Abstract
Study design
Single-subject-research-design.
Objectives
To improve seated postural control in a participant with spinal cord injury (SCI) with a robotic Trunk-Support-Trainer (TruST).
Setting
Laboratory.
Methods
TruST delivered “assist-as-needed” forces on the participant’s torso during a motor learning-and-control-based intervention (TruST-intervention). TruST-assistive forces were progressed and matched to the participant’s postural trunk control gains across six intervention sessions. The T-shirt test was used to capture functional improvements while dressing the upper body. Kinematics were used to compute upper body excursions (cm) and velocity (cm2), and sitting workspace area (cm2). Functional trunk dynamometry was used to examine muscle force (Kg). Surface electromyography (sEMG) was applied to measure trunk muscle activity. The Borg Rating of Perceived Exertion (RPE) was used to monitor physical exertion during TruST-intervention. A two-standard-deviation bandwidth method was adopted for data interpretation.
Results
After TruST-intervention, the participant halved the time needed to don and doff a T-shirt, increased muscle force of trunk muscles (mean = 3 kg), acquired a steadier postural sitting control without vision (mean excursion baseline: 76.0 ± 2 SD = 5.25 cm and post-intervention: 44.1 cm; and mean velocity baseline: 3.0 ± 2 SD = 0.2 cm/s and post-intervention: 1.8 cm/s), and expanded his sitting workspace area (mean baseline: 36.7 ± 2 SD = 36.6 cm2 and post-intervention: 419.2 cm2). The participant increased his tolerance to counteract greater TruST-force perturbations in lateral and posterior directions. Furthermore, abdominal muscle activity substantially augmented after completion of TruST-intervention across all perturbation directions.
Conclusions
Our data indicate a potential effectiveness of TruST-intervention to promote functional sitting in SCI.
Similar content being viewed by others
Introduction
In developed socioeconomic countries, high-energy (e.g., vehicle or sports accidents) and low-energy (e.g., falls) traumatisms are the leading cause of spinal cord injury (SCI). In the last five decades in the United States, there have been an increase in motor incomplete injuries, as defined by the American Spinal Injury Assessment (ASIA) Impairment Scale (AIS) [1]. People with SCI experience severe limitations in mobility, function, self-care, and participation [2,3,4]. Moreover, most of these individuals require wheelchairs to function and participate in society. Thus, effective neurorehabilitation strategies that maximize upper body control, as a whole, could promote functional independence in people with SCI who are wheelchair-users.
SCI causes a constellation of complex sensorimotor defects such as muscle tone dysregulation, flaccid paralysis, hyperreflexia and spasticity, and muscle dyscoordination of limbs and trunk musculature [3]. Without the active control of paraspinal muscles, the trunk is a multisegmented structure with a high level of intrinsic instability. As a result, intricate neuromuscular activations are required to control the center of mass (COM) of the upper body during actions [5,6,7,8]. People with SCI demonstrate significant postural-related impairments such as lack of multidirectional trunk control, inability to shift body weight in sitting, trunk balance deficits, and reduced sitting workspace—which in the present study is defined as the immediate peripersonal space in which a person can function without changing the base of support (BOS) or falling. For people with SCI, deficits in upper body control hamper their performance during activities of daily living (ADLs) such as dressing tasks [9,10,11]. Furthermore, poor trunk control in people with SCI results in frequent compensatory upper extremity strategies during ADLs [12]. In other words, people with SCI may overcome postural demands by reaching for support and expanding their BOS; which inevitably ends up limiting the use of arms and hands to interact with the environment.
Different neuro-rehabilitatory approaches are currently being investigated to improve seated trunk control in individuals with SCI. A study in people with chronic SCI (AIS A or C) showed that spinal stimulation induces short-term improvements in trunk muscle tone, pelvic alignment, and sitting stability [13]. Other studies involved activity-based approaches in which sensory-based movements were employed to restore the supraspinal and intrinsic control of the injured spine. Studies in rodents with SCI showed that electrochemical spinal neuromodulation (i.e., serotoninergic replacement therapy and epidural electrical stimulation) during trunk control via gravity-assisted robotics (i.e., adjustable body weight support during ambulation) enhanced cortico-reticulo-spinal connectivity, bilateral proprioceptive-mediated postural responses (i.e., coordinated muscles contractions between flexor-extensors muscles), and natural patterns (i.e., unconstrained stepping) in weight-bearing walking and swimming tasks [14, 15]. Similarly, in humans, the use of epidural and transcutaneous spinal electrical stimulation of the lumbosacral region results in partial recovery of over-ground ambulation and trunk control [13, 16]. These combinatorial rehabilitative strategies, including cutting-edge biomedical technology, share two common key factors: activity-based practice and trunk stability.
Activity-based interventions may enhance the sensorimotor system damaged after SCI [17]. Clinical research on this type of interventions show promise to improve postural control, gait, and functional capabilities in SCI [18, 19]. We believe that the profound trunk control impairment in people with SCI can be a substantial impediment for the proper implementation of intensive activity-based therapies. In line with other authors, we think the application of technology and robotics to implement motor learning and control principles, and intensive task-specific practice, are key to restore sensorimotor-related spinal networks and induce long-lasting functional benefits in complex motor actions and self-care behaviors [20].
