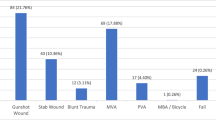
Abstract
Study design
Case–control study.
Objectives
To identify factors associated with neuropathic pain (NP) in patients with spinal cord injury of traumatic origin (TSCI).
Setting
University Hospital of Valle, Cali, Colombia.
Methods
Study participants were individuals with diagnosis of TSCI who visited a trauma referral center from January 1st, 2016, to December 31st, 2016. Information was retrospectively extracted from the Hospital’s Spinal Cord Injury registry and patients’ medical records. Cases were defined as patients with NP and controls were those without NP. The exposure of interest was intentional injuries. Individuals were matched by age and stratified into 11 groups of ±3 years each.
Results
We found 164 participants with an average age of 34 ± 13 years, of whom 95.1% were male, and 53.6% had NP. Neurogenic bladder and bowel occurred in 94.3% of NP patients. Cause of injury was not associated with NP. Older injuries were protective for NP (>10 years since injury OR = 0.10, 95% CI = 0.03–0.37, p < 0.0001) and neurogenic bladder and bowel were found as risk factors (OR = 5.89, 95% CI = 1.84–18.88; p = 0.003).
Conclusions
Our study uniquely shows time since injury as a protective factor for NP and neurogenic bladder and bowel as a risk factor, while violence was not found associated. This could help guide the scope of future research about NP secondary to SCI.
Similar content being viewed by others
Introduction
The effects of traumatic or nontraumatic spinal cord injury (SCI), have direct and indirect consequences on individual, family, and community levels [1]. The global annual incidence of SCI is estimated to be 23 SCI cases per million, corresponding to 179,312 new cases per year [2]. In Latin America, violence is a significant cause of SCI [2, 3], and Cali is one of the main cities of Colombia with the highest rates of violence, both within the country and worldwide [4].
The complications of SCI include neurogenic bladder and bowel, urinary tract infections, pressure ulcers, spasticity, depression, and pain [1, 5]. A significant proportion of people with SCI experience neuropathic pain (NP) [1], which can substantially impact quality of life and functionality.
The International Association for the Study of Pain defines NP as pain caused by a lesion or disease of the somatosensory nervous system [6, 7]. Its diagnosis is mainly clinical [8]. Symptoms can develop either at or below the level of the injury. At-level pain is defined by its presence anywhere within a region spanning one dermatome rostral and three dermatomes caudal to the neurological level of injury (NLI), whereas below-level pain occurs below three dermatomes caudal to the NLI. Central NP is due to injury or disease of the spinal cord or brain. In patients with incomplete SCI, below-level pain can also have an allodynic component, but a substantial proportion of patients with below-level pain have complete spinal lesions and no sensory function in the painful region [7, 9].
According to a systematic review and meta-analysis from high-income countries, the prevalence of NP secondary to SCI is 53% [10]. Factors including age, gender, trauma mechanism, type and level of SCI, its intensity, and early onset of dysesthetic symptoms, as well as associated complications such as pressure ulcers, constipation, spasticity, and infections [11] have been associated with NP in patients with SCI. NP has been shown to be more common at 1-year post-SCI [10], however there is a need for more research examining the relationship between time elapsed since SCI and onset of NP [12]. Moreover, the relationship between violence and NP has not been evaluated in low- to middle-income countries.
In Colombia, to the best of our knowledge, there are no studies specifically investigating the population of individuals with NP secondary to SCI. Therefore, the aim of this study is to identify associated factors with NP in patients with TSCI who were referred to a trauma referral center in southwestern Colombia during 2016.
Methods
Study design
Case–control study of patients with TSCI based on the Hospital’s Spinal Cord Injury registry and patients’ medical records. The study was approved by the Institutional Review Committee of Human Ethics of Universidad del Valle and the Board for Ethics Research of the Hospital Universitario del Valle (HUV) with code 070-018.
Study setting
The study was carried out with data from HUV. This is the main trauma referral center in the Colombian southwestern region as it is the referral center for 56 public primary centers in the Department of Valle del Cauca, a region that holds 42 cities/municipalities. Amongst various specialties, it contains a physical medicine and rehabilitation unit which serves patients with SCI. Overall, HUV serves an estimated number of 120,000 patients per year, out of which ~6% (≈7000) of consults are related to trauma [13].
