Abstract
Study design
This was a sub-group analysis of a multicentre, randomised, placebo-controlled, double-blind trial (ECLISP trial)
Objectives
To assess the efficacy of a probiotic containing at least 6.5 × 109 live Lactobacillus casei Shirota (LcS) in preventing antibiotic associated diarrhoea (AAD) in patients with spinal cord injury (SCI) who consumed proton pump inhibitor (PPI) regularly. LcS or placebo was given once daily for the duration of an antibiotic course and continued for 7 days thereafter. The trial was registered with ISRCTN:13119162.
Setting
Three SCI centres (National Spinal Injuries Centre, Midland Centre for Spinal Injuries and Princess Royal Spinal Cord Injuries Centre) in the United Kingdom
Methods
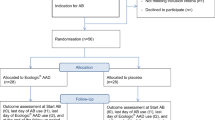
Between November 2014, and November 2019, 95 eligible consenting SCI patients (median age: 57; IQ range: 43-69) were randomly allocated to receive LcS (n = 50) or placebo (n = 45). The primary outcome is the occurrence of AAD up to 30 days after finishing LcS/placebo.
Results
The LcS group had a significantly lower incidence of AAD at 30 days after finishing the antibiotic course (28.0 v 53.3%, RR: 95% CI: 0.53, 0.31–0.89; z = 2.5, p = 0.01). Multivariate logistic regression analysis identified that LcS can reduce the risk of AAD at 30 days (OR: 0.36, 95% CI 0.13, 0.99, p < 0.05). No intervention-related adverse events were reported during the study.
Conclusions
LcS has the potential to prevent AAD in what could be considered a defined vulnerable group of SCI patients on regular PPI. A confirmatory, randomised, placebo-controlled study is needed to confirm this apparent therapeutic success to translate it into appropriate clinical outcomes.
Sponsorship
Yakult Honsha Co., Ltd.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) is a catastrophic condition that affects at least 2,500 people in the UK annually [1]. Neurogenic bladder dysfunction because of SCI often leads to increased risk of symptomatic urinary tract infections [2, 3]. The use of urinary catheters further increases the need for antibiotics and the risk of the undesirable effects like antibiotic associated diarrhoea (AAD) and Clostridioides difficile infection (CDI) [4]. In addition, diarrhoea can moreover delay rehabilitation, increase the risk of developing pressure ulcers/delay wound healing and reduce quality of life.
During the acute stage, people with SCI (PWSCI) require anticoagulation therapy to prevent venous thromboembolism. This and the increased risk of upper gastrointestinal haemorrhage due to spinal cord damage, patients are prescribed gastric protection, such as proton pump inhibitor (PPI). However, PPI exposure is also a risk factor for AAD/CDI. Literature reports show that patients on PPIs have a relative risk of 1.69 of contracting CDI compared to patients who are not taking the medication [5]. The prevalence of AAD and CDI in PWSCI are reported in the range 14.9–30.3% [6,7,8].
Our previous RCT [9], using a strict criteria for defining AAD (≥2 liquid stools using Bristol Stool Scale type 5, 6 or 7) over a 24 hours period, indicated that probiotic, Lactobacillus casei Shirota (LcS), may have a potential to prevent AAD in the subgroup of PWSCI on PPI.
There is growing interest in probiotics to reduce the risk of AAD/CDI in general. Probiotics, defined as ‘live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’, have been proposed to prevent AAD/CDI by restoring or maintaining a healthy gut microbiome in hospitalised patients on antibiotic therapy, particularly those on broad-spectrum antibiotics [10]. However, it is still unclear whether a specific probiotic strains is responsible to reduce the overall incidence of AAD/CDI. In order to confirms this effect, we carried out a sub-group analysis to assess the efficacy of live LcS in preventing AAD in people with SCI who are on PPI regularly.
