Abstract
Introduction
Autonomic dysreflexia is an uninhibited sympathetic response evoked by a strong sensory input below the level of the injury in patients with spinal cord injury. As presented in this case, autonomic dysreflexia can be associated with unusual symptoms such as Horner’s syndrome.
Case presentation
An 18-year-old man with a traumatic spinal cord injury (C7 AIS A) experienced symptoms of unilateral Horner’s syndrome: miosis, ptosis and anhidrosis which occurred simultaneously with symptoms of autonomic dysreflexia: severe headache accompanied by increasing right-sided diaphoresis, flushing, blurred vision, and increased blood pressure. These symptoms were triggered by bladder distention and were resolved after catheterisation.
Discussion
The patient experienced a transient Horner’s syndrome due to autonomic dysreflexia. Both Horner’s syndrome and symptoms of autonomic dysreflexia resolved when eliminating the eliciting stimulus, indicating that Horner’s syndrome occurred due to a transient pressure on the sympathetic fibres supplying the superior cervical ganglion. Autonomic dysreflexia may have caused increased pressure disrupting the sympathetic input, thus inducing unilateral miosis, ptosis, and facial anhidrosis.
Similar content being viewed by others
Spinal cord injuries (SCI) with a neurological level at or above the sixth thoracic level interrupt autonomic pathways leading to disturbed cardiovascular homeostasis. This deranged cardiovascular control has been attributed to multiple underlying mechanisms such as loss of tonic supraspinal inhibitory inputs, hyper-responsiveness of peripheral α-adrenoreceptors, and loss of baroreceptor reflexes [1]. Disturbance of the autonomic nervous system may result in a symptom complex commonly known as autonomic dysreflexia (AD) [1, 2]. Studies have found AD in 48 to 90% of individuals with tetraplegia or high paraplegia [1].
Clinically, the symptoms of AD vary from asymptomatic or mild to potentially life-threatening emergencies and occur due to an uninhibited sympathetic response evoked by a strong sensory input below the level of the injury. The severity of symptoms is associated with the level of the injury; higher levels of injury typically cause more severe episodes of AD [2]. AD is defined as “an increase in systolic BP (>20 mmHg above baseline), including headache, flushing, piloerection, stuffy nose, sweating above the level of the lesion, vasoconstriction below the level of the lesion, and dysrhythmias. This syndrome may or may not be symptomatic and may occur at any period following SCI.” [3]. AD is however, not easily recognised as symptoms vary between patients; some patients may also experience mydriasis, conjunctival congestion, lid lag, Horner’s syndrome, penile erection, and pallor [4]. In severe cases if left untreated AD may result in cardiovascular complications such as myocardial infarction, retinal haemorrhage, stroke and ultimately death [2].
Both non-noxious and noxious stimuli can trigger AD, although the most common triggers originate from irritation of the bladder and bowel [2]. When afferent nerve impulses from these stimuli ascend through the dorsal columns and the spinothalamic tract of the spinal cord, collateral connections excite the preganglionic sympathetic neurons in the intermediolateral column leading to high sympathetic outflow through release of noradrenaline below the level of the injury [1]. The loss of supraspinal control to counteract the reflex sympathetic response causes widespread vasoconstriction below the level of the injury leading to a paroxysmal increase in blood pressure. Consequently, the baroreceptor reflex system detects the increased blood pressure and compensate through increased parasympathetic activity. This increased parasympathetic activity lowers blood pressure by decreasing heart rate and reducing the activity of vasoconstrictor sympathetic preganglionic neurons. However, the compensatory parasympathetic response cannot travel below the level of the injury, therefore causing incomplete compensation with bradycardia and vasodilation above the level of the injury and continued uninhibited vasoconstriction below the level of the injury [1, 5, 6]. The chain of events leading to increased vasodilation as described before is well known in patients with low-level lesions. However, though vasodilation above the level of the injury is commonly seen in AD in patients with cervical cord injuries, the mechanism for this phenomenon is not well understood, as the spinal cord in these cases is isolated prior to the sympathetic outflow regulating the contractility of blood vessels [7].