Sample selection
During 2016, a total of 188 patients were seen at the SCI outpatient clinic; 2 records were excluded because they were duplicates, 6 records were incomplete, 18 were nontraumatic, including 5 oncological pathologies, 5 autoimmune, 3 infectious, 2 spinal stenosis, 2 congenital, and 1 vascular, thus leaving 164 (87.23%) patients to be included in the study.
The study population was composed of individuals who attended the HUV’s SCI outpatient clinic during 2016. Consecutive sampling was used, and all patients with TSCI were included without discrimination for age, gender, or ethnicity.
Exposure
The exposure of interest was interpersonal injury (intentional mechanism) as the cause of the SCI. This variable, namely the cause of SCI, was classified as either intentional injuries (violence) or unintentional injuries (falls and road traffic injuries [RTI]).
Outcome
The outcome of interest was the presence of NP. All patients with a diagnosis of NP between January 1st to December 31st of 2016 were included. NP had to be diagnosed by at least two different physicians specialized in Physical Medicine and Rehabilitation based on the ISCIP definition [7], and identified in the Registry after filtering by the International Classification of Disease 9 version (ICD-9) using the codes 729.2, 336.9, 337.2x, 337.1, 341.9, 353.x, 354.x, 355.x, 952.xx, and 953.4.
Each case was individually matched to one individual who did not have a diagnosis of NP by December 31st 2016 using a concurrent sampling strategy. Matching was on the basis of age within ±3 years range. Controls were identified from the Hospital’s Spinal Cord Injury registry as individuals who had consulted the clinic in the same year as the cases, thus comparing active patients of the clinic.
Potential confounding variables
Potential confounding variables with regards to the association between SCI caused by an intentional injury and NP were considered to be time since injury, neurogenic bladder or bowel, level of the injury, and American Spinal Injury Association (AIS) Grade Classification [14].
Power calculation
An a priori feasibility count from the Hospital’s Spinal Cord Injury registry estimated that 88 cases of NP due to traumatic SCI would be identified in the study period. Using a matching ratio for cases and controls of 1:1, assuming a rate of exposure to injuries caused by interpersonal violence in the control group of 45%, a correlation of exposure between cases and controls of 0.2%, and alpha of 5%, it was estimated that the study would have 88.6% power to detect an odds ratio of 3.
Data collection
Data was extracted from both the Hospital’s Spinal Cord Injury registry and patient’s medical records. Patient records were searched manually, and the data including demographics, etiology, and AIS grade of TSCI was collected systematically. Information was collected in a Microsoft Access® database. To verify the quality of the information, 10% of the collected records were randomly reviewed using a random number sequence in Microsoft Excel® and the accuracy of the information was verified using the registry and the individuals’ charts.
Explanatory variables
We collected sociodemographic variables including age, gender, and health insurance; and clinical variables such as SCI complications, level of injury, and AIS grade [15, 16]. The level of the injury was categorized as cervical, thoracic, and lumbar/sacral. Time since injury was recorded in <2 years, 3–5 years, 6–10 years, and >10 years. The presence of complications such as ulcers, spasticity, neurogenic bladder (bladder catheter users, overflow incontinence or lack of diuresis sensation) and bowel (the use of laxatives or medication to treat constipation or evacuation maneuvers) were collected.
Statistical analysis
The analyzes were performed in Stata 16.0 (STATA Corp., Texas, US)®. Data quality was assessed using univariate analysis. Missing values and extreme values were double checked using the registry information or the medical records.
Quantitative variables were described using central tendency and dispersion measurements, while categorical variables were described with frequencies and percentages. Bivariate analyses were performed to compare the groups of individuals with and without NP based on their sociodemographic and clinical variables. For categorical variables we used the Fisher’s exact test or the Chi-2 test, and for continuous variables we used t test. Differences with a value of p < 0.05 were considered statistically significant.
Unadjusted conditional logistic regressions with NP and the mechanism of injury that caused the SCI matched by age (±3 years) were used to assess the association with potential confounders. Variables with a p value <0.20 in the unadjusted models were included in a conditional multivariate logistic regression model using NP as dependent variable and with individuals matched by age (±3 years). Odds ratios (OR) and 95% confidence intervals (95%CI) were estimated. Collinearity was assessed using the Variance Inflation Factor (VIF). Sex had a VIF higher than 10 and it was not found associated with a p value < 0.20 therefore it was excluded from the final adjusted model. Interaction terms were created and assessed comparing the full adjusted model without the interaction term, and the full adjusted model with it—using the likelihood ratio test. The goodness-of-fit was assessed using the Akaike and Bayesian information criterions (AIC, BIC). Three sensitivity analysis were carried out: (1) comparing the full model with and without sex, (2) against a dichotomized time since injury into <6 and >5 years, and (3) comparing a logistic vs. a conditional logistic regression model. In the logistic model age was used as a covariate, instead of a matching variable. The AIC and BIC were used to assess the differences of the models.