Methods
Study design
This was a sub-group analysis from a prospective, multicentre, randomised, double-blind, placebo-controlled study (ECLISP) [9]. Patients who had been prescribed antibiotics were identified and approached for consent. After obtaining written informed consent, study data were collected at the time of prescribing antibiotics (baseline) and at follow up, set at 7 days and 30 days after the end of the antibiotic course (Abx + 7d, Abx + 30d). The study was conducted within the National Health Service in the UK. The three centres involved in this study are responsible for about 45–50% of all specialist SCI service in the UK.
Participants
The inclusion criteria of the present sub-group analysis included patients aged ≥18 years, who had sustained a SCI, had been admitted to one of the three investigatory centres, were due to receive antibiotics for an infection, who are taking PPI regularly and who were able to take the study drinks within 48 h of the first dose of antibiotic. Patients were excluded from the study for the following reasons: patients could not be recruited more than once and if they had antibiotic use in the 30 days prior to recruitment – although a single dose of prophylactic antibiotic given 14 to 30 days before recruitment was permitted. Also, patients with diarrhoea within the seven days prior to recruitment, and those with known gastrointestinal disease that could result in diarrhoea as well as patients with several other conditions and comorbidities, were excluded. (Supplementary Table: Appendix 1).
Between November 2014 to November 2019, 95 consenting SCI patients, who were within 48 h of commencing antibiotics and taking regular PPI, were randomly allocated to receive a fermented milk drink (Yakult®: 65 ml) containing a minimum of 6.5 ×109 colony-forming units (CFU) LcS/bottle, or placebo daily for the duration of the antibiotic course and for 7 days thereafter. The study drink was given at the drug round by nurses. Consumption was monitored on a daily basis by the study team. Minor non-compliance was defined as two consecutive days of not drinking the study intervention. Major non-compliance was defined when three or more consecutive days were missed. If participants missed the intervention for more than three days, they were withdrawn from the study.
The participants’ demographics, baseline clinical and nutritional information were collected. These included age, gender, level of SCI and completeness of injury using the International Standards for Neurological Classification of Spinal Cord Injury [11] and the cause of SCI. Information about nutritional factors, such as weight and height, route of nutrition, nutrient intake as estimated by food record charts (nil by mouth, less than half, half, more than half, and all eaten), interruptions and supplementation of nutrition (use of oral nutritional supplements and artificial nutrition support), were collected. Additional data, which included the use of mechanical ventilation, the history of intensive care unit stay, the number of medications, the indication, route and the antibiotic used as well as the use of laxatives, were recorded.
The perceived risks of the various antibiotics were used to categorise patients into three groups: “low-risk” antibiotics (metronidazole and parenteral aminoglycosides), “medium risk” antibiotics (tetracyclines, sulphonamides and macrolides) and “high risk” antibiotics (aminopenicillins, cephalosporins and quinolones) as described in previous studies [9, 12] and by the UK National Institute for Health and Care Excellence [10].
Primary outcome
The primary outcome was defined as the occurrence of AAD during and up to 30 days after the antibiotic course finished. The bowel movements were monitored routinely by the nursing staff on the ward using the Bristol stool scale [13]. Diarrhoea was defined as more than two liquid stools (Bristol stool scale type 5, 6 or 7) in any 24 h period.
Secondary outcomes
Whenever diarrhoea was reported, a stool sample was collected and sent to the hospital laboratory for the detection of C. difficile toxin. In the present study, CDI was defined by the hospital microbiology laboratory on confirmation of the presence of C. difficile toxin, but the method of C. difficile toxin detection varied between the laboratories: i.e., screening for glutamate dehydrogenase (GDH) antigen followed by toxin A and B detection, enzyme immunoassays for C. difficile toxin A and toxin B, or toxin-producing C. difficile gene detection by polymerase chain reaction testing, or a combination of these [14]. The study team recorded the occurrence of diarrhoea throughout the study. The census date was fixed 30 days after the antibiotic course had finished. The secondary outcomes were the occurrence of AAD during and up to 7 days after the antibiotic course finished with or without CDI being detected by the study site laboratory.
