Abstract
Study design
Cohort prospective study.
Objectives
Epidural spinal cord stimulation (eSCS) improves volitional motor and autonomic function after spinal cord injury (SCI). While eSCS has an established history of safety for chronic pain, it remains unclear if eSCS in the SCI population presents the same risk profile. We aimed to assess safety and autonomic monitoring data for the first 14 participants in the E-STAND trial.
Setting
Hennepin County Medical Center, Minneapolis and Minneapolis Veterans Affairs Medical Center, Minnesota, USA.
Methods
Monthly follow-up visits assessed surgical and medical device-related safety outcomes as well as stimulation usage. Beat-by-beat blood pressure (BP) and continuous electrocardiogram data were collected during head-up tilt-table testing with and without eSCS.
Results
All participants had a motor-complete SCI. Mean (SD) age and time since injury were 38 (10) and 7 (5) years, respectively. There were no surgical complications but one device malfunction 4 months post implantation. Stimulation was applied for up to 23 h/day, across a broad range of parameters: frequency (18–700 Hz), pulse width (100–600 µs), and amplitude (0.4–17 mA), with no adverse events reported. Tilt-table testing with eSCS demonstrated no significant increases in the incidence of elevated systolic BP or a greater frequency of arrhythmias.
Conclusions
eSCS to restore autonomic and volitional motor function following SCI has a similar safety profile as when used to treat chronic pain, despite the prevalence of significant comorbidities and the wide variety of stimulation parameters tested.
Similar content being viewed by others
Introduction
Spinal cord stimulation (SCS) has been used to treat chronic pain for the last 50 years. The use of SCS for modulating neuronal function has expanded beyond chronic pain to restoring function in patients with spinal cord injury (SCI). While stimulation for pain suppression is guided by induced paresthesia in the same area where pain is noted, the optimization for SCI patients requires electromyography and autonomic testing [1]. Preliminary studies have shown immediate and long-term benefits with training on motor function [2, 3], as well as the amelioration of autonomic deficits in cardiovascular (CV), bladder, bowel, and sexual function [1, 4, 5].
Safety has previously been demonstrated amongst individuals with chronic pain and spasticity [6]. Epidural SCS encompasses the risks of the surgical intervention itself, such as infection, hematoma formation, cerebrospinal fluid (CSF) leak, nerve root injury, and SCI, as well as the risks of hardware implantation, which include electrode migration, electrode/lead wire malfunction, early end of battery life, epidural electrode encapsulation, skin erosion or pain at implantable pulse generator (IPG) site [7]. The most common complications are electrode migration (1.5–27%) and infection (2.5–14%) [6,7,8]. Severe adverse events (AEs), such as spinal cord damage and large hematoma formation, are extremely rare [9].
The long-term safety profile in the SCI population, whereby a different SCS paradigm is used to restore supraspinal control of various body functions, such as volitional movement and autonomic functions, has not been assessed. Patients with SCI, specifically those with established chronicity, have unique comorbid conditions, such as muscle wasting, osteoporosis, neuropathic arthropathy, CV changes, a higher risk of infections, falls, and fractures [10, 11], all of which might contribute to a different spectrum of possible AEs. Moreover, individuals with injuries at or above the sixth thoracic level, even some as low as the tenth thoracic level, commonly experience episodes of autonomic dysreflexia (AD) [11]. This condition has been associated with notable cerebrovascular consequences such as hypertensive encephalopathy, seizure, and stroke as well as cardiac rhythm disturbances such as atrial fibrillation, bigeminy, premature atrial and ventricular contractions, and prominent T waves [12, 13]. Cardiovascular complications secondary to SCI dysregulation are amongst the leading causes of morbidity and mortality in these individuals [14].
Peripheral electrical stimuli have been shown to trigger AD in the SCI population [15]. Epidural SCS is applied below the level of injury and acts through dorsal afferents that may potentially elicit episodes of AD. To our knowledge, there has been only one report in the literature indicating that eSCS may induce AD [16]. However, the potential for eSCS to induce AD or arrhythmias has not been studied in larger cohorts. Heart rate (HR) and rhythm abnormalities are common among individuals with SCI due to the damage sustained to descending spinal sympathetic pathways and altered sympathovagal balance, including bradycardia, atrioventricular node block, and atrial and ventricular ectopics [17]. Episodes of AD can exacerbate arrhythmias due to variable sympathetic drive to the heart.
