Abstract
Study design
A cross-sectional study.
Objective
This study aimed to investigate the vitamin D status after acute spinal cord injury (SCI) onset.
Setting
Specialized SCI rehabilitation center in Switzerland.
Methods
Patients admitted to the center after an acute SCI onset were included. The prevalence of a deficient (25(OH)D ≤ 50 nmol/l), insufficient (50 < 25(OH)D ≤ 75 nmol/l) and sufficient (25(OH)D > 75 nmol/l) vitamin D status were determined after admission. Vitamin D status was compared between different patient groups based on demographic and SCI characteristics. The occurrence of bed rest, falls and pressure injuries were also assessed.
Results
In total, 87 patients (median (interquartile range); 53 (39–67) years, 25 females, 66 traumatic SCI, 54 paraplegia) were included. Assessed a median of 15 (9–22) days after SCI onset, median vitamin D status was 41 (26–57) (range 8–155) nmol/l. The majority of patients had a deficient (67%, 95% confidence interval (95% CI) 0.56–0.76) or insufficient (25%, 95% CI 0.17–0.36) vitamin D status. A moderate negative correlation was found between vitamin D status and body mass index (p = 0.003). A moderate positive correlation was found between vitamin D and calcium status (p = 0.01).
Conclusion
A deficient or insufficient vitamin D status directly after SCI onset is highly prevalent. Vitamin D status should be carefully observed during acute SCI rehabilitation. We recommend that all patients with recent SCI onset should receive vitamin D supplementation with a dosage depending on their actual vitamin D status.
Similar content being viewed by others
Introduction
Vitamin D is produced through solar ultraviolet B (UVB) radiation in the skin. It is an important hormone regulating body functions, including bone health and immune function [1]. In the general population, a high body mass index (BMI), clothing, low sun exposure, seasonality, or the excessive use of sunblock might lead to an insufficient vitamin D status [1]. Especially during the winter months, a high prevalence of vitamin D insufficiency is observed in the northern hemisphere [2]. Among patients undergoing acute rehabilitation after traumatic brain injury [3], stroke [4], or spinal cord injury (SCI) [5, 6], the prevalence of an insufficient vitamin D status is higher compared to able-bodied individuals [7]. In SCI, possible reasons for the excess prevalence are: specific physiological changes (for example an increase in fat mass which could store non-available vitamin D), the use of certain medication, bed rest, decreased sun exposure, and physical activity [8, 9].
Physical adaptations which initiate during the acute SCI phase are often life-lasting and irreversible, including a decrease in bone density, altered gastrointestinal functioning, and diminished immune system functioning [10, 11]. As a result, common secondary complications among individuals with SCI include pressure injuries, diminished nutrient absorption, reduced respiratory and immune system functioning, as well as osteoporosis. A relation between these complications and vitamin D status has been suggested, implicating the relevance of a sufficient vitamin D status, especially during the acute phase [8, 9]. Among the SCI population, a low vitamin D status has been related to impairments in immune function and functional independence during daily activities, as well as an increased risk for pressure injuries, fractures, and falls [8, 9, 12]. In the general population, a serum 25(OH)D concentration above 75 nmol/l (equivalent to 30 ng/ml) has been defined as sufficient in relation to several positive health outcomes regarding the immune system, musculoskeletal and cardiovascular health [13,14,15]. Even though no specific guidelines for an optimal vitamin D status in individuals with SCI exist, sufficient levels might reduce the risk for secondary complications. The relatively limited number of studies assessing vitamin D status among patients admitted to acute SCI rehabilitation reported a high prevalence of a deficient or insufficient status (70–98%) [5, 6, 16,17,18,19]. Besides demographic and lesion characteristics, no other secondary parameters like mobility or the occurrence of bed rest or pressure injuries were investigated in these mainly North-American studies. The primary aim of this cross-sectional study was to investigate the vitamin D status directly after SCI onset and to assess the prevalence of vitamin D insufficiency. Furthermore, the vitamin D status was compared between several patient groups based on secondary parameters including demographic and SCI characteristics, mobility, and the occurrence of pressure injuries, bed rest, and falls.