Statistical analysis
The primary statistical analysis was carried out on the basis of intention-to-treat, with all participants being analysed according to their allocated treatment group irrespective of what treatment they actually received.
Fisher’s exact test and χ2 test were used to compare rates of diarrhoea, as well as rates of AAD and CDI across categorical variables. Relative risks with 95% confidence intervals, were used to describe the treatment effects of LcS.
A series of screening univariate analyses were undertaken. Logistic regressions were used to establish which factors individually influenced the occurrence of diarrhoea and its duration as well as the development of CDI at Abx + 7d and Abx + 30d follow up. Linear regression was used for continuous outcome measures for the duration of diarrhoea and the number of episodes of diarrhoea. Thereafter, statistically significant univariate predictors were used in, multiple binary logistic regression and multiple regression analysis to determine statistically significant predictors for AAD, CDI and other secondary outcomes, after accounting for their relationship to other pertinent variables. No allowance for multiplicity was made for the secondary outcomes.
To reduce the bias implicit in utilising only complete cases, multiple imputation procedures for the data was used using the SPSS (SPSS version 25, Inc, Chicago, IL) multiple imputation function with fully conditional specification (maximum iterations of 500) using a Predictive Mean Modelling method (PMM) to produce 10 imputed datasets. The imputation model included all variables (demographic, clinical and outcomes) involved in the analyses, with PMM imputed variable limits set for imputed values to be within the range of available data. Main outcome variables i.e., AAD and CDI were not imputed. These 10 imputed datasets were then individually analysed as normal; thereafter standard multiple imputation procedures were used to combine the multiple scalar and multivariate estimates quantities [15, 16]. This reduces the bias of analysing incomplete datasets.
For logistic regression, odds ratio (OR), Nagelkerke’s (R) and correctly classified cases are reported. For linear regression, adjusted R2 and β coefficients with t-test significance are reported. For all tests, a p-value of 0.05 or less, or when the 95% CI for OR did not cross 1.0 were considered as statistically significant. Statistical analysis was performed using the Minitab statistical software (Version 25.0; Minitab, Inc.) and SPSS (Version 19; IBM Corporations).
Ethical consideration
The present study, conducted according to the guidelines laid down in the Declaration of Helsinki, received ethical approval from the National Research Ethics (REC) Committee (reference no. 14/SC/1101) and approval from the local research and development department at each participating site. After the study had been explained by a research coordinator and all questions had been answered, each participant signed an informed consent form prior to trial initiation.
The original study protocol was registered at ISRCTN in January 2015 (ISRCTN13119162). The study steering group was set up in July 2014 and the study commenced its recruitment in November 2014. Changes were recommended to the original, approved protocol to improve recruitment specifically in relation to the inclusion and exclusion criteria of patients’ enrolment. The new version of the protocol was developed in accordance with the consolidated standards of reporting trials 2010 guideline and subsequently approved by the sponsor and funder and the REC [Supplementary File].
A register was kept of all patients who withdrew from the study. The reason for consent withdrawal was documented, if provided by the participants, along with the following core patient characteristics: age, gender and level and severity of SCI. To monitor the progress and conduct of the study, all investigators attended meetings before the study and met for communal bi-annual updates and end of study meeting in Jan 2019.
The study was additionally monitored by an external Clinical Research Associate (PHARMExcel) according to applicable provisions of the sponsor’s subcontractors monitoring procedures, in conformance with ICH-GCP FDA guidelines, ISO 14155 and UK-specific laws/regulations.
Definition of undernutrition risk
Participants were considered at risk of undernutrition on the basis of the Spinal Nutrition Screening Tool (SNST) [17]. The SNST assesses eight criteria, of which the majority are recognised as predictors or symptoms of undernutrition: history of recent weight loss; body mass index; level of SCI; presence of co-morbidity; skin condition; appetite; ability to eat. Each step of screening has a score of up to 5 and the total score reflects the participant’s degree of risk. A score of 0–10 suggests a low risk, 11–15 a moderate risk and >15 suggests a high risk of undernutrition. Participants who had a SNST score ≤10 were considered at low risk, and all those with a SNST score ≥11 were considered at increased risk.