This study aims to assess the basic safety data, battery usage, and autonomic effects for the first 14 participants enrolled in the E-STAND clinical trial in order to determine the incidence of AEs due to changes in motor and autonomic functions.
Methods
Study design and research participants
This non-randomized, multi-institutional, interventional study was approved by the local Institutional Review Boards as well as the FDA for Investigational Device Exemption. The study protocol is registered with ClinicalTrials.gov (NCT03026816). Inclusion and exclusion criteria have been reported previously [1].
Surgical procedure
Same day surgery was performed under general anesthesia. A 16-contact epidural paddle lead (Tripole™, Abbott, Plano, TX) was implanted through a standard one-level laminectomy at approximately the twelfth thoracic vertebral level. On the day before surgery, patients were asked to shower with chlorhexidine wipes. In the operating room, surgical prep started with a chlorhexidine scrub dried with a sterile towel. Once completely dry, the area was saturated with alcohol and allowed to completely dry once again. Finally, a chlorhexidine/alcohol prep stick was used to prep the surgical field widely. Enough time was allowed for the solution to dry before placing surgical drapes. A single dose of intravenous cefazolin was given prior to the start of surgery. Prophylactic cephalexin was prescribed for 5 days after surgery.
Follow-up data and safety outcomes
Although safety was not a primary or secondary outcome in this study, AEs were monitored during every study visit. Postoperative complications, based on the Clavien-Dindo classification [18], were screened for during the first month after surgery and subsequent monthly follow-up study visits, as specified in the IRB-approved study protocol. Participants self-reported any medical events in the month prior and provided lab results, if applicable. Patients were allowed to use the eSCS outside of the scheduled daily exercises. Monthly usage logs from the implantable generator were extracted during visits. As this was implemented later on in the study, capture windows vary by participant enrollment time. Total energy delivered was calculated as the product of frequency, pulse width, amplitude, and time.
Autonomic function testing
With participants securely strapped in an automated tilt table (Hausmann Industries Model 6058 Northvale, NJ), continuous beat-to-beat blood pressure (BP) and heart function were assessed via finger photoplethysmography (Finometer PRO, Finapres Medicine Systems, Amsterdam, The Netherlands) from the right hand and electrocardiography (ECG) (ML 132; ADInstruments), respectively. Discrete brachial BP was recorded every minute from the left arm (BpTRU-BPM-100, Coquitlam; VSM Medical, Vancouver, BC, Canada). After 10 min of baseline recording while resting in the supine position, participants were passively moved to a ~70° head-up tilt (HUT). This position was maintained until the participant demonstrated orthostasis symptoms or signs. Participants were assigned to the autonomic arm of the study if they met orthostatic hypotension criteria during their initial assessment. Autonomic stimulation settings were chosen based on previous reports [19]. At follow-up autonomic visits, eSCS was applied until BP normalized and/or signs or symptoms of orthostatic intolerance were ameliorated. For analysis purposes, where systolic BP (SBP) remained >150 mmHg for ≥30 s this was deemed representative of an episode of AD.
Statistical analysis
Hemodynamic outcomes were analyzed with a mixed-effects generalized linear model (GLM) with correction for the nested random effects of individuals and number of trials, to assess significant differences between conditions: (1) supine baseline; (2) HUT without eSCS, and (3) HUT with eSCS. Seated brachial SBP values at the beginning and end of each follow-up laboratory visit were compared between participants in the autonomic and non-autonomic groups using an independent t-test. Chi-squared tests were used to assess the presence of arrhythmias during different stages of autonomic assessments (supine rest, HUT without eSCS and HUT with eSCS). Significance was set a priori at p < 0.05 for all statistical analyses. R Studio (R 3.0.1) was used for statistical modeling [20]. Further methodological details and information on data analysis can be found as supplementary material.