Horner’s syndrome describes the clinical combination of miosis, ptosis, and facial anhidrosis, and it occurs ipsilateral due to interruption of the sympathetic innervation to the eye through the oculosympathetic pathway [8, 9]. The oculosympathetic pathway consists of a three-neuron arc ranging from the hypothalamus to the eye. The first-order neuron originates in the posterolateral hypothalamus and descends to the synapse in the intermediolateral columns of spinal cord from C8-Th2. The second-order neuron is the sympathetic preganglionic neuron that leaves the spinal cord and travels to the superior cervical ganglion to synapse with the third-order neuron. Finally, the postganglionic fibres leave the superior cervical ganglion and form a plexus. Sudomotor and vasomotor fibres innervating the face branch off with the external carotid artery while the sympathetic fibres to the eye follows the internal carotid artery. The sympathetic fibres join the ophthalmic division of the trigeminal nerve and travel with the nasociliary nerve through the superior orbital fissure and the ciliary ganglion and divides into several fibres innervating orbital vasomotor function, the pupillary sphincter, and accessory muscles for eyelid retraction [9]. Horner’s syndrome may occur due to lesion at any of the involved neurons along the oculosympathetic pathway and symptoms may vary depending on the location of the lesion. Regardless of the neuron involved in the lesion, all patients experience ptosis due to lack of innervation to the Müller muscle, and miosis due to lack of sympathetic innervation to the iris dilator muscle. Anhidrosis, however, is typically not present in third-order neuron lesions as the sudomotor and vasomotor fibres to the face branch off [10].
Acknowledgement of AD is crucial to prevent severe cardiovascular complications. Thus, caregivers must be aware of potential signs of AD to reduce morbidity and mortality. This case report presents a rare association between AD and Horner’s syndrome that may be a more common association than previously though.
Case presentation
An 18-year-old Caucasian male with a traumatic SCI at C7, AIS A, experienced symptoms of Horner’s syndrome concurrent to episodes of AD [11]. The ISNCSCI revealed motor level at C7 and sensory level at C8 bilaterally. There was an altered sensory function at T1 level at the left side; no sensory function was preserved below this level. The transient episodes presented as progressive unilateral left-sided miosis, ptosis, and anhidrosis accompanied by severe headache and increasing symptoms of right-sided diaphoresis, flushing, and blurred vision. Figure 1 shows a picture of the patient during the episode. Several stimuli could trigger episodes of left-sided Horner’s syndrome and right-sided symptoms of AD in our patient. Most commonly, it was triggered by bladder distention; however, severe pain, urinary tract or other infections, or irritation from leg braces could also trigger these episodes. The patient suffered from SCI at the age of one and paediatric medical journal describe occurrence of AD with episodes of general discomfort, diaphoresis, and trembling from the age of three. According to the medical records, the parents reported a change of these symptoms when the patient at the age of six, but these changes were not described in further detail. At the age of 16, the patient was hospitalised due to continued occurrence of autonomic dysfunction; the patient also started noticing transient Horner’s syndrome concurrent to episodes of AD. He now suffers from daily episodes of AD and every episode is accompanied by unilateral left-sided Horner’s syndrome with no alternation of the affected side between episodes. On the day of observation, the patient had an average blood pressure of 102/55 mmHg (daytime: 104/59 mmHg) and average heart rate at 84 beats per minute (bpm) (daytime: 90 bpm). At the occurrence of the symptoms, the patient’s blood pressure increased to 144/79 mmHg with a heart rate at 80 bpm. According to the patient, ignoring a prolonged episode of AD would cause bilateral sweating in addition to Horner’s syndrome. Eliminating the eliciting stimulus, i.e. emptying the bladder, would quickly resolve the transient symptoms of both Horner’s syndrome and AD. Likewise, the patient reported that extreme pain stimuli such as a broken leg would trigger AD accompanied by bilateral sweating and Horner’s syndrome.
Discussion
The patient experiences a transient Horner’s syndrome because of AD. In this case, Horner’s syndrome and symptoms of AD resolves when eliminating the eliciting stimulus e.g. emptying of the bladder. This may indicate that Horner’s syndrome occurs due to a transient pressure on the sympathetic fibres involved in the oculosympathetic pathway. AD may induce increased pressure leading to disruption of sympathetic input thus inducing unilateral miosis, ptosis, and anhidrosis of the facial skin. By removing the eliciting stimulus, pressure on the fragile nerve fibres resolves thus causing the disappearance of Horner’s syndrome.