Materials and methods
Setting and study population
Individuals with an acute SCI between 18 and 85 years old who were admitted to the specialized rehabilitation center between October 2017 and February 2020 were included in this cross-sectional study. Patients receiving daily vitamin D supplementation with a dosage > 400 IU were not eligible. The maximum dosage was set to 400 IU/day since this vitamin D quantity is included in the multivitamin that is commonly administered among patients in our center. This dosage is still below the recommended maintenance dosage of 600 IU/day [20]. Patients having thyroid gland, renal or malabsorption disorders were not eligible. Patients admitted to the center more than 28 days after SCI onset were excluded since a longer hospitalization implies reduced sun exposure, which in turn could influence vitamin D status [9]. Study participation merely entailed access to medical records and no compensation was provided. All patients provided written informed consent. The study was conducted according to the Declaration of Helsinki and Swiss law, and approval of the local ethics committee was obtained (BASEC-Number: 2017-01108).
Data collection
The first vitamin D assessment that was available after admission to the center was extracted from the patient’s records and all other data were also extracted from the same time point. All assessments were performed by trained medical personnel following routine clinical procedures.
Demographic characteristics, including age, sex, BMI, and smoking status, as well as lesion characteristics, including onset date and lesion level, were collected. From a 17.3 ml venous sample, the following parameters were assessed: vitamin D (25(OH)D), calcium, parathyroid hormone (PTH), and phosphate (Table 1). The intake of vitamin D and calcium supplements was recorded. The presence (yes or no) of pressure injuries according to the European Pressure Ulcer Advisory Panel (EPUAP) classification system [21], bed rest, and falls were documented. To evaluate mobility and potential movement during activities of daily living, the Spinal Cord Independence Measure (SCIM-III) score for the subcategory “indoor and outdoor mobility” (six items, total range 0–30) was collected [22].
Data preparation
Smoking status was categorized into “never”, “former” or “current” smoker. SCI etiology was categorized into “non-traumatic” (caused by a medical event) or “traumatic” (caused by trauma). The neurological level of injury, defined as the highest motor level, was categorized into “tetraplegia” (C1-C8) or “paraplegia” (T1 or lower) [23]. The American Spinal Injury Association Impairment Scale (AIS) was used to categorize the level of impairment into motor “complete” (A or B) or “incomplete” (C or D) [23]. The season of SCI onset was categorized into “winter” (October-March) or “summer” (April-September) [24]. The duration between SCI onset and admission to the center was categorized into “within two weeks” or “more than two weeks”. The same categorization was made for the duration between SCI onset and the time point of vitamin D assessment. SCIM mobility score was categorized into “less active” (0-3 points) or “active” (>3 points). Vitamin D status was categorized in “deficient” (25(OH)D ≤ 50 nmol/l), “insufficient” (50 nmol/l < 25(OH)D ≤ 75 nmol/l) or “sufficient” (25(OH)D > 75 nmol/l) [8, 13,14,15]. A PTH concentration of < 5 ng/l was set to 1 ng/l.
Data analyses
Data were described by reporting median with interquartile range (IQR), mean with standard deviation (SD), range, and proportions with exact Clopper-Pearson 95% confidence intervals (95% CI). Differences were tested using Kruskal-Wallis H tests and Fisher’s exact tests. Associations were assessed by Spearman’s correlation. A p-value below 0.05 (two-tailed) was considered statistically significant. Due to the exploratory nature of the study, no adjustments for multiplicity were applied. Analyses were performed using Stata (StataCorp. 2017, Stata Statistical Software: Release 16.0. College Station, TX: StataCorp LLC).