Results
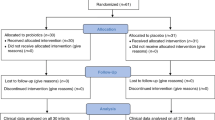
Over the 60 months of the study period, 359 patients were approached by the study team; 48 (11%) patients refused to participate in the study, 44 (9.6%) potentially eligible patients were missed because of the tight recruitment timeframe, and eight (1.7%) were excluded for logistical reasons. Of the 359 patients recruited into ECLISP study, 95 patients (27%) of patients met the inclusion of this sub-group analysis. The participants flow is summarised in Fig. 1.
The baseline characteristics of the 95 patients (median age: 57, IQ range: 25; 23% female; 88% Caucasian), and the outcomes are summarised in Table 1. SCI was traumatic in origin (n = 68, 67%) and non-traumatic in 27 case (28%). The median onset of SCI was 104 days (IQ range: 54–469).
The prevalence of risk for undernutrition was 41% (n = 39) at the time of recruitment and 16 (17%) were malnourished on dietitian assessment.
Most participants (67%) received one antibiotic as their entry criterion to the study, but 21% received two, 7.4% received three and 4.2% received four or more antibiotics. A total of 19 different antibiotics were recorded in the present study: the oral route was used in 48% and the intravenous route was used in 52% of participants. The median length of antibiotic course was 7 days (IQ range: 6–11); no statistically significant differences was found with regard to nature or duration of antibiotic intake. The indications for antibiotic treatment were: urinary tract infections (n = 49, 47%), respiratory tract infections (n = 20, 19%), wound infections (8, 7.7%), pressure ulcer infections (7, 6.7%), post-operative infections (n = 4, 3.8%), sepsis (n = 4, 3.8%), eye infections (n = 2, 1.9%), surgical implant infection (n = 1, 0.9%) and others (n = 9, 8.6%).
At baseline, the LcS and placebo groups were similar with respect to demographic and clinical characteristics, which included: age, onset of SCI, those with tetraplegia, the percentage who were on mechanical ventilation, the percentage with pressure ulcers, the percentage with previous history of Intensive Therapy Unit (ITU) stay; laxative use; body mass index (BMI), the percentage of those nil-by-mouth and those with enteral feeding tubes (Table 1). The number of participants on high-risk antibiotics was higher in the placebo group (64% placebo group and 44% in LcS group, p < 0.05).
Two (2.1%) severe adverse events were reported in this sub-group analysis and they were not related to the use of investigational product (Table 2).
Primary outcome
Antibiotic associated diarrhoea
The overall prevalence of AAD was 40% at 30 days follow up. This is a statistically significant difference between the LcS and placebo groups (28 v 53%, RR: 0.53, 0.31–0.89; z = 2.5, p = 0.01) in regard to the intention to treat that included all patients with available end-point data (Fig. 2 and Table 3).
Secondary outcomes and predetermined subgroup analysis
There was a statistically significant difference between the LcS and placebo groups for the prevalence of AAD at 7 days follow up (19% v 36%, RR: 0.53, 95% CI: 0.29–0.99, z = 2.0; p = 0.04) but there was no significance observed in the duration of diarrhoea, the number of episodes of diarrhoea and the occurrence of CDI at 7 days follow up, nor at 30 days follow up for CDI (Table 3).
Risk factors for antibiotic associated diarrhoea/Clostridioides difficile infection
The risk factors for AAD at Abx + 30d were being in control group (taking placebo) and study site as the unique risk factors of AAD at 30 days follow up. The use of LcS was associated with a lower risk of AAD at 30 days follow up (28% v 53%, RR: 0.53, 95% CI: 0.31–0.89) (Table 4.1).
