB. While cooperation with AOA can be simulated in co-diffusion situations, by limiting ammonia, this restricts the ammonia reaching the anoxic AnAOB layer, and thus the oxygen and ammonia need to be tightly adjusted to show this cooperation.
While counter-diffusion biofilms may not be significant to global nitrogen cycling, analogous situations of counter-diffusion occur within large regions of the marine water column. Taking the Black Sea example reported by Lam et al. 5, oxygen diffuses down from the oxygenated surface waters, while ammonia diffuses up from decay byproducts in the anoxic lower ocean. This leads to higher ammonia and lower oxygen levels in the deeper ocean, more favorable to AnAOB, and lower ammonia and higher oxygen levels in the upper ocean, more favorable to AOA. Similar concentration gradients exist in freshwater environments showing similar population responses [29, 30], and we attribute cooperation in these environments to the counter-diffusion of oxygen and ammonia. The topic of applying Anammox systems to mainstream wastewater treatment is also of interest due to the potential for low energy nitrogen removal; however, achieving low effluent nitrogen concentrations is a challenge [28]. What this analysis suggests is that at low ammonia concentrations, AOA enrichments should be more efficient than AOB, however if AOA and AnAOB are present in a biofilm, the most ideal scenario would be to provide the oxygen and ammonia counter-diffusionally to allow AnAOB activity (e.g., a membrane biofilm reactor [31]). Common biofilm reactors (granular sludge-based or moving bed biofilm reactors), however, are more difficult to control.
The affinity results presented in this study generally agree with published affinity km/i values for AOA and AnAOB. What has not been established, however, is the impact affinity has on AOA/AnAOB cooperation. Because of the very high affinity of AOA, we propound that it is environment of oxygen/ammonia counter-diffusion that encourage AOA/AnAOB cooperation. To further demonstrate this, growing AOA and AnAOB in a membrane biofilm reactor could be tried or populations in environment where partial nitritation and Anammox co-occure could be surveyed. Furthermore, there is a large diversity of AOA and AnAOB whose kinetics have not been explored and would be invaluable to this work.
References
Gao D, Wang X, Liang H, Wei Q, Dou Y, Li L. Anaerobic ammonia oxidizing bacteria: ecological distribution, metabolism, and microbial interactions. Front Environ Sci Eng. 2018;12:1–15.
Oshiki M, Satoh H, Okabe S. Ecology and physiology of anaerobic ammonium oxidizing bacteria. Environ Microbiol. 2016;18:2784–96.
Babbin AR, Keil RG, Devol AH, Ward BB. Oxygen control nitrogen loss in the ocean. Science (80-). 2014;344:406–8.
Kuypers MMM, Marchant HK, Kartal B. The microbial nitrogen-cycling network. Nat Rev Microbiol. 2018;16:263–76.
Lam P, Jensen MM, Lavik G, McGinnis DF, Muller B, Schubert CJ, et al. Linking crenarchaeal and bacterial nitrification to anammox in the Black Sea. Proc Natl Acad Sci. 2007;104:7104–9.
Winkler MKH, Kleerebezem R, Van Loosdrecht MCM. Integration of anammox into the aerobic granular sludge process for main stream wastewater treatment at ambient temperatures. Water Res. 2012;46:136–44.
Verhamme DT, Prosser JI, Nicol GW. Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J. 2011;5:1067–71.
Limpiyakorn T, Fürhacker M, Haberl R, Chodanon T, Srithep P, Sonthiphand P. AmoA-encoding archaea in wastewater treatment plants: a review. Appl Microbiol Biotechnol. 2013;97:1425–39.
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, et al. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature. 2006;442:806–9.
Wuchter C, Abbas B, Coolen MJL, Herfort L, van Bleijswijk J, Timmers P, et al. Archaeal nitrification in the ocean. Proc Natl Acad Sci USA. 2006;103:12317–22.
Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature. 2009;461:976–9.
Konneke M, Schubert DM, Brown PC, Hugler M, Standfest S, Schwander T, et al. Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation. Proc Natl Acad Sci USA. 2014;111:8239–44.
Zhang L, Narita Y, Gao L, Ali M, Oshiki M, Ishii S, et al. Microbial competition among anammox bacteria in nitrite-limited bioreactors. Water Res. 2017;125:249–58.
Tourna M, Stieglmeier M, Spang A, Könneke M, Schintlmeister A, Urich T, et al. Nitrosophaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci USA. 2011;108:8420–5.