There exist different conventional postural systems in rehabilitation to statically support the trunk after SCI (e.g., torso orthoses and wheelchair accessories). In the field of technology, some of the current systems consist of instrumented seats embedded on movable platforms that rotate across different planes of motion while the user performs movements or interfaces with screens that deliver visual feedback [21, 22]. Nonetheless, conventional or mechanical systems do not allow the clinician to systematically implement dynamic postural support on specific regions of the trunk during motor practice. Most importantly, these trunk support systems do not allow the implementation of postural task-progression—i.e., trunk control assistance that is progressively reduced to add postural challenge during motor practice. Assist-as-needed force field technology is a solution to address progressive postural support. In brief, assistive-force fields can be defined as a field of vectors that interact with a specific body part and are configured to assist the person to move beyond a predefined space region. The robotic trunk-support-trainer (TruST) applies assistive-force fields in real-time so that users can actively move beyond their sitting stability boundaries and expand their sitting workspace [9]. Furthermore, the convergent application of assistive-force fields and activity-based intervention acts synergistically to improve postural strategies [23].
The present proof-of-concept and feasibility single-subject-research-design study investigates the applicability of TruST as a means to implement an activity-based intervention (TruST-intervention) to train goal-oriented postural and reaching tasks in sitting for a person with SCI. The activity-based tasks are founded on motor learning and control parameters. We hypothesized that TruST-intervention would ameliorate functional limitations (reduced dressing time) and improve upper body control (postural-related kinematics) in a participant with SCI.
Material and methods
Clinical and anthropometric features of the participant
Table 1 summarizes the participant’s de-identified demographic and clinical information. The participant with SCI presented with poor trunk control, as defined by the asymmetric and reduced workspace present during baseline sessions. Medical records were used to characterize the SCI following the Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) [24]. AIS was used to define the severity of the SCI as complete (AIS A), sensory incomplete (AIS B), motor incomplete (AIS C or D), or normal (AIS E) [24]. The Spinal Cord Independence Measure-III (SCIM-III) was used to score the level of functional independence during ADLs, in which a score of 100 indicates maximum independence [25]. Inability of walking was classified with the Walking Index for Spinal Cord Injury-II (WISCI-II), which determines the person’s ability to ambulate 10 m on a scale from 0 (inability to stand and/or participate in assisted walking) to 20 (ambulation without devices, braces, or physical assistance) [26]. Upper body dressing was assessed with the T-shirt test, which measures the time a person takes to don and doff a t-shirt [27]. The Borg Rating of Perceived Exertion-Category Ratio (BRPE) was used as an indicator of physical tolerance and to monitor activity intensity during TruST-intervention [28]. A BRPE of 9-10 was indicative of physical intolerance during TruST-intervention.
Additionally, the trunk muscle force-generation ability of the participant was measured with functional dynamometry (kg) in sitting position. As in our previous study, we used a “make test approach” with a hand-held dynamometer (Lafayette Manual Muscle Tester), in which the participant had to exert maximal isometric contractions with the dynamometer held stationary [9]. We tested overall activity of the muscle groups of the torso: flexion (rectus abdominis), extension (bilateral erector spinae), lateral flexion (quadratus lumborum and ipsilateral erector spinae) and rotations (abdominal external and internal obliques). The position of the hand-held dynamometer was on the chest (sternum, below the sternal notch) for trunk flexion, on lateral shoulder (lateral deltoid region) for trunk lateral flexion, inter-scapular region (between the superior angle of scapulae) for trunk extension, and on anterior deltoid region for measuring trunk rotations. The participant was sitting with arms crossed over the chest and was instructed to perform maximum isometric contractions during 3 s in each trunk direction. The average of three dynamometry trials was computed.
Inclusion and exclusion criteria
The inclusion criteria were: (1) traumatic SCI, (2) chronic SCI (>1 yr), and (3) WISCI-II ≤ 2. Exclusion criteria included: (1) surgeries 6mos before participation, (2) any medical condition that contraindicates intense physical exertion (e.g., heart, pulmonary, liver or renal disease), and (3) uncontrolled neurologic or musculoskeletal pain by medication.
Study design
Approval for this study was obtained by the IRB for Human Research at Columbia University (Protocol IRB# AAAQ7781). The participant was informed about the research features, goal, commitment, and then consented prior to start the study.
Figure 1 outlines the study phases of our ABA single-subject-research-design study (with a 1 week withdrawal phase). The study had a total of 10 sessions: three baselines, six intervention sessions, and one 1 week post-intervention session. The study was completed in 5 weeks. In the first week, we collected three baseline assessments to establish stability of the data. Baseline and intervention sessions occurred every other day without including weekends. The post-intervention assessment was scheduled one week after the last intervention session. We used a 2 SD bandwidth method to interpret outcomes as substantial improvements or detriments, or unchanged outcomes, with respect to baseline. At the time of the study, the participant was only receiving TruST-intervention; which prevented potential interferences from other therapies or physical activities [29]. CARE guidelines were followed to report the present study.
We collected three baselines during the first week to establish measurement stability of clinical (T-shirt test) and kinematic (sitting workspace) data. TruST-intervention consisted of six training sessions spread across two weeks. Each session lasted 60–90 min, including assessments and breaks. Thus, effective training (i.e., active movement practice) was approximately 60–70 min. After 1week withdrawal, the participant was re-assessed (post-intervention assessment). BL1, BL2, or BL3 Baseline1, Baseline2, or Baseline3, respectively. 2SD 2 Standard deviations.
