Abstract
Study design
Preclinical pharmacology.
Objectives
To determine whether blocking substance P signaling attenuates the hypertension and bradycardia evoked by colorectal distension (CRD) in spinal cord injured (SCI) rats.
Setting
University laboratory in Pennsylvania, U.S.A.
Methods
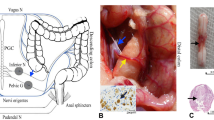
Tachykinin NK1 receptor antagonists were administered 30 min prior to CRD three weeks after complete spinal cord transection at the 4th thoracic (T4) level. The dose range, route of administration, and pretreatment time was based on published data demonstrating occupancy of brain NK1 receptors in rodents.
Results
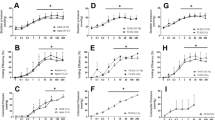
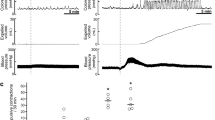
Subcutaneous (SC) administration of 10–30 mg/kg GR205171 ((2S,3S)-N-[[2-methoxy-5-[5-(trifluoromethyl)tetrazol-1-yl]phenyl]methyl]-2-phenylpiperidin-3-amine dihydrochloride) reduced CRD-induced hypertension and bradycardia by 55 and 49%, respectively, compared with pretreatment values. There was no effect of GR205171 on resting blood pressure or heart rate. In contrast, the same dose range of CP-99,994 ((2S,3S)-N-[(2-methoxyphenyl)methyl]-2-phenyl-3-piperidinamine dihydrochloride) had no effect on CRD-induced cardiovascular responses.
Conclusions
The effective dose range of GR205171 to alleviate autonomic dysreflexia is consistent with the blockade of NK1 receptors on pelvic sensory afferents in the lumbosacral spinal cord, which may in turn prevent the over-excitation of sympathetic preganglionic neurons (SPNs) that regulate blood pressure and heart rate. The findings provide preclinical support for the utility of NK1 receptor antagonists to treat autonomic dysreflexia in people with SCI. The difference in the effects of the two NK1 receptor antagonists may reflect the ~200-fold lower affinity of CP-99,994 than GR205171 for the rat NK1 receptor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data generated and analyzed during this study are available upon reasonable request from Dr. Hou at Drexel University.
References
Kurnick NB. Autonomic hyperreflexia and its control in patients with spinal cord lesions. Ann Intern Med. 1956;44:678–86.
Faaborg PM, Christensen P, Krassioukov A, Laurberg S, Frandsen E, Krogh K. Autonomic dysreflexia during bowel evacuation procedures and bladder filling in subjects with spinal cord injury. Spinal Cord. 2014;52:494–8. https://doi.org/10.1038/sc.2014.45.
Krassioukov A, Warburton DE, Teasell R, Eng JJ. Spinal Cord Injury Rehabilitation Evidence Research Team A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil. 2009;90:682–95.
Blackmer J. Rehabilitation medicine: 1. Autonomic dysreflexia. CMAJ 2020;169:931–5.
de Groat WC, Yoshimura N. Changes in afferent activity after spinal cord injury. Neurourol Urodyn. 2010;29:63–76.
Giannantoni A, Di Stasi SM, Stephen RL, Navarra P, Scivoletto G, Mearini E, et al. Intravesical capsaicin versus resiniferatoxin in patients with detrusor hyperreflexia: a prospective randomized study. J Urol. 2002;167:1710–4.
Igawa Y, Satoh T, Mizusawa H, Seki S, Kato H, Ishizuka O, et al. The role of capsaicin-sensitive afferents in autonomic dysreflexia in patients with spinal cord injury. BJU Int. 2003;91:637–41.
Holzer P, Bucsics A, Lembeck F. Distribution of capsaicin-sensitive nerve fibres containing immunoreactive substance P in cutaneous and visceral tissues of the rat. Neurosci Lett. 1982;31:253–7.
Shaker H, Wang Y, Loung D, Balbaa L, Fehlings MG, Hassouna MM. Role of C-afferent fibres in the mechanism of action of sacral nerve root neuromodulation in chronic spinal cord injury. BJU Int. 2000;85:905–10.
Green SA, Alon A, Ianus J, McNaughton KS, Tozzi CA, Reiss TF. Efficacy and safety of a neurokinin-1 receptor antagonist in postmenopausal women with overactive bladder with urge urinary incontinence. J Urol. 2006;176:2535–40.
Frenkl TL, Zhu H, Reiss T, Seltzer O, Rosenberg E, Green S. A multicenter, double-blind, randomized, placebo-controlled trial of a neurokinin-1 receptor antagonist for overactive bladder. J Urol. 2010;184:616–22.
Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81:506–16.
Hou S, Duale H, Cameron AA, Abshire SM, Lyttle TS, Rabchevsky AG. Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection. J Comp Neurol. 2008;509:382–99.
Llewellyn-Smith IJ, Martin CL, Minson JB, Pilowsky PM, Arnolda LF, Basbaum AI, et al. Neurokinin-1 receptor-immunoreactive sympathetic preganglionic neurons: target specificity and ultrastructure. Neuroscience. 1997;77:1137–49.