French E, Kozlowski JA, Mukherjee M, Bullerjahn G, Bollmann A. Ecophysiological characterization of ammonia-oxidizing archaea and bacteria from freshwater. Appl Environ Microbiol. 2012;78:5773–80.
van der Star WRL, Abma WR, Blommers D, Mulder JW, Tokutomi T, Strous M, et al. Startup of reactors for anoxic ammonium oxidation: experiences from the first full-scale anammox reactor in Rotterdam. Water Res. 2007;41:4149–63.
AWWA. Standard Methods for the Examination of Water and Wastewater, 20th ed. American Public Health Association; 1999.
Reichert P. AQUASIM - a tool for simulation and data analysis of aquatic systems. Water Sci Technol. 1994;30:21–30.
Volcke EI, Picioreanu C, De Baets B, van Loosdrecht MC. Effect of granule size on autotrophic nitrogen removal in a granular sludge reactor. Environ Technol. 2010;31:1271–80.
Rittmann BE, McCarty PL. Environmental biotechnology: principles and applications. McGraw-Hill; 2001.
Jung MY, Park SJ, Min D, Kim JS, Rijpstra WIC, Damste JSS, et al. Enrichment and characterization of an autotrophic ammonia-oxidizing archaeon of mesophilic crenarchaeal group I.1a from an agricultural soil. Appl Environ Microbiol. 2011;77:8635–47.
Kits KD, Sedlacek CJ, Lebedeva EV, Han P, Bulaev A, Pjevac P, et al. Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature. 2017;549:269–72.
Li PN, Herrmann J, Tolar BB, Poitevin F, Ramdasi R, Bargar JR, et al. Nutrient transport suggests an evolutionary basis for charged archaeal surface layer proteins. ISME J. 2018;12:2389–402.
Hao X, Heijnen JJ, Van Loosdrecht MCM. Sensitivity analysis of a biofilm model describing a one-stage completely autotrophic nitrogen removal (CANON) process. Biotechnol Bioeng. 2001;77:266–77.
Lotti T, Kleerebezem R, Lubello C, van Loosdrecht MCM. Physiological and kinetic characterization of a suspended cell anammox culture. Water Res. 2014;60:1–14.
Strous M, Heijnen JJ, Kuenen JG, Jetten MSM. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl Microbiol Biotechnol. 1998;50:589–96.
Nowka B, Daims H, Spieck E. Comparison of oxidation kinetics of nitrite-oxidizing bacteria: Nitrite availability as a key factor in niche differentiation. Appl Environ Microbiol. 2015;81:745–53.
Tomaszewski M, Cema G, Ziembińska-Buczyńska A. Influence of temperature and pH on the anammox process: a review and meta-analysis. Chemosphere. 2017;182:203–14.
Schubert CJ, Durisch-kaiser E, Wehrli B, Thamdrup B, Lam P, Kuypers MMM. Anaerobic ammonium oxidation in a tropical freshwater system (Lake Tanganyika). Env Microbiol. 2006;8:1857–63.
Wenk CB, Blees J, Zopfi J, Veronesi M, Bourbonnais A, Schubert CJ, et al. Anaerobic ammonium oxidation (anammox) bacteria and sulfide-dependent denitrifiers coexist in the water column of a meromictic south-alpine lake. Limnol Oceanogr. 2013;58:1–12.
Nerenberg R. The membrane-biofilm reactor (MBfR) as a counter-diffusional biofilm process. Curr Opin Biotechnol. 2016;38:131–6.
Acknowledgments
This work was sponsored by the Defense Advanced Research Projects Agency (Contract Number: HR0011-17-2-0064), the content of the information does not necessarily reflect the position or the policy of the government, and no official endorsement should be inferred. The authors also thank Cristian Picioreanu for assistance in the model development.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Straka, L.L., Meinhardt, K.A., Bollmann, A. et al. Affinity informs environmental cooperation between ammonia-oxidizing archaea (AOA) and anaerobic ammonia-oxidizing (Anammox) bacteria. ISME J 13, 1997–2004 (2019). https://doi.org/10.1038/s41396-019-0408-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41396-019-0408-x
This article is cited by
-
Nitrification and beyond: metabolic versatility of ammonia oxidising archaea
The ISME Journal (2023)
-
Competitive and substrate limited environments drive metabolic heterogeneity for comammox Nitrospira
ISME Communications (2023)
-
Role of archaea in aquaculture: prospects and challenges
Aquaculture International (2023)
-
A general approach to explore prokaryotic protein glycosylation reveals the unique surface layer modulation of an anammox bacterium
The ISME Journal (2022)
-
Nitrogen cycling and microbial cooperation in the terrestrial subsurface
The ISME Journal (2022)