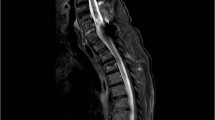
To our knowledge, transient Horner’s syndrome in relation to an episode of AD has only been described once before in 2013 [12]. The patient with a complete cervical C5 SCI included in the previous study had a history of progressive cervical cord syringomyelia. The authors speculate that the occurrence of Horner’s syndrome in their case may have been caused by an AD-induced hypertension that resulted in subsequent increase in intramedullary and thereby the pressure within the syrinx. The authors suggested that the increased intraspinal pressure was distributed unevenly causing a greater compression of the sympathetic fibres in one side therefore showing unilateral symptoms of Horner’s syndrome. As seen in this case, symptoms resolved after removing the eliciting stimulus initially causing AD [12]. Tumour, haemorrhage, or infarct along the oculosympathetic pathway may also cause Horner’s syndrome. This includes lung tumours (Pancoast), schwannoma, thyroid tumours, damage to the aorta, surgery in the chest cavity, jugular venous ectasia, and subclavian artery aneurism, lesions that dilates or compresses the carotid artery, carotid dissection, and traumas [13]. Our patient was investigated regarding alternative causes of Horner’s syndrome using a CT, which excluded causes such as aneurisms. Due to assumed non-compatible surgical implantations, an MRI for exclusion of syringomyelia could not be performed at the time of evaluation of autonomic dysfunction. However, information of an existing MRI at the age of five showed extradural liquid collection at the level of the injury along with an 8 cm long syrinx distal to the liquid. Though CT-scans are not optimal for diagnosing syringomyelia, the scan performed 11 years following the MRI showed no signs of such. Hence, the transient Horner’s syndrome in this case may be due to the increased vasodilation above the level of the injury during an episode of AD. This vasodilation may compromise the fragile nerves somewhere along the three-neuron arch in the oculosympathetic pathway. One can speculate that the possible syringomyelia and the patient’s autonomic dysfunction with sensory asymmetry are probable contributors to the observed phenomenon of unilateral Horner’s syndrome. Our patient experienced anhidrosis, suggestive of temporary nerve compression occurring somewhere along the first- or the second-order neuron [9].
Early identification of AD in patients with SCIs is crucial to initiate early treatment and reduce the risk of cardiovascular complications. This case shows Horner’s syndrome as a rare presentation of AD, which may facilitate early recognition of AD and thereby reduce morbidity and mortality of this severe condition.
References
Teasell RW, O Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81:506–16.
Krassioukov A, Warburton DE, Teasell R, Eng JJ. A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil. 2009;90:682–95.
Krassioukov A, Biering-Sorensen CF, Donovan W, Kennelly M, Kirshblum S, Krogh K, et al. International Standards to Document Remaining Autonomic Function after Spinal Cord Injury (ISAFSCI), First Edition 2012. Top Spinal Cord Inj Rehabil. 2012;18:282–96.
Krassioukov AV, Furlan JC, Fehlings MG. Autonomic dysreflexia in acute spinal cord injury: an under-recognized clinical entity. J Neurotrauma. 2003;20:707–16.
Eldahan KC, Rabchevsky AG. Autonomic Dysreflexia after Spinal Cord Injury: Systemic Pathophysiology and Methods of Management. Auton Neurosci. 2018;209:59–70.
Karlsson AK. Autonomic dysreflexia. Spinal Cord. 1999;37:383–91.
Cunningham DJC, Guttmann L, Whitteridge D, Wyndham CH. Cardiovascular responses to bladder distension in paraplegic patients. J Physiol. 1953;121:581–92.
Martin TJ. Horner syndrome: a clinical review. ACS Chem Neurosci. 2018;9:177–86.
Walton KA, Buono LM. Horner syndrome. Curr Opin Ophthalmol. 2003;14:357–63.
Kanagalingam S, Miller NR. Horner syndrome: clinical perspectives. Eye Brain. 2015;7:35–46.
ASIA and ISCoS International Standards Committee. The 2019 revision of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)-What’s new? Spinal Cord. 2019;57:815–7.
Cragg JJ, Krassioukov AV. Pearls and oysters: transient Horner syndrome associated with autonomic dysreflexia. Neurology. 2013;81:35.
Reede D, Garcon E, Smoker W, Kardon R. Horner’s syndrome: clinical and radiographic evaluation. Neuroimaging Clin North Am. 2008;18:369–85. xi
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Harsfort, D., Hagen, E.M. & Hansen, R.M. Autonomic dysreflexia and concurrent Horner’s Syndrome: a rare presentation in a patient with spinal cord injury. Spinal Cord Ser Cases 7, 47 (2021). https://doi.org/10.1038/s41394-021-00410-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-021-00410-9