Results
Study population
Fourteen participants were enrolled starting in 2017, 11 males and 3 females, with a mean (SD) age of 38 (10) years. Mean (SD) time since injury at enrollment was 7 (5) years. All participants had SCIs that were classified as American Spinal Injury Association impairment scale (AIS) grade A or B (Table 1). None of the participants underwent formal rehabilitative exercise training during the study.
Adverse events
There were no postoperative complications. There was one medical device-related AE: an IPG early end of battery life after 4 months of stimulation. There were also two AEs unrelated to research interventions. Participant 2 reported a metatarsal fracture during the seventh month of enrollment after her wheelchair slipped in the snow. This event was not related to intervention-related increased mobility and eSCS was turned off at the time. Participant 5 developed an intergluteal cleft pressure ulcer during the eleventh month of enrollment, which resolved within 4 days. The pressure ulcer was distant to the IPG implant site. There were no falls or injuries related to SCS increased mobility.
Bladder function
There were seven reported urinary tract infections (UTI) confirmed with urine cultures. Participant 2 had 3 UTIs at postoperative month 5, 7, and 8. Participant 11 had 3 UTIs at postoperative month 1, 2, and 4. Participant 7 had 1 UTI at postoperative month 1. All UTIs resolved following treatment with oral antibiotics. The incidence of UTIs was 6%. These events were mild AEs that are unlikely to be related to research interventions. There were no reports of other bladder-related complications, such as changes in incontinence, urgency, urinary retention or inability to self-catheterize.
Cardiovascular safety
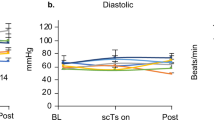
Over the course of 23 autonomic visits, >11 h of continuous CV monitoring with eSCS were collected from ten participants with autonomic dysregulation (total ~65 min per autonomic participant, range: 31–129 min). Frequencies tested ranged from 30 to 740 Hz, pulse widths ranged from 200 to 550 µs, and amplitudes up to 14 mA. All tested eSCS parameters were analyzed for CV safety, irrespective of whether they were configured to stabilize BP or delivered as a sham eSCS. Figure 1 shows a representative trace for a standard autonomic assessment.
Upon transitioning to a 70° HUT the participant steadily experienced a drop in BP and concomitant rise in HR (indicative of postural orthostatic tachycardia syndrome), accompanied by 5/10 lightheadedness and perceived loss of hearing. To mitigate these cardiovascular responses, the participant was returned to the supine position after 9 min of HUT. These findings were then replicated during a second HUT. However, rather than returning the participant to the supine position, the application of eSCS (frequency of 200 Hz, pulse width of 420 µs, up to 4 mA) immediately normalized cardiovascular outcomes. These responses were maintained for 24 min with sustained eSCS, without the presence of notable skeletal muscle tone (assessed anecdotally through investigator palpation). Despite the stimulator being turned off, there appeared to be a residual effect on BP, while HR steadily increased mimicking a similar response to that observed when the participant was tilted without eSCS (indicative of orthostatic intolerance). HR is again modulated upon the application of a second eSCS program (frequency of 640 Hz, pulse width of 500 µs, up to 4 mA). BP blood pressure, DBP diastolic blood pressure eSCS epidural spinal cord stimulation, HR heart rate, HUT head-up tilt, SBP systolic blood pressure.
One testing session for one participant was excluded from the CV safety analysis due an elevated average supine baseline SBP (155 mmHg) and AD symptoms (headache, goosebumps, sweating). Maximum continuous SBP readings (mean (SD)) during supine (132 (11) mmHg), HUT (mean (SD) 127 (16) mmHg), and HUT with eSCS conditions (mean (SD) 128 (14) mmHg) were comparable (Fig. 2A). The mean (SD) change in SBP between the end and start of each eSCS program at maximum intensity was 1 (8) mmHg (Fig. 2B). Percentage time with SBP > 150 mmHg for ≥ 30 s was not significantly different between supine, HUT, and HUT with eSCS conditions in the GLM (Fig. 2C). Two out of ten participants experienced elevations in SBP > 150 mmHg for ≥30 s during the application of eSCS. More information on the eSCS configurations used during these AD episodes and possible causes can be found as supplementary material. Seated SBP measured at the beginning and end of experimental visits was significantly lower (p < 0.0001) in the autonomic group compared to the non-autonomic group (Fig. 2D).