Results
Patient and clinical characteristics
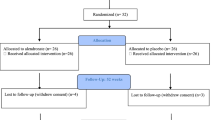
A total of 169 patients with an acute SCI were admitted to the center, of whom 90 fulfilled the inclusion criteria and signed the informed consent (Fig. 1). Vitamin D status data were missing for three patients. Thus, they were excluded from the analyses. The remaining 87 patients (median age 53 (39–67) years, range 18–85 years, 25 females) were admitted to the center within 28 (median 8 (4–14)) days after SCI (Table 2). Data were collected a median of 15 (9–22, range 2–42) days after SCI onset. Traumatic SCI (76%), paraplegia (62%), and incomplete lesion (63%) were the most common lesion characteristics. When comparing demographic characteristics between patients with a tetraplegic and a paraplegic lesion, only AIS score differed (p = 0.04). Slightly more patients had an SCI onset during winter (58%) or had their vitamin D status assessed during winter (58%). All patients were Caucasian.
Pressure injuries were present in eight (9%) patients. A fall was documented in one (1%) patient. Bed rest was prescribed in seven (8%) patients, and five of these patients also had a pressure injury. Calcium status between patients with or without bed rest was similar (p = 0.3).
Vitamin D status
The median vitamin D status was 41 (IQR 26–57, range 8–155) nmol/l and the mean vitamin D status was 44 [25] (range 8–155) nmol/l. The majority of patients had a deficient (58 patients, 67%, 95% CI 0.56–0.76) or insufficient (22 patients, 25%, 95% CI 0.17–0.36) vitamin D status (Table 3). Two patients received a daily multivitamin including calcium (120 mg) and vitamin D (200 IU). One of these patients started taking the multivitamin six days before the vitamin D assessment and had a sufficient vitamin D status (100 nmol/l), while the other patient started taking the multivitamin three days before the vitamin D assessment and had insufficient vitamin D status (57 nmol/l). Seven (8%, 95% CI 0.03–0.16) patients had a sufficient vitamin status. Median PTH (27, IQR 18-37, range 13–48 ng/l), calcium (2.2, IQR 2.1–2.3, range 1.9–2.6 mmol/l), and phosphate (1.2, IQR 1.0–1.4, range 0.6–1.9 mmol/l) concentrations were within normal ranges.
A moderate negative correlation was found between vitamin D status and BMI (rs = −0.33, p = 0.003, n = 80), but not for age (rs = −0.09 p = 0.4, n = 87, Supplement 1). A moderate positive correlation was found between vitamin D and calcium status (rs = 0.34, p = 0.01, n = 52), though not for either PTH (rs = −0.20, p = 0.4, n = 23) or phosphate (rs = −0.05, p = 0.7, n = 57) status.
Vitamin D status among different clinical outcomes is displayed in Fig. 2. Patients with bed rest had a lower vitamin D status (p = 0.02). Vitamin D status was similar when comparing winter versus summer values for season of SCI onset (p = 0.3) and season of assessment (p = 0.3 with mean 41 (23) versus 47 (28) nmol/l). Patients admitted to the center within two weeks after SCI onset tended to have a higher vitamin D status compared to patients admitted to the center more than two weeks after SCI onset (n = 66, median 45 (26–62) nmol/l) versus n = 21, median 40 (26–41) nmol/l, p = 0.07). For the duration between SCI and the time point of vitamin D assessment, the difference was negligible (n = 43, median 41 (23–56) nmol/l versus n = 44, median 40 (27–59) nmol/l, p = 1.0).
Boxplots are displayed for vitamin D status stratified by A sex, B smoking status, C SCI etiology, D month of SCI onset, E AIS score, F) SCIM mobility score, G pressure injuries, and H bed rest. AIS American Spinal Injury Association Impairment Scale, SCI spinal cord injury, SCIM Spinal Cord Independence Measure.
When comparing the categorized vitamin D status between different patient groups, no differences (p > 0.06) were found (Table 3).
Discussion
At a median of 15 (9–22) days after SCI onset, we found a median vitamin D status of 41 (26–57) nmol/l. The majority (92%) of patients had a deficient or insufficient vitamin D status, independent of lesion as well as most demographic characteristics.