Results
We found 164 individuals with TSCI, distributed as 76 (46.34%) without NP (controls) and 88 (53.66%) with NP (cases). Out of the study population, 95.12% were men and 4.88% were women. The average age was 34 ± 13 ranging from 9 to 82 years of age.
There were no statistical differences when comparing gender, age, insurance status, injury intentionality, and numbers of visits by NP presence (Table 1). Unintentional injuries were caused by falls (n = 14, 8.54%) or RTIs (n = 19, 11.59%). Among those with SCI due to falls, 3 (21.43%) had NP and for RTI, 13 (68.42%) had NP.
Individuals with NP were found to have a shorter time elapsed since injury (Fig. 1); however, this difference was not significant (p = 0.07). Most of the individuals were classified as AIS A without statistically significant differences between those with and without NP. Having neurogenic bladder and bowel was found in higher proportion amongst individuals with NP (p < 0.0001, Fig. 2 and Table 2). Further details about the individuals and their clinical status can be found in Tables 1, 2.
Bar graph showing percentage of patients with and without neuropathic pain, grouped by time since injury of <2, 3–5, 6–10, and >10 years. In all groups except for patients with >10 years since injury, there were more patients with neuropathic pain than without. Amongst those with >10 years since injury, <40% had neuropathic pain and more than 60% did not have neuropathic pain.
Bar graph showing rates of neuropathic pain grouped by the presence or absence of neurogenic bladder and bowel. The majority of patients had neurogenic bladder and bowel, and out of those, most of them had neuropathic pain. In the patients without neurogenic bladder and bowel, most of them did not have neuropathic pain.
Crude and adjusted analyses
The multivariate conditional logistic model matched by age was adjusted by injury intentionality, time since injury, and neurogenic bladder and bowel. Cause of injury, sex, level of injury, and AIS grade did not have statistical significance with regard to NP (p > 0.05, see Table 3). In a model adjusted by intentionality, the risk for NP decreased as the time since the injury increased. Patient groups with 3–5 years and 6–10 years since the injury had a nonsignificant protective factor, which only became significant in the >10 years since the injury patient group.
There was a significant difference between the two groups observed regarding the presence of neurogenic bladder and bowel. The results indicate that the odds of having NP is 5.89 times higher (OR = 5.89, IC95% = 1.84–18.88) in patients with this complication compared to patients without them.
Finally, the following interaction terms were created: neurogenic bladder/bowel by time since injury ≤5 or >5 years and violence by time since injury ≤5 or >5 years. The likelihood ratio tests were not significant (p > 0.05) when comparing the full model vs. the full model with one interaction term at a time. Therefore, the interactions were not included in the analysis of the final model. In the sensitivity analysis, the model with the sex variable had a slightly higher AIC and BIC than the model without it (with sex: AIC = 172.42, BIC = 190.80 vs. without sex: AIC = 171.95, BIC = 187.27). Similar findings were recorded for a model with a dichotomized time since injury except for the BIC, which was lower in this model (AIC = 173.60, BIC = 182.72), and a model with multivariate logistic regression instead of a conditional regression with age as continuous variable as a covariate (AIC = 203.74, BIC = 225.40).
Discussion
We found that 59% of the patient with SCI had NP and associated factors identified in an adjusted and multivariate conditional logistic regression included time since injury (>10 years since injury OR = 0.10, 95% CI = 0.03–0.37, p < 0.0001) and neurogenic bladder and bowel (OR = 5.89, 95% CI = 1.84–18.88; p = 0.003). There was no association found for sex, intentional injury, level of the injury, and AIS grade.