Cassam AK, Llewellyn-Smith IJ, Weaver LC. Catecholamine enzymes and neuropeptides are expressed in fibres and somata in the intermediate gray matter in chronic spinal rats. Neuroscience. 1997;78:829–41.
Klimaschewski L. Increased innervation of rat preganglionic sympathetic neurons by substance P containing nerve fibers in response to spinal cord injury. Neurosci Lett. 2001;307:73–76.
Rabchevsky AG, Patel SP, Duale H, Lyttle TS, O’Dell CR, Kitzman PH. Gabapentin for spasticity and autonomic dysreflexia after severe spinal cord injury. Spinal Cord. 2012;49:99–105.
Hou S, Blesch A, Lu P. A radio-telemetric system to monitor cardiovascular function in rats with spinal cord transection and embryonic neural stem cell grafts. J Vis Exp. 2014;92:e51914.
Rupniak NM, Carlson EJ, Shepheard S, Bentley G, Williams AR, Hill A, et al. Comparison of the functional blockade of rat substance P (NK1) receptors by GR205171, RP67580, SR140333 and NKP-608. Neuropharmacology. 2003;45:231–41.
Hutson PH, Patel S, Jay MT, Barton CL. Stress-induced increase of cortical dopamine metabolism: attenuation by a tachykinin NK1 receptor antagonist. Eur J Pharm. 2004;484:57–64.
Jung M, Calassi R, Maruani J, Barnouin MC, Souilhac J, Poncelet M, et al. Neuropharmacological characterization of SR 140333, a non-peptide antagonist of NK1 receptors. Neuropharmacology. 1994;33:167–79.
Lombet A, Spedding M. Differential effects of non-peptidic tachykinin receptor antagonists on Ca2 + channels. Eur J Pharm. 1994;267:113–5.
Varty GB, Cohen-Williams ME, Morgan CA, Pylak U, Duffy RA, Lachowicz JE, et al. The gerbil elevated plus-maze II: anxiolytic-like effects of selective neurokinin NK1 receptor antagonists. Neuropsychopharmacology. 2002;27:371–9.
Smith G, Harrison S, Bowers J, Wiseman J, Birch P. Non-specific effects of the tachykinin NK1 receptor antagonist, CP-99,994, in antinociceptive tests in rat, mouse and gerbil. Eur J Pharm. 1994;271:481–7.
Brocco M, Dekeyne A, Mannoury la Cour C, Touzard M, Girardon S, Veiga S, et al. Cellular and behavioural profile of the novel selective neurokinin 1 receptor antagonist vestipitant: a comparison to other agents. Eur Neuropsychopharmacol. 2008;18:729–50.
Rupniak NM, Webb JK, Williams AR, Carlson E, Boyce S, Hill RG. Antinociceptive activity of the tachykinin NK1 receptor antagonist, CP-99,994, in conscious gerbils. Br J Pharm. 1995;116:1937–43.
McLean S, Ganong A, Seymour PA, Snider RM, Desai MC, Rosen T, et al. Pharmacology of CP-99,994; a nonpeptide antagonist of the tachykinin neurokinin-1 receptor. J Pharm Exp Ther. 1993;267:472–9.
Gardner CJ, Armour DR, Beattie DT, Gale JD, Hawcock AB, Kilpatrick GJ, et al. GR205171: a novel antagonist with high affinity for the tachykinin NK1 receptor, and potent broad-spectrum anti-emetic activity. Regul Pept. 1996;65:45–53.
Sharif H, Hou S. Autonomic dysreflexia: a cardiovascular disorder following spinal cord injury. Neural Regen Res. 2017;12:1390–1400.
Bycroft J, Shergill IS, Choong EAL, Arya N, Shah PJR. Autonomic dysreflexia: a medical emergency. Postgrad Med J. 2005;81:232–5.
Acknowledgements
The authors would like to thank Jason Cook for assistance with statistical analysis.
Funding
Studies were supported by the National Institute of Neurological Disorders and Stroke under award number R41NS117205-01.
Author information
Authors and Affiliations
Contributions
NMJR and KBT developed the concept and hypotheses underlying the studies. The design of the experiments was developed by NMJR, SH, SF, LM, and KBT. SF conducted the experiments and analyzed the data with LM. NMJR wrote the manuscript with contributions from all coauthors.
Corresponding author
Ethics declarations
Competing interests
NMJR, KBT, and LM are employees and shareholders in Dignify Therapeutics LLC. SF, and SH have no competing interests to declare.
Ethical approval
Institutional Animal Care and Use Committee and National Institutes of Health guidelines on animal care to minimize suffering were followed (IACUC PHS Animal Welfare Assurance #A-3222-01, Protocol No: 20839).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rupniak, N.M.J., Fernandes, S., Hou, S. et al. Effect of GR205171 on autonomic dysreflexia induced by colorectal distension in spinal cord injured rats. Spinal Cord 61, 499–504 (2023). https://doi.org/10.1038/s41393-023-00918-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-023-00918-x