We found a higher prevalence of a deficient vitamin D status (67%) than reported in the general population (24–40%) [7]. Other studies also reported a high prevalence of vitamin D deficiency or insufficiency in patients admitted to acute SCI inpatient rehabilitation [5, 6, 16,17,18,19]. All of these studies were executed in the northern hemisphere, mostly in the United States, and studies from the southern hemisphere are not available. Similar to our study, patients in these previous studies were recruited throughout the year, accounting for the seasonal variability in vitamin D status [1]. Among patients measured within one week after SCI onset, mean vitamin D status was 51 (28) nmol/l, with 76% (65/85) having a deficient or insufficient status [6]. Within one month after SCI onset, a mean vitamin D status of 36 (18) nmol/l was found with 98% (44/45) patients having a deficient or insufficient status [5]. With a time since injury ranging from 15-92 days, a mean vitamin D status of 40 (17) nmol/l was found whereas 93% (27/29) of patients had an insufficient status [17]. In patients with a subacute SCI (2-6 months after onset), 71% (30/42) had a vitamin D status below 80 nmol/l [19]. Though exact details regarding the duration between SCI and measurement of vitamin D status are not provided, Ehsanian et al. [18] reported a deficient or insufficient status in 80% (227/282) of patients admitted to an acute SCI rehabilitation center. In 70% (14/20) of patients with an acute SCI admitted to a general rehabilitation center during summer, a deficient or insufficient status was reported [16]. These patients were hospitalized for approximately eight days before having their vitamin D status assessed, but the exact time since injury was not provided.
Similar to the previous studies, the cross-sectional and post-injury data available in our study makes it difficult to differentiate the exact cause of the high prevalence of vitamin insufficiency shortly after SCI onset. The reported prevalence of a deficient or insufficient vitamin D status in individuals with an acute SCI between studies ranges from 70−98% [5, 6, 16,17,18,19]. A first explanation for the high but also varying prevalence might be the acute disease itself. Altered intestinal motility, immune and endocrine system functioning are known to occur after SCI onset and could also lower vitamin D status [9]. Micronutrient concentrations, including several vitamins, are lowered as part of the systemic inflammatory response after acute and chronic tissue injury [25]. This systemic inflammatory response, measured by serum C-reactive protein, has also been reported after SCI onset and could (partially) explain a lowered vitamin D status in this population [17]. A high prevalence of vitamin D deficiency or insufficiency was also found among patients undergoing acute rehabilitation after traumatic brain injury (61%) [3] and stroke (77%) [4]. Similar prevalences were reported in another study shortly after the occurrence of brain injury (78%) or a stroke (85%) [16]. A prospective cohort study concluded that a lower vitamin D status did not lead to a higher stroke risk but is instead a consequence of stroke [26].
A second and third explanation might be found in the vitamin D status before disease onset as well as the hospitalization. Vitamin D status is influenced by the production in the skin through UVB radiation, nutritional intake of vitamin D-rich foods and vitamin D supplementation during the previous 2-3 months [17, 19]. Therefore, reduced sun exposure and or hospitalization duration before the vitamin D assessment could be relevant [9]. Although not significantly, patients with a longer duration between SCI and admission to the center in our study had a lower vitamin D status whereas the duration between SCI onset and assessment seemed less important. Another study compared the vitamin D status of patients with SCI onset duration within one month, between 1-3 months, or more than three months [5]. Though not significant (p > 0.05), both the mean vitamin D status (36 (18) nmol/l, 44 (19) nmol/l, 46 (23) nmol/l) as well as the number of patients with a sufficient status (1/45 (2%), 2/16 (13%), 30/39 (77%)), increased as the duration between SCI onset and measurement increased. No information on vitamin D supplementation was provided for this study. In patients with a subacute SCI (2–6 months after onset), significantly higher (p = 0.003) vitamin levels were found compared to patients with a chronic SCI ( >12 months after onset) [19]. Patients with subacute SCI did receive a daily vitamin D supplementation of 400 IU whereas only a “few” patients with a chronic SCI took regular supplementation, which could have possibly distorted the findings. Only two patients included in our study started receiving a (minimal) vitamin D supplementation dosage (200 IU/day) a few days before the vitamin D assessment, and therefore we did not further examine this parameter. Nevertheless, we speculate that prior hospitalization, the duration between SCI and admission to the center, and intake of vitamin D supplements prior to SCI onset, might be clinically relevant factors when deciding on vitamin D supplementation.