A Peak SBP data, taken as maximum 30 s rolling averages per testing condition (supine, HUT, and HUT with eSCS), and represented as mean with standard deviation. Each data point represents the maximum rolling average during the latter half of a given testing condition, with a variable number of HUT and eSCS conditions occurring per participant visit and per participant. B Difference between 30 s averages of SBP at the end and start of maximal tolerated eSCS intensity, represented as mean with standard deviation. Each data point represents one instance of testing an eSCS program. C Percentage time where SBP was >150 mmHg for ≥30 s during supine, HUT, and HUT with eSCS conditions, with each data point representing an individual participant visit. Data represented as median with interquartile range. Pooled means were 2.1%, 1.0%, and 0.5%, respectively. D Seated SBP measurements for participants in the autonomic and non-autonomic groups of the trial. The autonomic group had significantly lower SBP (p < 0.0001). 7.5% of the non-autonomic group SBP readings and 2.9% of the autonomic group readings were >150 mmHg.
Heart rate responses to HUT (represented as percent change from supine) were reduced (p = 0.01) with eSCS (Fig. 3A, B). Notable arrhythmias observed during the autonomic testing sessions are displayed in Fig. 3C–E. In summary, 5/10 participants demonstrated some form of arrhythmia during supine rest. During HUT without eSCS, 4 participants developed sinus tachycardia and 1 had premature ventricular contractions. In one case, sinus tachycardia was ameliorated by eSCS. Epidural SCS did not appear to affect the development of arrhythmia in other participants.
A Representative trace showing HR response to tilt and eSCS in one study participant (Participant 2). B Summary HR data showing percent change in HR from supine rest in response to HUT and HUT with eSCS. Each data point is an average of multiple tilt tests during each testing condition and represents an individual participant testing session. Data represented as means with standard deviations and analyzed by paired t-test. C Summary data demonstrating the number of participants that experienced abnormal heart rhythms before and during eSCS. P > 0.05 for all comparisons by Chi-squared test. D Representative arrhythmic event in one participant before stimulation. Red circles indicate regular sinus P-waves followed by a premature junctional complex (PJC) that occurs outside of normal sinus rhythm. E Representative unfiltered ECG trace demonstrating stimulation artifact. Red arrows point to low amplitude stimulation artifacts evident in the isoelectric line. Black arrows show the underlying T-wave which is covered by stimulation artifacts. Black stars demonstrate regular QRS complexes.
Stimulator use
Each study participant used chronic stimulation therapy, individualized to their preferences/needs by adjusting stimulation time and amplitude. As such, these two factors reflected inter-participant differences in daily stimulation energy delivered (Fig. 4). Across all participants, mean (SD) frequencies tested were 136 (175) Hz (range: 18–700 Hz). Mean (SD) pulse widths tested were 249 (130) µs (range: 100–600 µs). Mean (SD) amplitudes selected by the participants were 4.5 (2.6) mA (range: 0.4–17 mA). Participants used stimulation for a mean (SD) of 16.2 (7.7) hours/day (range: 0–23 h/day). There were no AEs related to these ranges of stimulation parameters. Due to participant 8’s use of higher energy settings for longer periods of time, settings were adjusted to preserve battery life. This intervention is reflected in this participant’s fourth follow-up visit in Fig. 4. Figure 5 illustrates the range of stimulation parameter exploration for one participant during 5 months of study enrollment. There is not sufficient data at this point in the study to identify patterns in stimulation use across participants.