Methods
Experimental setup and procedures
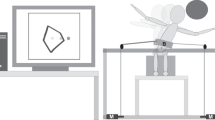
The participant sat on a bench (Kaye products Inc., North Carolina) with pelvic straps over the iliac crests for safety purposes. A light harness, anchored to the ceiling, was attached to the participant’s torso to prevent falls from the bench. This harness was slack and did not provide any additional weight support. A restraint system composed of four passive strings was added to the robotic TruST system as an additional safety barrier to prevent potential falls during the motor intervention (Fig. 2).
The picture shows the participant sitting on the bench and wearing the TruST-belt while practicing a boxing activity. The black lines depict two of the four cables controlled by the motors to deliver assistive-force fields (1). The red dotted lines highlight two of the four passive cables connected to the belt to prevent potential falls in the horizontal plane (2). The yellow dotted line represents the used slack harness to prevent potential falls in the vertical plane (3).
Kinematics were recorded with 19 infrared motion capture cameras (Vicon Vero 2.2, Denver). Reflective markers were secured with hypoallergic tape on the skin. As defined by de Leva (1996), markers were placed on anatomical landmarks to recreate the participant’s upper body as a 8 linked-system (head, upper thorax, lower thorax, pelvis, arms, and forearms) and estimate its center of mass (COM) [30]. We recorded surface electromyography (sEMG) (Delsys Trigno Wireless System, Massachusetts). The sEMG electrodes (Trigno Avanti, rectangular sensors with a dimension of 27 × 37 × 13 mm and a weight of 14 g) were placed bilaterally on rectus abdominis (RA)—1 cm above and lateral to the umbilicus—and paravertebral lumbar and thoracic muscles—latissimus dorsi, iliocostalis, longissimus, spinalis, and quadratus lumborum [31].
To study reactive seated postural control, TruST was configured to deliver perturbative forces through the belt toward anterior, posterior, right, and left directions. The perturbations were delivered as pulse forces that lasted 450 ms (150 ms ramp up, 150 ms of steady phase, and 150 ms ramp down). Postural kinematics and sEMG of trunk muscles were analyzed during the first two seconds after the perturbation onset. A progressive 2%incremental protocol based on the participant´s body weight (%BW) was used to measure the participant’s tolerance and reactive postural control capability against the progressive application of unforthcoming perturbations. Maximum tolerance was defined as the need of the participant to use the hands for postural support. In other words, the participant showed inability to counteract the perturbative force by eliciting an “in-place” trunk control strategy (i.e., without changing or expanding the BOS).
The robotic trunk-support-trainer (TruST)
The engineering and technological details of TruST have been previously published and validated [32, 33]. The robotic TruST is a motorized-cable driven system that applies forces in the horizontal plane in any direction to actively manipulate the participant’s position. TruST comprises four cables that are connected to a belt, which is placed on the participant’s torso (thoracic region: T9–12). In our previous SCI studies, and people with other neuromotor disorders such as cerebral palsy, we have configured TruST to create a force field that corresponds to the participants’ postural stability boundaries [9, 23, 32,33,34]. The functional translation of this robotic setting is that participants can actively control their upper body within and beyond their sitting stability boundaries while using upper extremities to perform goal-oriented motor activities. Specifically with TruST, when participants move beyond their sitting stability boundaries, the system provides direction-specific assistive-force fields toward the predefined boundaries. TruST-generated assistive-force fields supplement the lack of neuromuscular control and balance, and prevent the participant from falling or engaging the upper extremities for postural support during motor practice. The intensity of the assistive-force field is set as a percentage of the participant’s BW. Nonetheless, TruST-force field boundaries are expanded as the participant progressively gains postural trunk control across the intervention sessions.
The postural star-sitting test and TruST-force field configuration
The postural star-sitting test is a customized test based on the Star Excursion Balance Test for lower extremity injuries and other SCI-related balance tests [35,36,37]. The postural star-sitting test has been satisfactorily implemented for evaluative purposes in adults with SCI and children with cerebral palsy [9, 33]. The participant is instructed to maintain a sitting position while performing maximal trunk excursions along eight 45°star-radiated trajectories. Once at maximum amplitude, the participant has to return to sitting posture without hands assistance. The examiner places a ball close to the participant’s forehead and displaces it along the examined trajectory just to guide the trunk movements [9]. The data is used to configure force field boundaries that match the participant’s sitting stability limits. It is also used to objectively measure sitting workspace area (cm2) in which the participant demonstrates active trunk control. The goal is to implement objective and systematic postural-task progression during TruST-intervention. In other words, we optimize the level of postural training during motor practice based on the trunk control status of the participant in each intervention session.
TruST-intervention
In TruST-intervention, postural control is conceptualized as four major dimensions. Steady-state postural control is the ability to cope with the body’s center of mass relative to the base of support (BOS). Active postural control is defined as the ability to elicit continuous compensatory postural adjustments during an action. Proactive postural control is defined as the anticipatory muscle control strategies executed prior to the action in order to minimize the expected loss of balance. Reactive postural control is the ability to execute rapid corrective muscle strategies during unpredicted perturbations [38, 39]. These subdivisions in postural control allow us to target postural strategies via specific actions as well as what control parameters to modulate during TruST-intervention (Fig. 3).