In contrast to other studies, we found a moderate negative correlation between vitamin D status and BMI (p = 0.003), which has also been found among individuals with a chronic SCI [8]. Two other studies among individuals with an acute SCI did not find a significant difference when comparing the BMI among those with an insufficient versus sufficient vitamin D status [16, 17]. The lower vitamin D status among individuals with a higher BMI could be caused by the storage of vitamin D in body fat tissue but also by a volume diluting effect [9]. Studies in the general population have shown obesity can decrease the bioavailability of vitamin D due to deposit in fat tissue [27]. After SCI onset, skeletal muscle reduces while body fat percentage increases leading to increased BMI [28, 29]. Due to the increased body fat, individuals with SCI likely need higher vitamin D supplementation dosages to correct an insufficient status.
We did not find significant differences in vitamin D status for sex, age, or smoking status. In contrast, age was associated with a low vitamin D status (p < 0.001), and vitamin D deficiency was proportionally higher among younger patients compared to older patients (p < 0.001) in patients admitted to a general acute rehabilitation center [16]. The mean duration between injury and admission was only nine days in this study, and the authors suggest a premorbid low vitamin D status as a probable cause for the high rate of deficiency that was found in the entire study population (76%). For SCI characteristics, including lesion level and completeness, we also did not find differences in vitamin D status. Previous studies among individuals with SCI have reported mixed results: while some reported a significantly lower vitamin D status among individuals with a tetraplegia [8], other studies found no significant differences. A significantly higher mean vitamin D status has been found in patients with an acute complete lesion compared to patients with an acute incomplete lesion (45 (20) versus 37 (18) nmol/l, p = 0.02) [5]. On the other hand, Waliullah et al. [6]. also didn’t find any significant differences in sex, age, SCI etiology, or AIS score in the acute SCI phase. They did, however, find significantly lower vitamin D values in serum samples taken during winter compared to summer (41 (21) nmol/l versus 59 (30) nmol/l, p = 0.003). Also among individuals with a subacute or chronic SCI, a significant main effect for season (p = 0.02) has been reported, with a higher vitamin D status during summer (55 (57.3) nmol/l) compared to winter (41 (42.7) nmol/l) [19]. Interestingly, in both of these studies [6, 19] the vitamin D status during the winter months was similar to our study (41 (23) nmol/l), but the status during the summer months was higher than we found in our study (47 (28) nmol/l). In our study, we assessed slightly more patients during winter (58%), probably since the recruitment took place during two summers and three winters. This might have lowered the overall vitamin D status, as we found no difference in vitamin status for either the season of SCI onset or the season of assessment.