The multivariate logistic/regression analysis revealed independent risk factors for AAD at Abx + 7d: use of high-risk antibiotics (OR: 6.2; 95% CI: 1.5–25.4), study site (OR: 57.9; 95% CI: 4.1–829) and number of drugs (OR: 1.3; 95% CI: 1.1–1.5) (Table 4.2).
Discussion
Optimisation of people with SCI’s experience in SCI rehabilitation and neurogenic bowel & bladder management has been a priority for both clinical [18] and research [19]. In 2014, the James Lind Alliance research priority setting exercise reported an improved bladder management, urinary tract infection and intervention in bowel management were listed within the top 10 research priorities for PWSCI [19].
People with SCI may be particularly vulnerable to diarrhoea and its consequences because of their long hospital stays for acute care and rehabilitation. The present study found the incidence of AAD similar to previous reports in the range of 15–36% [6,7,8]. but seems higher than studies conducted in general populations (11–18%) [20, 21]. This may be attributed to a longer follow-up period (30 days) than in many of the other published trials (often only 7–14 days) [20]. It is reported that diarrhoea may occur up to 2 months after discontinuing antibiotic treatment [22].
This sub-group analysis suggested that the LcS could reduce the risk of AAD in patients with SCI who consumed PPI regularly. These finding are similar to our earlier open-labelled study [6].
The present study defined AAD as ≥2 liquid stools (Bristol Stool Scale type 5, 6 or 7) for a 24-h period, whereas the previous trial required ≥3 days [6]. This altered definition may have led to a failure in distinction between clinically relevant AAD and loose stool due to neurogenic bowel as a result of SCI. Indeed, the definition of AAD varies widely between published studies. For example, Rajkumar et al. [20] defined diarrhoea as ≥2 loose stools, Bristol 6 or 7 a day for ≥3 days, whereas Allen et al. [21] and Helps et al. [23] defined diarrhoea from ≥3 loose stools, Bristol 5, 6 or 7 in a single 24-h period. The definition for CDI also varies. The use of standardised definitions of AAD/CDI will greatly improve the quality and the interpretation of newer research studies, especially important for systematic reviews and meta-analyses.
There are some limitations in this study. The inclusion of patients treated in different SCI centres could be considered a strength, but can also be regarded as a weakness. Infection control policies, AAD/CDI definition were different in the participating SCI centres, thus the influence of these factors on the study results could not be excluded. In addition, different SCI centre may have different policies on antibiotic prescribing and different catheters and bowel management programme. Nevertheless, the selection of the SCI centres was at the discretion of the authors; and those selected represented approximately 45–50% of the SCI centre beds in the UK, therefore, we would assume the result derived from this study can be considered representative.
The present study did not judge whether antibiotics were prescribed appropriately; there may be differences between centres in their antibiotics prescribing and bowel management programme.
Current evidence remains equivocal in whether probiotics could reduce the incidence of AAD/CDI either in the general hospitalised or SCI populations [20, 21, 24]. The complexity of probiotic use is not just strain-, product-, dose- and disease specific, but also includes defining when the probiotic should be administered and the duration of its use; all these factors need to be considered. The present study dose of a minimum of 6.5 ×109 CFU LcS was selected based on the previous trial’s data [6]. LcS is well tolerated in clinical settings and has been used in a broad range of patients [25, 26]. However, dose and type of probiotic vary between published studies. For example, Allen et al. [21] used a mixed strains probiotic (L. acidophilus CUL60, CUL21, B. bifidum CUL20, B. lactis CUL34 in 6 ×109 CFU/day), Helps et al. [23] used a single strain probiotic (LcS in 13 ×109 CFU/day), and Rajkumar et al. [20] used mixed strains (L. casei immunitas DN-114001 in 10 ×109 CFU/day) as did Selinger et al. [27] (VSL#3 in 900 ×109 CFU/day). It has been suggested that the dose of probiotic should >1010 CFU/ day in order to prevent AAD [28]. The dose of 6.5 ×109 CFU LcS was selected on pragmatically to limit the volume to one bottle to aid compliance, and it is possible that a higher does could have yielded even greater benefits. However, the efficacy of increasing the dose of the probiotic LcS should be carefully monitored to avoid unexpected adverse effect.