A The motor intervention follows a 360-degree approach around the subject by addressing 8 star-radiated directions from the participant’s sitting position. B Motor task features can be discrete (characterized by a defined start and end), serial (group of discrete tasks), or continuous (tasks that stop arbitrarily). C1 and C2 Definition and criterion of postural control strategies trained without and with assistive TruST-force fields (within-boundaries and beyond-boundaries, respectively). D Motor control parameters to be applied during C2. Multilimb coordination: ability to move both upper limbs simultaneously. Control precision: ability to perform rapid and precise movements to control devices (i.e., games or toys). Response orientation: ability to move to specific direction/s. Reaction time: ability to respond rapidly to an external signal. Arm movement speed: ability to perform fast movements with the upper extremities. Rate of control: ability to time anticipatory and compensatory postural and arm adjustments in response to speed and/or direction changes of a continuously moving target. This parameter is of critical importance in TruST-intervention. Manual dexterity: ability to perform skillful hand-arm movements with objects. Finger dexterity: Ability to perform skillful finger movements with small objects such as coins/checkers. Arm-hand steadiness: ability to maintain steady hand-arm and postural positions. Wrist, finger speed: ability to perform rapid and repetitive wrist/finger movements. Aiming: ability to move the hand/finger to a small target that can be static or moving.
During TruST-intervention, the system delivers real-time visual feedback of the participant’s trunk position with respect to the predefined stability boundaries. This information is displayed on a computer screen that is used by the clinician to objectively target seated postural control strategies within stability boundaries (i.e., inactive TruST-force field) or beyond stability boundaries (i.e., active TruST-force field). Figure 2 depicts our TruST-intervention algorithm. We have applied successfully the motor learning and control parameters used in this study in SCI and other neuromotor conditions. Similarly, other researchers have applied similar training parameters in other motor learning-based approaches [18, 23, 33, 40, 41]. The participant practiced (i) pointing tasks with buzzers, (ii) reaching for balls of different sizes and small checkers, (iii) bimanual holding or catching, (iv) visual aiming skills, (v) bimanual or unimanual throwing, and (vi) adapted boxing. The intervention sessions were 90 minutes long, including a 10–15 min break. The motor activities practiced during TruST-intervention follow the same star-shaped scheme as in the postural-star sitting test (Fig. 2). The participant practiced a range of 15–20 repetitions in each one of the eight directions practiced (a total of 120–160 repetitions). The goal of TruST-intervention in each motor task is to make the participant practice postural strategies within stability boundaries; and then, to practice postural control beyond stability boundaries while receiving assistive-force fields. The intensity of TruST-force fields was set at 15%BW and it remained constant across intervention sessions. However, the assistive-force field boundaries, as determined by the postural-star sitting test before the intervention, were adjusted to the improved sitting stability boundaries of the participant.
Data reduction
MATLAB (R2021a, Mathworks Inc), was used to filter and process kinematic and sEMG data. Kinematics (100 Hz) were smoothed with a zero time-lag 4th order Butterworth filter at 6 Hz cutoff. Surface EMG signals (1000 Hz) were band-pass filtered (60–500 Hz), rectified, and low-pass filtered at 100 Hz. We estimated the upper body COM based on the participant’s anthropometric data [30]. We computed total COM excursions across sagittal and frontal planes during 30 s of resting sitting (i.e., steady-state balance control) with and without vision. As in previous studies, we computed workspace area (cm2) based on maximal trunk excursions during the postural star-sitting test with the in-built MATLAB function boundary(x,y, 0.05) [9, 23, 33].
Results
T-shirt test
As Table 2 displays, the participant substantially reduced the amount of time required for donning and doffing a t-shirt while sitting in the wheelchair.
Functional dynamometry
The participant increased the muscle-force generation of trunk muscles after TruST-intervention (Table 3).
Steady-state postural sitting control
The participant acquired the ability to maintain a still sitting posture, with and without vision, after TruST-intervention (Fig. 4). Compared to baseline values, COM upper body excursions and velocity were reduced during steady-state seated postural control with vision (COM excursions, mean baseline: 92.8 ± 2 SD = 204.5 cm, post-intervention: 21.2 cm, and 1week post-Intervention: 43.7 cm; COM velocity, mean baseline: 3.7 ± 2 SD = 8.2 cm/s, post-intervention: 0.9 cm/s, and 1week post-intervention: 1.7 cm/s) and without vision (COM excursion, mean baseline: 76.0 ± 2 SD = 5.3 cm, post-intervention: 44.1 cm, and 1week post-intervention: 15.1 cm; COM velocity, mean baseline: 3.0 ± 2 SD = 0.2 cm/s, post-intervention: 1.8 cm/s, 1week post-intervention: 0.6 cm/s). Nonetheless, the great amount of variability (SD) observed during baseline obscured the interpretation of this improvement as substantial.
Three-dimensional paths (blue lines) representing stability of upper body COM (red circle) while sitting with and without vision before TruST-Intervention (A, B) and after TruST-Intervention (C, D), respectively. Note the reduction in upper body COM, one week after the completion of TruST-intervention.