Vitamin D status was not different between active or less active patients. We did find a lower vitamin D status in patients with bed rest compared to patients without bed rest (p = 0.02), though our findings should be interpreted carefully since bed rest only occurred in seven patients. Nevertheless, we speculate that the overall health status including comorbidities might be relevant in relation to vitamin D status since most of the patients with bed rest also had a pressure injury. The effect of bed rest could be compared with other forms of unloading the skeleton or weightlessness and is known to lead to increased bone resorption and decreased bone formation [30]. Furthermore, serum and urinary calcium levels increase, while intestinal calcium absorption, PTH, and vitamin D levels decrease [30]. Even though unloading is relevant in the development of osteoporosis, hormonal changes contribute too since bone loss is also present in the upper extremities after SCI [10]. We found no difference in calcium status for patients with versus without bed rest, but we did find a correlation between vitamin D and calcium status (p = 0.01). A negative calcium balance, as well as decreased vitamin D and PTH levels during the acute SCI phase, play a role in the pathogenesis of osteoporosis, which is a very frequent secondary complication in SCI [10]. Besides osteoporosis, an insufficient vitamin D status has also been associated with other common secondary complications among chronic SCI patients including reduced mobility, chronic pain, urinary tract infections, depressive symptoms, musculoskeletal health, chest illness, and pressure injuries [8,9,10, 12, 31,32,33,34]. This highlights the potential importance of a sufficient vitamin D status at the beginning of the rehabilitation phase. Rather unexpectedly, we did not find a correlation between vitamin D status and PTH or phosphate status. This might be caused by the relatively large variation in serum statuses among our study population, but also since PTH status was only available for 23 patients. PTH might also take some time before hormonal feedback leads to an increased PTH level, as studies among individuals with a chronic SCI found that those with a lower vitamin D status had a higher PTH status [8].
Unfortunately, no supplementation guidelines are available for the SCI population, but routine monitoring of vitamin D status and treatment of a suboptimal status are recommended [9]. Among acute traumatic SCI patients, combined progesterone (0.5 mg/kg body mass) and vitamin D (200 IU/kg body mass) supplementation twice a day for five days after admission, lead to higher AIS motor and sensory scores compared to placebo [35]. Unfortunately, vitamin D status was not assessed either before or after the intervention in this study. Daily supplementation with 1000 IU for one month proved unsuccessful in correcting the vitamin D status of acute SCI patients [17] and hence a higher dose seems warranted. It might be advisable to supplement all patients at the beginning of the rehabilitation phase of an acute SCI, with a dosage depending on the recently measured vitamin D status. Physical activity during early rehabilitation could improve fitness and BMI, which may also support the promotion of vitamin D from fat tissue [36].
Strengths and limitations
As vitamin D status is affected by vitamin D production through UVB radiation, seasonal fluctuations of vitamin D status are known [19]. By consecutively recruiting patients for more than two years, we were able to assess the association between vitamin D status and seasonality. Nevertheless, we did not assess sun exposure by either duration or amount of body surface exposed, which is a limitation of this study. Our study population was very homogenous regarding skin pigmentation, which limits the generalization of our results to individuals with other non-Caucasian skin pigmentations. Though the SCIM assessments were performed by clinical professionals as part of the daily routine and provide some insight into the patient’s mobility abilities and potential daily movement patterns, we acknowledge that these assessments provide no direct measurement of mobility or activity status. As fortification of food products with vitamin D is not common in Switzerland and patients follow a standardized diet during rehabilitation, food intake was not assessed in this study. However, patients might have ingested additional vitamin D through dietary intake, which could have slightly influenced our findings [37].
As data were collected from clinical routine assessments, there is missing data for certain outcomes leading to different sample sizes for some of the analyses. Though we did investigate the occurrence of pressure injuries, bed rest, and falls, our cross-sectional study design and relatively small study population prevented us to perform more in-depth analyses or to make causal statements about vitamin D status and secondary parameters. Other secondary complications in SCI that might be linked to vitamin D status, including reduced bone density and heterotopic ossifications, could not be investigated in this study as such assessments were not part of the clinical routine in our rehabilitation center. Interventional studies during acute SCI rehabilitation are needed to assess the optimal dose of vitamin D supplementation, as well as the effect on secondary complications while controlling for potential biases including sun exposure and activity status.
Conclusion and clinical implications
A high prevalence of vitamin D insufficiency was found in the early stages after SCI. We recommend that all patients admitted to the first rehabilitation after the onset of SCI should be supplemented with a dosage that is dependent on their actual vitamin D status. The dose and time interval needed to correct a deficient or insufficient vitamin D status warrants further investigation.