In a meta-analysis of NP in SCI, Burk et al. found a prevalence of 53% for NP post SCI [10]. As Burk et al. conducted a meta-analysis including studies from high-income countries where the provision of healthcare services are greater than in Colombia, a middle-income country, results may be not comparable. However, a study conducted at the San Vicente de Paul University Hospital in Medellin, northwestern Colombia found a 47% prevalence of pain in patients hospitalized because of SCI [14]. Although this is close to the value that we found, it is not possible to make a clear comparison given that the authors did not clearly define the criteria for NP.
Regarding the etiology of the SCI, a SCI was the result of violence in 80% of the studied population. This can be compared with the data found by Cripps et al. whereby the authors reported violence and self-inflicted injuries as a cause of SCI in 15% in North America, 6% in Western Europe, and 2% in Australia [17]. This marked difference could be a result of the high rates of interpersonal violence prevalent in Colombia, especially in Cali [18, 19], therefore, further research on these issues is vital in the improvement of patient care, patient outcomes, and quality of healthcare provision. However, being exposed to an intentional injury was not found to be associated with the presence of NP thus providing relevant information in terms of patient care after the injury.
Additionally, although factors including age, gender, and level of SCI [11], have been associated with NP in patients with SCI, we did not find such associations in our study. These findings contrast with those described in the study by Andresen et al. where NP was more frequent in patients with incomplete injury and tetraplegia [11].
The presence of neurogenic bladder and bowel and a time of injury greater than 10 years—were found to be associated with the presence of NP. Patients with a neurogenic bladder and bowel were subject to almost 6 times the odds of having NP compared to those without neurogenic bladder and bowel. This reinforces the findings of the study by Widerström-Noga and Turk which found that prolonged afferent activity, due to having a full bladder and impacted intestine, was significantly and frequently associated with pain aggregation, which, in turn, is associated with the development of NP [20]. However, as the findings show a correlation between NP and neurogenic bladder and bowel, in addition to the pathophysiology of pain as a therapeutic target, clinical issues such as spasticity should also be considered [21], as this could precede the presence of NP or increase its intensity. Current clinical evidence surrounding the management of NP in SCI patients is focused on the use of drugs and medical procedures [22]. As there are various interventions for neurogenic bladder and bowel [23, 24], our findings suggest that these should be taken into account when initiating a rehabilitation plan in patients with SCI with or without NP.
The study by Widerström-Noga and Turk was unable to provide data to describe the relationship between onset of injury and NP diagnosis [20]. However, we found an inverse relationship between NP and time since the injury. Patients who have had more years since the injury were less likely to have NP. On the contrary, the literature suggests that the greater the amount of time passed since injury, the greater the presence of pain and sensory alterations [12, 25]. A systematic review found higher pain prevalence in studies performed longer after the initial SCI event [26]. Furthermore, Finnerup et al. in their cohort study with 1 year of follow-up, found that the prevalence of NP increased with time [27]; however, it is important to note that their study only included intervals of up to 12 months since injury. Nagoshi et al. in a population of 72 patients, found that NP becomes more severe when the time passed since the injury is >1 year [28]. On the other hand, Adrianssen et al. found that there has been no correlation between the presence of NP and musculoskeletal pain and the time of evolution of the injury [5].
It has been suggested that pain after SCI may vary with age at SCI onset [29], and therefore the differences between our findings and the existing literature could be a result of the demographic differences between individual with SCI in Colombia and globally, as our study had a markedly younger population (33.63 years old) compared to the aforementioned study by Nagoshi et al.(mean = 56 years old) [28], Adrianssen et al. (median = 47.8 years old) [5], and Finnerup et al. (mean = 48.9 years old) [27]. Furthermore, another Colombian study also found a mean age of 33 years amongst individuals with SCI [30]. It is worth mentioning that, to the best of our knowledge, there is no information about life expectancy after a SCI in Colombia. Therefore, if expected post-TSCI survival rates are lower in Colombia, we could have underestimated the rates of NP and the association with time after the injury due to survival bias. Cohort studies of patients with SCI over a longer period of time are required to identify whether or not time passed since injury has an effect on the presence of NP.
Other protective factors for NP after SCI should be explored in prospective studies. In a meta-analysis of NP in animal models of SCI it was found that exercise can have a positive impact on mechanical, spontaneous, and thermal and cold nociception pain; however, authors found high heterogeneity levels among the studies [31]. On the other side, in a cross-sectional study of 60 participants with SCI, resilience was explored with no association with the degree of NP [32]. Larger sample sizes are required to conclude about psychological protective factors in NP.