Active-proactive postural sitting
The participant substantially expanded his sitting workspace area (mean baseline: 36.7 ± 2 SD = 36.6 cm2) after completion of TruST-intervention (post intervention: 419.2 cm2 and 1 week post intervention = 215.1 cm2) (Fig. 5).
Sitting balance boundaries (red lines) and volitional trunk excursions (blue paths) during the postural star-sitting test in baseline (A), post-intervention (B), and 1week post-intervention (C). Sitting workspace area (cm2) are represented for each study session to display the substantial increase in workspace area (D): baseline and 1st pre-intervention session (red squares), 2nd–6th intervention sessions (blue circles), and 1 week after TruST intervention (green triangle).
Reactive postural sitting control
The outcomes from the 2%increment-protocol to investigate reactive postural control showed that the participant increased his motor capability to counteract the reactive forces delivered by the robotic TruST. These improvements were observed in all directions with the exception of forward perturbations (Fig. 6A). The kinematic analysis showed an improvement trend in the control of the upper body, as indicated by a decrease in COM excursions (cm) and COM velocity (cm/s) (Fig. 6B, C).
Graphs display maximum intensity tolerated by the participant in each perturbation direction at baseline and 1 week after receiving TruST-intervention. The participant acquired the ability to receive a perturbation force intensity equivalent to 20%BW post-intervention, except in forward perturbations (A). We can note an improving trend in upper body control, as determined by a decrease in upper body COM excursions (B) and velocity (C). However, these improvements did not achieve the 2 SD bandwidth criterion to be interpreted as substantial. sEMG analysis showed a substantial increase of bilateral thoracic muscles (5, 6) during anterior perturbations and augmented activity of the right abdominal muscle group (1) across all directions (D). %BW = perturbative force intensity scaled to the participant’s body weight %. EMG electromyography. mV millivolts. Right abdominal muscle group = 1. Left abdominal muscle group = 2. Right lumbar muscle group = 3. Left lumbar muscle group = 4. Right thoracic muscle group = 5. Left thoracic muscle group = 6.
EMG responses were highly variable within and across study sessions. The EMG analysis displayed augmented muscle activity of right abdominal in all directions and of paravertebral thoracic muscles (right and left) during anterior perturbations (Fig. 6D).
Physical exertion tolerance & pain
No adverse events were found. The participant categorized the physical demands of TruST-intervention as “sort of hard, hard, or really hard” (BRPE range = 4–8), across sessions. Moreover, TruST-intervention did not cause any pain or discomfort at any point of the study. The participant only reported one-point increase in the pain visual analogue scale (1/10 points) after the motor intervention in the first two sessions and in the last session.
Discussion
Our single-subject-research-design study shows the feasibility and effectiveness of TruST-intervention to improve upper body dressing skills, seated postural control strategies, and force-generation capacity of trunk muscles in a participant with SCI. The participant completed all scheduled intervention sessions, showed acceptable physical exertion during motor practice, and did not report musculoskeletal pain aside from muscle fatigue.
During TruST-intervention, the participant was continuously challenged to achieve trunk amplitudes beyond sitting stability boundaries. We believe TruST-intervention improved the muscle condition of the trunk (as indicated by the increase in functional dynamometry) and promoted activity-dependent plasticity through a continuous bidirectional communication between the brain and the injured spinal cord. Following this same concept, in our previous study with an ambulatory participant with SCI, we showed that robotic-mediated assistive and reactive forces during task-specific motor practice in standing improved functional balance, proprioceptive-mediated postural responses, ankle control, activity of stabilizing hip muscles, and cardiovascular endurance [23]. Similarly, a study investigating an activity-based intervention involving stepping tasks showed modulations in the physiological status of spinal circuits and activity-dependent plasticity [42]. Another study also demonstrated how the delivery of visual feedback during trial-and-error practice of postural tasks via video games synchronized with a force plate improved sitting balance [43].
Authors have reported the need to improve the therapeutic design of activity-based interventions in people with SCI [44]. In a randomized controlled trial, the authors investigated a task-specific training program during unsupported sitting in people with SCI. The sessions were an hour long and scheduled 3 times per week, across 6 weeks. The authors created three variants of the motor tasks depending on the participant’s sitting abilities. They found improvements (2.0–10.8 cm) in the maximal balance range test of trunk motion. Nonetheless, they did not find functional post-intervention effects in the T-shirt test [18]. In our study, the participant did not only acquire effective seated postural control strategies and greater workspace area but also showed a substantial reduction in both donning and doffing a T-shirt. We believe that there are two main reasons why prior activity-based interventions for individuals with SCI may not cause functional improvements, aside from the severity of the spinal injury. One is the lack of a structured intervention protocol based on motor learning and control parameters. Indeed, a study of children with cerebral palsy found greater improvements in motor control of the trunk and arm when participants followed structured skill practice (i.e., increasing repetitions and skillful features of motor tasks) compared to unstructured practice [45]. Another critical aspect is the absence of task-progression—the systematic addition of task complexity or challenge during motor practice. In TruST-intervention, the clinician provided both motor- and postural-task progression. Motor practice started within boundaries, then moved to beyond-boundaries, and finally several motor control parameters were modulated and randomized around the 360° peripersonal space of the participant. Most importantly, TruST-intervention delivered an assistive-force field to the person’s trunk to practice motor tasks beyond stability boundaries. Then, the diameter of this assistive-force field was expanded as the person gained sitting workspace. We assume that maximizing postural control by achieving greater trunk amplitudes might be enhancing the proprioceptive system (e.g., muscle spindles and Golgi tendon organs). Mechanistically, as the participant actively expanded his sitting stability boundaries, the trunk muscle fibers adopted new lengthened configurations that demanded higher level of sensorimotor control and rate of force development. Hence, proprioception may be critical in these improvements; although, further studies are required to confirm this interpretation.