Data availability
The data that support the findings of this study are available upon reasonable request.
References
Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S.
Macdonald HM. Contributions of sunlight and diet to vitamin D status. Calcif tissue Int. 2013;92:163–76.
Dubiel R, Williams B, Sullivan E, Callender L, Bennett M, Driver S, et al. Prevalence of 25-hydroxyvitamin D deficiency in the acute rehabilitation population following traumatic brain injury. NeuroRehabilitation 2019;45:513–7.
Poole KE, Loveridge N, Barker PJ, Halsall DJ, Rose C, Reeve J, et al. Reduced vitamin D in acute stroke. Stroke. 2006;37:243–5.
Nemunaitis GA, Mejia M, Nagy JA, Johnson T, Chae J, Roach MJ, et al. A Descriptive Study on Vitamin D Levels in Individuals With Spinal Cord Injury in an Acute Inpatient Rehabilitation Setting. PMR. 2010;2:202–8.
Waliullah S, Kumar D, Kumar D, Tewari PG, Kumar V, Srivastava RN, et al. Prevalence of Vitamin D Deficiency in a Young Adult With Acute Spinal Cord Injury. Cureus 2021;13:e13791.
Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Köstenberger M, Tmava Berisha A, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498–513.
Flueck JL, Perret C. Vitamin D deficiency in individuals with a spinal cord injury: a literature review. Spinal Cord. 2017;55:428–34.
Lamarche J, Mailhot G. Vitamin D and spinal cord injury: should we care? Spinal Cord. 2016;54:1060–75.
Jiang SD, Jiang LS, Dai LY. Mechanisms of osteoporosis in spinal cord injury. Clin Endocrinol. 2006;65:555–65.
Berlowitz DJ, Wadsworth B, Ross J. Respiratory problems and management in people with spinal cord injury. Breathe (Sheff). 2016;12:328–40.
Zhou XJ, Vaziri ND, Segal JL, Winer RL, Eltorai I, Brunnemann SR, et al. Effects of chronic spinal cord injury and pressure ulcer on 25(OH)-vitamin D levels. J Am Paraplegia Soc. 1993;16:9–13.
Vieth R. Why the minimum desirable serum 25-hydroxyvitamin D level should be 75 nmol/L (30 ng/ml). Best Pract Res: Clin Endocrinol Metab. 2011;25:681–91.
Souberbielle JC, Body JJ, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ, et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun Rev. 2010;9:709–15.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The. J Clin Endocrinol Metab. 2011;96:1911–30.
Wu J, Chavez-Arom V, Han JJ, Yeh BY. High Rates of Vitamin D Deficiency in Acute Rehabilitation Patients. Arch Rehabil Res Clin Transl. 2021;3:100137.
Mailhot G, Lamarche J, Gagnon DH. Effectiveness of two vitamin D(3) repletion protocols on the vitamin D status of adults with a recent spinal cord injury undergoing inpatient rehabilitation: a prospective case series. Spinal Cord Ser Cases. 2018;4:96.
Ehsanian R, Timmerman MA, Wright JM, McKenna S, Dirlikov B, Crew J, et al. Venous Thromboembolism is Associated With Lack of Vitamin D Supplementation in Patients With Spinal Cord Injury and Low Vitamin D Levels. PM R. 2019;11:125–34.
Oleson CV, Patel PH, Wuermser LA. Influence of season, ethnicity, and chronicity on vitamin D deficiency in traumatic spinal cord injury. J Spinal Cord Med. 2010;33:202–13.
European Food Safety Authority. Scientific opinion on dietary reference values for vitamin D. In: FSA NDA Panel (EFSA Panel on Dietetic Products NaA, editor. EFSA Journal 2016.
National Pressure Ulcer Advisory Panel EPUAPaPP, Alliance. PI. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. In: Haesler E, editor. 2019 ed. Osborne Park, Australia: Cambridge Media; 2014.