The compliance with LcS therapy in the present study was good (92%), with no known adverse events directly related to LcS.
Another important criterion for any probiotic used is that the strains survive the passage through the stomach and arrive in a viable state in the small intestine and colon. LcS has been shown to survive and is well tolerated in the upper gastrointestinal tract and reach the large intestine in a viable state [29, 30].
Due to global concern of antibiotic resistance as infections become drug resistant and antibiotics becoming less effective at treating infections, and in an effort to prevent such occurrence, we assume that a non-antibiotic therapy, such as probiotic supplement is highly desirable if this could prevent AAD/CDI. Indeed, reducing antibiotic use could improve quality of life, save money and help preserve the usefulness of existing antibiotics.
For gastric protection in the acute phase after SCI, approximately 1 in 3 people are prescribed PPI. We believe that the number of patients prescribed PPI instead is much higher due to national shortage of H2 blocker. The present sub-group analysis suggests that the daily consumption of probiotic LcS has the potential to prevent AAD in the higher risk group patients on regular PPI. In order to translate into improved clinical outcome, a confirmatory randomised, placebo-controlled study is recommended to confirm this apparent therapeutic success.
Data availability
The study is registered with ISRCTN, number: 13119162. In accordance with the protocol, no investigators will have access to participant data with identifiers. De-identified participant data that underlie the results reported in this article will be made available as supplementary material.
References
Spinal cord injury paralyses someone every four hours, new estimates reveal. Spinal Injury Association. 2022. https://www.spinal.co.uk/news/spinal-cord-injury-paralyses-someone-every-four-hours-new-estimates-reveal/. Accessed 16th January 2023.
Wong S, Santullo P, Hirani SP, Kumar N, Chowdhury JR, Carcia-Forcada A, et al. Use of antibiotic and the prevalence of antibiotic-associated dirrhoea in patients with spinal cord injuries: an international, multi-centre study. J Hosp Infect. 2017;97:146–52.
Vigil HR, Hickling DR. Urinary tract infection in the neurogenic bladder. Transl Androl Urol. 2016;5:72–87.
Meddings J, Rogers MAM, Macy M, Saint S. Systematic review and meta-analysis: reminder system to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51:550–60.
Janarthanan S, Ditah, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107:1001–10.
Wong S, Jamous A, O’Driscoll J, Sekhar R, Weldon M, Yau C, et al. A Lactobacillus casei Shirota probiotic drink reduces antibiotic-associated diarrhoea in patients with spinal cord injuries: a randomised controlled trial. Br J Nutr. 2014;111:672–8.
Wong S, Santullo P, O’Driscoll J, Jamous A, Hirani SP, Saif M. Use of antibiotic and prevalence of antibiotic-associated diarrhoea in patients with spinal cord injuries: a UK national spinal injury centre experience. Spinal Cord. 2017;55:483–587.
Wong S, Santullo P, Hirani SP, Kumar N, Chowdhury JR, Gracia-Forcada A, et al. Use of antibiotics and the prevalence of antibiotic-associated diarrhoea in patients with spinal cord injuries: an international, multi-centre study. J Hosp Infect. 2017;97:146–52.
Wong S, Hirani SP, Forbes A, Kumar N, Hariharan R, O’Driscoll J, et al. A study into the effect of Lactobacillus casei Shirota in preventing antibiotic associated diarrhoea including Clostridioides difficile infection in patients with spinal cord injuries: a multicentre randomised, double-blind, placebo-controlled trial. EClinicalmedicine. 2021;40:101098. https://doi.org/10.1016/j.eclinm.2021.101098.
National Institute for Health and Care Excellence. Clostridium difficile infection: risk with broad-spectrum antibiotics. Evidence summary. 2015. https://www.nice.org.uk/advice/esmpb1/chapter/Key-points-from-the-evidence. Accessed 20th June 2020.