Study limitations
The main limitation of this study is the fact that data was retrospectively obtained from a registry and clinical records; hence, relevant variables that were related to the presence of NP may be missing as they could have been omitted from the initial clinical reports. This did not allow discrimination of the type of NP in patients with SCI, as proposed by the ISCIP [7]. Furthermore, due to the retrospective nature of the study, we were not able to define the presence of urinary tract infection. Therefore, we could not make a correlation between this variable, neurogenic bladder, and NP, in order to analyze what was reported by Joseph and Wikmar regarding urinary tract infection as a risk factor for NP in SCI [33]. Furthermore, as we included only a single center with mostly male patients from low-income settings (based on the percentage of subsidized insurance holders) the external validity of our data may be limited. Further multicenter research is required to adjust for this gender difference. However, from the best of our knowledge, this is the first study in Colombia about this situation and it describes relevant information towards future research areas.
Strengths
One strength of the study is the number of records that were captured in a short period of time and in the same center. This increased the homogeneity of the population as they were evaluated and followed by the same providers. In this way, selection bias was decreased. Additionally, the study got enough power to identify the associations described. The regression model fit was tested and the results were positive.
Conclusion
In Latin America, there is a lack of epidemiological data about SCI and its associated factors. We used a case–control study design to investigate the presence of NP amongst patients with SCI. We found that about half of study participants had NP. Our study uniquely shows time since injury as a protective factor NP, while violence as a cause of injury was not found to be associated. Furthermore, the importance of the clinical issue of spasticity was reinforced in our study, as neurogenic bladder and bowel were found as risk factors for NP. This could help guide future research about NP secondary to SCI as well as improve the provision of post-SCI care by equipping clinicians with a more thorough understanding of the social and clinical situations that complicate patient outcomes. The importance of our research lies in the implications that NP has in terms of patients’ quality of life [34], the associations that NP has with other post-SCI complications [11], and the treatment implications that NP has especially when considered with in conjunction with complications such as neurogenic bladder and bowel. This study provides valuable information that can guide the scope of future research about NP secondary to SCI. Further research about SCI and NP, as well as SCI in low- to middle-income countries is required [1].
Disclaimer
The views expressed in the article are own by the authors and not an official position of the institution or funder.
Data availability
Data is available per request to the corresponding author.
References
World Health Organization, International Spinal Cord Society (ISCOS). International perspectives on spinal cord injury. World Health Organization; 2013.
Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52:110–6.
Instituto Nacional de Medicina Legal y Forense. Datos para la vida. Herramienta para la interpretación, intervención y prevención de lesiones a causa externa en Colombia. Forensis. 2014;16:319–50.
Seguridad, Justicia Y Paz. Consejo Ciudadano para la Seguridad Pública y la Justicia Penal AC. Ciudades Más Violentas Del Mundo, 2017. Ciudad de Mexico. Seguridad, Justicia Y Paz.
Adriaansen JJ, Ruijs LE, van Koppenhagen CF, van Asbeck FW, Snoek GJ, van Kuppevelt D, et al. Secondary health conditions and quality of life in persons living with spinal cord injury for at least ten years. J Rehabil Med. 2016;48:853–60.
Merskey HA. Pain terms: a list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain. 1979;6:249.
Bryce TN, Biering-Sørensen F, Finnerup NB, Cardenas DD, Defrin R, Lundeberg T, et al. International spinal cord injury pain classification: part I. Background and description. Spinal Cord. 2012;50:413–7.
Widerström-Noga E, Loeser JD, Jensen TS, Finnerup NB. AAPT diagnostic criteria for central neuropathic pain. J Pain. 2017;18:1417–26.
Shiao R, Lee-Kubli CA. Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics. 2018;15:635–53.
Burke D, Fullen B, Stokes D, Lennon O. Neuropathic pain prevalence following spinal cord injury: A systematic review and meta‐analysis. Eur J Pain. 2017;21:29–44.
Andresen SR, Biering-Sørensen F, Hagen EM, Nielsen JF, Bach FW, Finnerup NB, et al. Pain, spasticity and quality of life in individuals with traumatic spinal cord injury in Denmark. Spinal Cord. 2016;54:973.
Werhagen L, Budh C, Hultling C, Molander C. Neuropathic pain after traumatic spinal cord injury—relations to gender, spinal level, completeness, and age at the time of injury. Spinal Cord. 2004;42:665.