Catz A, Itzkovich M, Tesio L, Biering-Sorensen F, Weeks C, Laramee MT, et al. A multicenter international study on the Spinal Cord Independence Measure, version III: Rasch psychometric validation. Spinal Cord. 2007;45:275–91.
Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34:535–46.
Cashman KD, Dowling KG, Skrabakova Z, Gonzalez-Gross M, Valtuena J, De Henauw S, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103:1033–44.
McMillan DC, Maguire D, Talwar D. Relationship between nutritional status and the systemic inflammatory response: micronutrients. Proc Nutr Soc. 2019;78:56–67.
Berghout BP, Fani L, Heshmatollah A, Koudstaal PJ, Ikram MA, Zillikens MC, et al. Vitamin D Status and Risk of Stroke: The Rotterdam Study. Stroke. 2019;50:2293–8.
Walsh JS, Bowles S, Evans AL. Vitamin D in obesity. Curr Opin Endocrinol Diabetes Obes. 2017;24:389–94.
Silveira SL, Ledoux TA, Robinson-Whelen S, Stough R, Nosek MA. Methods for classifying obesity in spinal cord injury: a review. Spinal Cord. 2017;55:812–7.
Laughton GE, Buchholz AC, Martin Ginis KA, Goy RE. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord. 2009;47:757–62.
Iwamoto J, Takeda T, Sato Y. Interventions to prevent bone loss in astronauts during space flight. Keio J Med. 2005;54:55–9.
Clark K, Goldstein RL, Hart JE, Teylan M, Lazzari AA, Gagnon DR, et al. Plasma vitamin D, past chest illness, and risk of future chest illness in chronic spinal cord injury (SCI): a longitudinal observational study. Spinal Cord. 2020;58:504–12.
Barbonetti A, Cavallo F, D’Andrea S, Muselli M, Felzani G, Francavilla S, et al. Lower vitamin D levels are associated with depression in people with chronic spinal cord injury. Arch Phys Med Rehabil. 2017;98:940–6.
Wu Z, Malihi Z, Stewart AW, Lawes CM, Scragg R. Effect of vitamin D supplementation on pain: A systematic review and meta-analysis. Pain Physician. 2016;19:415–27.
Deng QF, Chu H, Wen Z, Cao YS. Vitamin D and Urinary Tract Infection: A Systematic Review and Meta-Analysis. Ann Clin Lab Sci. 2019;49:134–42.
Aminmansour B, Asnaashari A, Rezvani M, Ghaffarpasand F, Amin Noorian SM, Saboori M, et al. Effects of progesterone and vitamin D on outcome of patients with acute traumatic spinal cord injury; a randomized, double-blind, placebo controlled study. J spinal cord Med. 2016;39:272–80.
Hengist A, Perkin O, Gonzalez JT, Betts JA, Hewison M, Manolopoulos KN, et al. Mobilising vitamin D from adipose tissue: The potential impact of exercise. Nutr Bull. 2019;44:25–35.
Beal C, Gorgey A, Moore P, Wong N, Adler RA, Gater D, et al. Higher dietary intake of vitamin D may influence total cholesterol and carbohydrate profile independent of body composition in men with Chronic Spinal Cord Injury. J spinal cord Med. 2018;41:459–70.
Acknowledgements
We kindly thank all patients who agreed to participate in this study. We thank Angela Frotzler and Yvonne Häberli for their valuable input during the conceptualization of the study.
Author information
Authors and Affiliations
Contributions
JF, AS, CP, AJ, and DL conceived the study. YW and AH performed the data collection. AS and AJ provided clinical expertise. DL and JK provided expertise in statistics. AH and JF prepared the first draft of the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the local ethics committee (BASEC-Number: 2017-01108).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hertig-Godeschalk, A., Scheel-Sailer, A., Wey, Y. et al. Prevalence of an insufficient vitamin D status at the onset of a spinal cord injury – a cross-sectional study. Spinal Cord 61, 211–217 (2023). https://doi.org/10.1038/s41393-022-00873-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-022-00873-z