Total daily stimulation energy delivered is obtained as a function of frequency, pulse width, amplitude, and time for each participant. Daily energy delivered is highly variable between participants as a result of preferences and goals. Daily energy delivered is highly variable within participants as their physiologies and daily activities may vary. For example, Participant 8 modified stimulation use in month 7 and 8 after receiving high battery expenditure warnings. The dashed “pain” line is based on the expected daily energy that would be delivered in a pain patient using the nominal settings established by the manufacturer. This is plotted as a reference for how markedly different eSCS therapy in this study is from regular eSCS therapy. A cumulative percentage of energy delivered is also plotted with the connected point lines to exemplify how each patient’s specific usage can burden the total capacity of the IPG. However, since this estimate is not entirely reflective of the dynamic ageing process of implantable batteries, a prediction of battery life expectancy cannot be made.
Above: Stimulation settings are plotted on the frequency/pulse width parameter space. The gray space below 60 Hz represents settings intended for volitional control whereas the white space above 60 Hz represents settings intended for autonomic functions. Frequency is set at a logarithmic scale due to a higher clustering of volitional settings on the low-frequency spectrum. Each circle represents a setting tested at home and concentric circles represent repeated uses of the same setting. Circle size is proportional to the electric charge delivered per second. Higher frequency and pulse width are fixed values that increase electric charge. Amplitude is a patient-controlled value that increases the charge and is therefore visualized by concentric circles that have different diameters. Circles are also color coded by time, see below. Below: In the span of 5 months, each setting change (setting switch count) is plotted by the current amplitude (mA) that it was used at and the energy that was delivered at each use. Energy delivered per use is dependent on fixed factors such as frequency and amplitudes and participant determined factors such as amplitude and time used. The color spectrum represents time (Dark blue: Month 1, Dark red: Month 6) and is used to code for the point in the study when each setting was tested in the plot above. Dotted lines mark the monthly follow-up visits. There is not sufficient data to identify trends across different patients in terms of stimulation use.
Discussion
Although eSCS in SCI patients has been used to treat pain and spasticity with acceptable safety results that are comparable to larger SCS cohorts [21], SCI patients with eSCS for restoration of function have unique underlying comorbidities and undergo a different stimulation paradigm. This must be taken into consideration when extrapolating safety data from the SCS pain literature. The safety results from the first 14 participants of the E-STAND trial help validate eSCS as a safe intervention in individuals with SCI. Recommendations based on our experience can be found in Table 2.
Surgical and medical device risks
Lead migration is the most common complication associated with SCS (more so with percutaneous leads) [8]. Therefore, stimulation therapy in our study was delayed for 1 month after implantation on all patients to ensure adequate scarring around the implant. Although there was no scheduled follow-up imaging, such as spine x-rays, to assess lead migration, there were no clinical indicators for such complication in any of the enrolled participants. The second most common complication from spinal cord stimulator placement is infection [8] and SCI patients are at a higher risk for wound infections well as have a higher incidence of being overweight or obese [11]. Two surgical infections and 3 wound dehiscences have been reported in a cohort of 11 patients receiving eSCS therapy for volitional movement [22]. As our study included 4 obese and 4 overweight participants, the risk of infection was addressed by standardizing preoperative care and surgical technique, as well as starting postoperative prophylactic antibiotic coverage for 5 days.
Risks of stimulation: rehabilitation/falls
Patients with SCI are at a higher risk for fractures during rehabilitative therapy due to loss of muscle mass and bone density from lack of weight-bearing activity. This translates to an incidence of fragility fractures of 30% [23]. There have been reported cases of hip and femur fractures when coupling electrical stimulation with rehabilitative therapy [2, 24]. Although patients in this trial did not undergo intensive rehabilitation as part of the study design, there were no AEs including fractures or falls with the use of eSCS during activities of daily living.
Risks of stimulation: bladder
The benefits of eSCS for treating neurogenic detrusor overactivity in SCI patients with adult neurogenic lower urinary tract dysfunction have been reported [25]. On the other hand, case reports have described worsening of lower urinary tract symptoms with eSCS associated with increased urethral sphincter tone and bladder wall compliance [26]. In our study, there were no patient-reported bladder function AEs that could be associated with research interventions. However, electrophysiological and urodynamic testing with chronic stimulation is warranted to adequately assess the urologic effects of stimulation over time.