Ordoñez CA, Morales M, Rojas-Mirquez JC, Bonilla-Escobar FJ, Badiel M, Miñán Arana F, et al. Trauma Registry of the Pan-American Trauma Society: One year of experience in two hospitals in southwest Colombia. Colomb Med. 2016;47:148–54.
Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34:535–46.
Kretzer RM. A clinical perspective and definition of spinal cord injury. Spine 2016;41:S27.
Montoya J, Lugo L. Complicaciones en pacientes hospitalizados con diagnóstico de Trauma Raquimedular en el Hospital Universitarios San Vicente de Paúl. Rev Colomb Med Fis Rehabil. 2002;13:31–8.
Cripps R, Lee B, Wing P, Weerts E, Mackay J, Brown D, et al. A global map for traumatic spinal cord injury epidemiology: towards a living data repository for injury prevention. Spinal Cord. 2011;49:493.
Instituto Nacional de Medicina Legal y Forense: Forensis. Datos para la vida. Herramienta para la interpretación, intervención y prevención de lesiones a causa externa en Colombia, Bogotá DC: Imprenta Nacional; 2016.
Bonilla-Escobar FJ, Gutiérrez MI. Injuries are not accidents: towards a culture of prevention. Colomb Med. 2014;45:132–5.
Widerström-Noga EG, Turk DC. Exacerbation of chronic pain following spinal cord injury. J Neurotrauma. 2004;21:1384–95.
Finnerup NB. Neuropathic pain and spasticity: intricate consequences of spinal cord injury. Spinal Cord. 2017;55:1046.
Mehta S, Teasell R, Loh E, Short C, Wolfe D, Hsieh J, et al. Pain following spinal cord injury. Spinal Cord. Inj Rehabil Evid. 2014;5:1–79.
Deng Y, Dong Y, Liu Y, Zhang Q, Guan X, Chen X, et al. A systematic review of clinical studies on electrical stimulation therapy for patients with neurogenic bowel dysfunction after spinal cord injury. Medicine. 2018;97:e12778.
Qi Z, Middleton JW, Malcolm A. Bowel dysfunction in spinal cord injury. Curr Gastroenterol Rep. 2018;20:47.
Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–57.
van Gorp S, Kessels AG, Joosten EA, van Kleef M, Patijn J. Pain prevalence and its determinants after spinal cord injury: a systematic review. Eur J Pain. 2015;19:5–14.
Finnerup NB, Norrbrink C, Trok K, Piehl F, Johannesen IL, Sørensen JC, et al. Phenotypes and predictors of pain following traumatic spinal cord injury: a prospective study. J Pain. 2014;15:40–8.
Nagoshi N, Kaneko S, Fujiyoshi K, Takemitsu M, Yagi M, Iizuka S, et al. Characteristics of neuropathic pain and its relationship with quality of life in 72 patients with spinal cord injury. Spinal Cord. 2016;54:656.
Dijkers MPF, Bryce TMD, Zanca JPMPT. Prevalence of chronic pain after traumatic spinal cord injury: a systematic review. J Rehabil Res Dev. 2009;46:13–29.
Giraldo YA, Castro JL, Tovar-Sánchez MA, Kumar AA, Pacichana-Quinayáz SG, Bonilla-Escobar FJ, et al. Epidemiology of traumatic spinal cord injuries in Colombia. Spinal Cord Ser Cases. 2021;7:42.
Palandi J, Bobinski F, Martins de Oliveira G, Ilha J. Neuropathic pain after spinal cord injury and physical exercise in animal models: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2020;108:781–95.
Kilic SA, Dorstyn DS, Guiver NG. Examining factors that contribute to the process of resilience following spinal cord injury. Spinal Cord. 2013;51:553–7.
Joseph C, Wikmar LN. Prevalence of secondary medical complications and risk factors for pressure ulcers after traumatic spinal cord injury during acute care in South Africa. Spinal Cord. 2016;54:535.
Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology. 2007;68:1178–82.
Funding
This study was supported by Universidad del Valle, Cali Colombia, through the 2018 internal call for research projects, project CI. 1845.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reyes-Campo, A., Pacichana-Quinayás, S.G., Kumar, A.A. et al. Factors associated with neuropathic pain in Colombian patients with spinal cord injury of traumatic origin: case–control study. Spinal Cord Ser Cases 8, 27 (2022). https://doi.org/10.1038/s41394-022-00494-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-022-00494-x