A typical finding in people with SCI, and other neuromotor conditions, is muscle paresis. An impairment in the force-generation capability of the muscular system causes detriments in muscle strength (maximum peak force that a muscle group can develop in a specified task) and muscle power (rate of force development) [46]. TruST-intervention is not built around muscle training principles—tone, strength, or endurance. However, our participant substantially improved trunk strength of all muscle groups in a range of 2–5 kg. Our muscle force testing approach was not a standardized protocol; however, the dynamometry provided us with an approximation on the potential effect of TruST-intervention for improving muscle force. It is noteworthy to mention that this outcome was observed despite the muscle atrophy present in our participant due to the chronicity of his spinal lesion (33 yrs). Other studies have investigated how to address muscle paresis with the use of neurostimulation. A study investigated several combinatory neurostimulation protocols with magnetic stimulation, non-invasive transcutaneous spinal stimulation, and peripheral nerve stimulation. The authors found a facilitation in the excitability of the corticospinal system, motor evoked potentials of distal muscles, and H-reflexes that were associated with increased dorsiflexion force [47]. While these results emphasize the residual sensorimotor plasticity of the injured spine and its potential for motor recovery, muscle control benefits were only observed during stimulation or briefly after its application. Based on our results, the retention of motor outcomes would yet require intensive motor practice alongside neurostimulation.
As in bipedal stance, postural sway in sitting may be controlled through small-amplitude sensory-mediated corrective torques [48]. In our present study, we examined quiet sitting without vision to test improvements in proprioceptive-mediated postural trunk responses during unsupported sitting. Our kinematic analysis revealed a reduction in excursions and velocity of the upper body COM after TruST-intervention. These postural kinematics may be indicative of a more efficient use of somatosensory information to maintain sitting posture. In addition to prolonged muscle force, the active and automatic stabilization of the spine would be partially controlled by the processing of sensory-based information (muscle spindles) in a coordinated fashion across intersegmental spinal networks [49, 50].
The participant of our study improved his tolerance to react against direction-specific perturbations during unsupported sitting—“in-place” postural control strategies. A study showed that in-place trunk control responses require a high level of force, joint control, and neuromuscular coordination to counteract the reactive torques and kinetic energy secondary to external forces [51]. In our study, perturbative forces equivalent to 10-15% of the participant’s body weight were sufficient to observe sitting control failure during baseline. However, with the exception of forward perturbations, the participant was capable to respond to perturbative forces as intense as 20% of the participant’s body weight after TruST-intervention. In baseline, the greatest lack of trunk control was observed in the frontal plane during leftward and rightward perturbations. However, the participant increased his threshold to tolerate lateral forces and reduced upper body COM excursions after TruST-intervention. Trunk control in the frontal plane would require the coordinated activation of right and left muscle groups within the thorax to prevent postural failure. Our participant showed a decreasing trend in upper body COM excursions and velocity in all perturbation directions despite showing an increase only on his right abdominal muscle group and a slight increase on his right and left thoracic muscles. Probably, the spinal insult in our participant affected at greater extent the reactive control of the left dorsal and ventral muscles of the thorax. Still, partial neuromuscular recovery was possible after TruST-intervention.
Future line of research and limitations
In this study, we gathered postural-related kinematics and functional data to demonstrate the synergistic effect of a structured motor learning-and-control-based intervention with force-based postural-task progression through the robotic TruST platform. Overall, the results show potential effectiveness of TruST-intervention to train seated postural control abilities in people with SCI and trunk control dysfunction. Nonetheless, a parallel intervention study with a larger sample size will be needed to generalize our outcomes and address intervention effect sizes.
Data availability
The data that support the findings of this study will be available on request from the corresponding author, SA.
References
Chen Y, He Y, DeVivo MJ. Changing demographics and injury profile of new traumatic spinal cord injuries in the United States, 1972–2014. Arch Phys Med Rehabil. 2016;97:1610–9.
Tederko P, Middleton J, Mycielski J, Joseph C, Pagliacci MC, Rapidi CA, et al. Relationship between level of economic development, age, and etiology of spinal cord injury: a cross-sectional survey from 22 countries. Arch Phys Med Rehabil. 2021;102:1947–58.
Fulk GD, Behrman AL, Schmitz TJ. Traumatic spinal cord injury. In: O’Sullivan SB, Schmitz TJ, Fulk GD, editors. Physical rehabilitation. Philadelphia: F.A. Davis Company PA; 2014. p. 889–964.
Carpenter C, Forwell SJ, Jongbloed LE, Backman CL. Community participation after spinal cord injury. Arch Phys Med Rehabil. 2007;88:427–33.