American Spinal Injury Association. International standards for neurological classification of spinal cord injury. Richmond (VA): American Spinal Injury Association; 2019.
Kelly CP, LaMont JT. Clostridium difficile infection. Annu Rev Med. 1998;49:375–90.
O’Donnell LD, Virjee J, Heaton KW. Detection of pseudo diarrhoea by simple clinical assessment of intestinal transit time. Br Med J. 1990;300:439–40.
Wong S, Saif M, O’Driscoll J, Sekhar R, Saif M, O’Driscoll S, et al. Use of probiotic in preventing antibiotic associated diarrhoea and Clostridium difficile associated diarrhoea in spinal injury centres: an international multicentre survey. Int J Probiotics Prebiotics. 2015;10:85–90.
Rubin D. Multiple imputation for nonresponse in surveys. John Wiley & Sons, InC, New York, 1987.
Schafer JL, Olsen MK. Multiple Imputation for Multivariate Missing-Data Problems: A Data Analyst’s Perspective. Multivar Behav Res. 1998;33:545–71. https://doi.org/10.1207/s15327906mbr3304_5.
Wong S, Derry F, Jamous A, Hirani SP, Grimble G, Forbes A. Validation of the Spinal Nutrition Screening Tool (SNST) in patients with spinal cord injuries (SCI) – result from a multicentre study. Eur J Clin Nutr. 2012;66:382–7.
NHS England. NHS Five Year Forward View. 2017. https://www.england.nhs.uk/five-year-forward-view/. Accessed 30th November 2022.
Van Middendorp J, Allison H, Cowan K, for the Spinal Cord Injury Priority Setting Partnership. Top ten research priorities for spinal cord injury. Lancet Neurol. 2014;13:1167.
Rajkumar C, Wilks M, Islam J, Ali K, Rafterj J, Davies KA, et al. Do probiotics prevent antibiotic-associated diarrhoea? Results of a multicentre randomized placebo-controlled trial. J Hosp Infect. 2020;105:280–8.
Allen SJ, Wareham K, Wang D, Bradley C, Hutchings H, Harris W, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-assocaited diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249–57.
McFarland LV. Update on the changing epidemiology of Clostridium difficile-associated disease. Nat Clin Pr Gastroenterol Hepatol. 2008;5:40–48.
Helps A, Bell E, Mactier R. Prospective randomized dibble-blind study of efficacy of probiotic milk drink in reducing the incidence of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea. Int J Probiotic Prebiotics. 2015;4:145–52.
Johnston BC, Lytvyn L, Ka-Fung C, Allen S, Wang D, Szajewska H, et al. Clostridium difficile Infection in Adults and Children: An individual patient data meta-analysis of 6,851 participants. Infect Control Hosp Epidemiol. 2018;39:771–81.
Matsuzaki T, Saito M, Usuku K, Nose H, Izumo S, Arimura K, et al. A prospective uncontrolled trial of fermented milk drink containing viable Lactobacillus casei strain Shirota in the treatment of HTLV-1 associated myelopathy / tropical spastic paraparesis. J Neuro Sci. 2005;237:75–81.
Stadlbauer V, Moorkerjee RP, Hodges SJ, Wright GA, Davies N, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2006;48:945–51.
Selinger C, Bell A, Cairns A, Lockett M, Sebastian S, Halasm N. Probiotic VSL#3 prevent antibiotic-associated diarrhoea in a double-blind, randomized, placebo-controlled clinical trial. J Hosp Infect. 2013;84:159–65.
McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhoea and the treatment of Clostridium difficile diseases. Am J Gastroenterol. 2006;101:812–22.
Yuki N, Watanabe K, Miller A, Tagami Y, Tanaka R, Ohwaki M, et al. Survival of a probiotic, Lactobacillus casei strain Shirota, in the gastrointestinal tract: selective isolation from faeces and identification using monoclonal antibodies. Int J Food Microbiol. 1999;48:51–57.