Panjabi M, Albumi K, Duranceau J, Oxland T. Spinal stability and intersegmental muscle forces. A bimechanical model. Spine. 1989;14:194–200.
Park RJ, Tsao H, Cresswell AG, Hodges PW. Anticipatory postural activity of the deep trunk muscles differs between anatomical regions based on their mechanical advantage. Neuroscience. 2014;261:161–72.
Chiou SY, Gottardi SEA, Hodges PW, Strutton PH. Corticospinal excitability of trunk muscles during different postural tasks. PLoS ONE. 2016;11:1–13.
Gurfinkel V, Cacciatore TW, Cordo P, Horak F, Nutt J, Skoss R, et al. Humans postural muscle tone in the body axis of healthy the influence of education and exercise on neck pain transition from sitting to standing. J Neurophysiol. 2006;96:2678–87.
Santamaria V, Luna T, Khan M, Agrawal S. The robotic Trunk-Support-Trainer (TruST) to measure and increase postural workspace during sitting in people with spinal cord injury. Spinal Cord Ser Cases. 2020;6:1–7.
Chen CL, Yeung KT, Bih LI, Wang CH, Chen MI, Chien JC, et al. The relationship between sitting stability and functional performance in patients with paraplegia. Arch Phys Med Rehabil. 2003;84:1276–81.
Serra-Añó P, Pellicer-Chenoll M, Garcia-Massó X, Brizuela G, García-Lucerga C, González Moreno LM, et al. Sitting balance and limits of stability in persons with paraplegia. Spinal Cord. 2013;51:267–72.
Moynahan M. Postural responses during standing in subjects with spinal-cord injury. Gait Posture. 1995;3:156–65.
Rath M, Vette AH, Ramasubramaniam S, Li K, Burdick J, Edgerton VR, et al. Trunk stability enabled by noninvasive spinal electrical stimulation after spinal cord injury. J Neurotrauma. 2018;35:2540–53.
Martin Moraud E, von Zitzewitz J, Miehlbradt J, Wurth S, Formento E, DiGiovanna J, et al. Closed-loop control of trunk posture improves locomotion through the regulation of leg proprioceptive feedback after spinal cord injury. Sci Rep. 2018;8:1–12.
Asboth L, Friedli L, Beauparlant J, Martinez-Gonzalez C, Anil S, Rey E, et al. Cortico–reticulo–spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat Neurosci. 2018;21:576–88.
Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med. 2018;379:1244–50.
Roy RR, Harkema SJ, Edgerton VR. Basic concepts of activity-based interventions for improved recovery of motor function after spinal cord injury. Arch Phys Med Rehab. 2012;93:1487–97.
Boswell-Ruys C, Harvey L, Barker J, Ben M, Middleton J, Lord S, et al. Training unsupported sitting in people with chronic spinal cord injuries: a randomized controlled trial. Spinal Cord. 2010;48:138–43.
Lotter JK, Henderson CE, Plawecki A, Holthus ME, Lucas EH, Ardestani MM, et al. Task-specific versus impairment-based training on locomotor performance in individuals with chronic spinal cord injury: a randomized crossover study. Neurorehabil Neural Repair. 2020;34:627–39.
Ragnarsson KT. Functional electrical stimulation after spinal cord injury: current use, therapeutic effects and future directions. Spinal Cord. 2008;46:255–74.
Marchesi G, Ricaldone E, De Luca A, Torre K, Quinland E, Bellitto A, et al. A robot-based assessment of trunk control in spinal cord injured athletes. In: 2020 8th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob); 2020. p. 497–502.
Eizad A, Lee H, Pyo S, Afzal MR, Lyu SK, Yoon J. A novel trunk rehabilitation robot based evaluation of seated balance under varying seat surface and visual conditions. IEEE Access. 2020;8:204902–13.
Santamaria V, Luna TD, Agrawal SK. Feasibility and tolerance of a robotic postural training to improve standing in a person with ambulatory spinal cord injury. Spinal Cord Ser Cases. 2021;7:1–9.
Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, et al. Reference for the 2011 revision of the international standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2011;34:547–54.
Catz A, Itzkovich M, Tesio L, Biering-Sorensen F, Weeks C, Laramee MT, et al. A multicenter international study on the Spinal Cord Independence Measure, version III: Rasch psychometric validation. Spinal Cord. 2007;45:275–91.
Ditunno PL, Ditunno JF. Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord. 2001;39:654–6.
Abou L, Ribeiro de Freitas G, Palandi J, Ilha J. Clinical instruments for measuring unsupported sitting balance in subjects with spinal cord injury: a systematic review. Top Spinal Cord Inj Rehabil. 2018;24:177–93.
Borg G. Psychophysical bases of perceived exertion. Med Sci Sport Exerc. 1982;14:377–81.
Perdices M, Tate RL. Single-subject designs as a tool for evidence-based clinical practice: are they unrecognised and undervalued? Neuropsychol Rehabil. 2009;19:904–27.
de Leva P. Adjustments to Zatsiorsky-Seluyanov’s segment inertia parameters. J Biomech. 1996;29:1223–30.
Spratt J. Thorax. In: Standring S (ed). Gray’s Anatomy: The Anatomical Basis of Clinical Practice (40th edition). Churchill Livingstone/Elsevier, Edinburgh: 2008, pp 953–975.