Khine WWT, Ang XJ, Chang YS, Lee WQ, Quek SY, Tan SH, et al. Recovery of Lactobacillus casei strain Shirota (LcS) from faeces of healthy Singapore adults after intake of fermented milk. Beneficial Microbes. 2019;10:721–8.
Acknowledgements
The authors would like to thank the patients and staffs from all three SCI centres. We are also grateful to the medical consultants of the National Spinal Injuries Centre (Dr Allison Graham, Mr Maurizio Belci, Mr Mofid Saif and Mr Wail Ahmed), Midland Centre of Spinal Injury (Mr Joy Roy Chowdhury) and Princess Royal Spinal Injury Centre (Dr K.M Mathew and Dr Pradeep Thumbikat) for allowing us to recruit patients under their care for the study. The authors also grateful to Yakult Europe B.V. for kindly providing the study drink/placebo free of charge. Thank to Dr Kenji Oishi, Dr Junji Fujimoto, Dr Kazunori Matsuda, Dr Akria Shigehisa, Dave Pritchard, Edmund Chiu, Dr Catherine Whittall, Theresa Garratt, Janine Turner, Alka Pandey, Meena Ganesan, Dr Joost van Middendorp, Pauline Cato, Florinda Sonon, Anthony Twist, Dr Anitha Naidoo, Paul Subong, Chiyuki Kajita, Takeshi Yoshimoto, Dr Kaori Suzuki, Dr Louise Durrant and Dr Linda Thomas who supported the trial design.
Funding
This study was funded by Yakult Honsha Co. Ltd., reference number: 13UK-1-SMH-AAD2 with the title “Efficacy of Consuming LcS In Spinal cord injury Patients (ECLISP): Effect of Lactobacillus casei Shirota in preventing antibiotic associated diarrhoea (AAD) including Clostridium difficile infection (CDI) in patients with spinal cord injuries: a multicentre, randomised, double-blind, placebo-controlled trial”. The study drink and placebo were provided by the manufacturing company, Yakult Europe B.V, a subsidiary of Yakult Honsha, Japan. The funder did not have any role in the design of the study, the collection, analysis, or interpretation of the data, the writing of this manuscript, or the decision to submit this manuscript for publication.
Author information
Authors and Affiliations
Contributions
SW- study chief investigator, protocol development, data collection, data analysis, manuscript preparation. SPH- protocol development, data evaluation, statistical support / analyst, manuscript revision. AF – protocol development, data evaluation, gastroenterology adviser, manuscript revision. NK – site principal investigator, protocol development, manuscript revision. RH – site principal investigator, data evaluation, gastroenterology adviser, manuscript revision. JOD – protocol development, clinical supervision, validation and guardian of laboratory data, manuscript revision. AV- data collection, data evaluation, manuscript revision. GH- protocol development, validation and guardian of laboratory data, manuscript revision. RS – protocol development, data evaluation, manuscript revision. AJ – protocol development, clinical supervision, data evaluation, manuscript revision.
Corresponding author
Ethics declarations
Competing interests
SW has received funding support from Yakult Honsha Co Ltd. Yakult Europe B.V. provided free LcS/Placebo and the necessary equipment to maintain the cold chain. The company was aware of, but did not influence the trial design, and had no role in data analysis and interpretation. AF has received honoraria form Fresenius Kabi, Takeda and Dr Falk Pharma. SW as chief investigator and corresponding author had full access to the final dataset and made final decision to submit for publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wong, S., Hirani, S.P., Forbes, A. et al. Lactobacillus casei Shirota probiotic drinks reduce antibiotic associated diarrhoea in patients with spinal cord injuries who regularly consume proton pump inhibitors: a subgroup analysis of the ECLISP multicentre RCT. Spinal Cord (2024). https://doi.org/10.1038/s41393-024-00983-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41393-024-00983-w