Khan MI, Santamaria V, Kang J, Bradley BM, Dutkowsky JP, Gordon AM, et al. Enhancing seated stability using trunk support trainer (TruST). IEEE Robot Autom Lett. 2016;2:1609–16.
Santamaria V, Khan M, Luna T, Kang J, Dutkowsky J, Gordon AM, et al. Promoting functional and independent sitting in children with cerebral palsy using the robotic trunk support trainer. IEEE Trans Neural Syst Rehabil Eng. 2020;28:2995–3004.
Khan M, Santamaria V, Agrawal S. Improving trunk-pelvis stability using active force control at the trunk and passive resistance at the pelvis. IEEE Robot Autom Lett. 2018;3:2569–75.
Gribble PA, Hertel J, Facsm À, Plisky P. Using the star excursion balance test to assess dynamic postural-control deficits and outcomes in lower extremity injury: a literature and systematic review. J Athl Train. 2012;47:339–57.
Gao KL, Chan KM, Purves S, Tsang WWN. Reliability of dynamic sitting balance tests and their correlations with functional mobility for wheelchair users with chronic spinal cord injury. J Orthop Transl. 2015;3:44–49.
Quinzaños J, Villa AR, Flores AA, Pérez R. Proposal and validation of a clinical trunk control test in individuals with spinal cord injury. Spinal Cord. 2014. https://doi.org/10.1038/sc.2014.34.
Shumway-Cook A, Woollacott MH. Postural control. In: Motor control: translating research into clinical practice. Philadelphia, PA: Wolters Kluwer; 2017. p. 153–82.
Santamaria V, Rachwani J, Saussez G, Bleyenheuft Y, Dutkowsky J, Gordon AM, et al. The seated postural & reaching control test in cerebral palsy: a validation study. Phys Occup Ther Pediatr. 2020;40:441–69.
Gordon AM, Hung Y-C, Brandao M, Ferre CL, Kuo H-C, Friel K, et al. Bimanual training and constraint-induced movement therapy in children with hemiplegic cerebral palsy. Neurorehabil Neural Repair. 2011;25:692–702.
Bleyenheuft Y, Ebner-Karestinos D, Surana B, Paradis J, Sidiropoulos A, Renders A, et al. Intensive upper- and lower-extremity training for children with bilateral cerebral palsy: a quasi-randomized trial. Dev Med Child Neurol. 2017;59:625–33.
Behrman A, Argetsinger L, Roberts M, Stout D, Thompson J, Ugiliweneza B, et al. Activity-based therapy targeting neuromuscular capacity after pediatric-onset spinal cord injury. Top Spinal Cord Inj Rehabil. 2019;25:132–49.
Sayenko DG, Alekhina MI, Masani K, Vette AH, Obata H, Popovic MR, et al. Positive effect of balance training with visual feedback on standing balance abilities in people with incomplete spinal cord injury. Spinal Cord. 2010;48:886–93.
Tse CM, Chisholm AE, Lam T, Eng JJ. A systematic review of the effectiveness of task-specific rehabilitation interventions for improving independent sitting and standing function in spinal cord injury. J Spinal Cord Med. 2018;41:254–66.
Hung YC, Brandão MB, Gordon AM. Structured skill practice during intensive bimanual training leads to better trunk and arm control than unstructured practice in children with unilateral spastic cerebral palsy. Res Dev Disabil. 2017;60:65–76.
Mentiplay BF, Perraton LG, Bower KJ, Adair B, Pua YH, Williams GP, et al. Assessment of lower limb muscle strength and power using hand-held and fixed dynamometry: a reliability and validity study. PLoS ONE. 2015;10:1–18.
Al’joboori Y, Hannah R, Lenham F, Borgas P, Kremers CJP, Bunday KL, et al. The immediate and short-term effects of transcutaneous spinal cord stimulation and peripheral nerve stimulation on corticospinal excitability. Front Neurosci. 2021;15:1–17.
Maurer C, Peterka RJ. A new interpretation of spontaneous sway measures based on a simple model of human postural control. J Neurophysiol. 2005;93:189–200.
Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain Res Bull. 2007;73:155–202.
Macefield VG, Knellwolf TP. Functional properties of human muscle spindles. J Neurophysiol. 2018;120:452–67.
Vette AH, Thrasher TA, Sin VW, Masani K, Craven BC, Popovic MR, et al. Responses of the trunk to multidirectional perturbations during unsupported sitting in normal adults. J Appl Biomech. 2010;26:332–40.
Acknowledgements
We thank the valuable collaboration of the participant. This research was funded by NYS research funding DOH01-C31290GG, SKA principal investigator.
Author information
Authors and Affiliations
Contributions
VS, XA, and SA designed the study and analyzed and interpreted the data. During data collection, XA was the bioengineer and VS delivered the motor intervention. VS, XA, and SA collaborated in the write-up and revisions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santamaria, V., Ai, X. & Agrawal, S.K. A motor learning-based postural intervention with a robotic trunk support trainer to improve functional sitting in spinal cord injury: case report. Spinal Cord Ser Cases 8, 88 (2022). https://doi.org/10.1038/s41394-022-00554-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-022-00554-2