Risk of stimulation: cardiovascular
Epidural SCS appears safe from a CV perspective, with few AEs observed across a wide range of stimulation parameters (both those optimized for CV control and the restoration of motor function). During autonomic testing, sustained (>30 s) increases in SBP > 150 mmHg were rarely observed during eSCS (representing 0.5% of pooled eSCS time for all participants), and were not increased in frequency during eSCS compared to HUT and supine conditions. It should be noted that the definition of AD is now constrained to uncontrolled elevations in BP, so as to differentiate it from well-controlled therapeutic interventions purposefully intended to increase BP [27]. Systolic BP > 150 mmHg was chosen as the safety threshold based on the clinical practice guidelines for when pharmacological management of AD is advised and has been previously used in the literature to indicate an episode of AD [28]. Systolic BP was also relatively stable when maximum tolerated eSCS amplitude was held constant, with mean (SD) change between end and beginning of maximum stimulation of 1 (8) mmHg. Approximately 75–85% of AD episodes are a result of bladder-related causes [29]. By nature of the study design, CV responses to eSCS were tested after HUT responses were characterized, with autonomic sessions lasting in some instances up to three hours. Nevertheless, despite possible AD triggers developing over time (i.e. bladder distension, neurogenic detrusor overactivity, irritation from straps, pain), we did not observe an increased frequency or severity of AD with eSCS. Importantly, participants with significant OH benefited from eSCS (Fig. 1) and those without substantial autonomic dysautonomia in response to a HUT were not harmed by the application of eSCS. Although eSCS-induced AD has previously been reported [16], our study supports the safe stabilization of BP, akin to what has been observed by other studies [1, 30]. Amongst both groups, autonomic and non-autonomic, sitting SBP means and SDs did not exceed 150 mmHg. There were no negative effects on CV measures as a result of interventions during study visits or from chronic eSCS over time.
Additionally, eSCS in SCI patients did not exacerbate the occurrence of arrhythmic events in any of the study’s participants. In one participant, eSCS prevented orthostatic tachycardia. Chronic mid-thoracic eSCS in canine non-SCI models of heart failure [31] and tachypacing induced atrial fibrillation [32] have shown beneficial antiarrhythmic effects due to improved autonomic regulation of cardiac electrophysiology. In conjunction with the other beneficial effects of eSCS in SCI patients, this may suggest that longer-term stimulation could be beneficial at offsetting the risk of arrhythmia development in SCI.
Stimulation usage/range: safety and costs
Trials assessing eSCS use in SCI patients have tested frequencies ranging from 0.5 to 130 Hz, pulse widths ranging from 180 to 800 µs, and amplitudes up to 10.5 V or 16 mA [3, 33]. Only one study addressed the amount of time patients used eSCS; stimulation time ranged from 40–120 min exclusively during study rehabilitation sessions [34]. In our study, a broader range of stimulation parameters was tested and patients adapted therapy to their daily activities. As a result, they utilized eSCS for a mean of 16.2 h/day.
In chronic pain management, eSCS has been deemed cost-effective. The expected duration of eSCS battery life is 6.5 years when used for 12 h a day (St. Jude Implantable Pulse Generator Proclaim™ Clinician manual), with a 3% incidence of battery end of life at 1 year post implantation attributed to higher stimulation requirements. It is unclear whether the device malfunction in our study was due to an early end of battery life. Nevertheless, the existing literature and our experience in this study support that participant needs are highly variable and may require higher energy expenditures. Patients utilize stimulation during the daily exercise routines to maximize motor function. However, improvement in functions such as sexual, bladder, and bowel control, which remain the highest valued in paraplegics [35], require daily and constant use of eSCS. When considering long-term management for patients with SCI who have spinal cord stimulators implanted, either the parameters set for best clinical response must be weighed by their concomitant energy expenditure to prolong battery life or there must be a shift to using IPGs with capacities for high-frequency settings.
Strengths and limitations
While eSCS has been used to treat pain after SCI for many years, this manuscript presents a safety analysis from the largest cohort of SCI participants undergoing eSCS for the purpose of restoring function published to date. Surgical complications are discussed, and a comprehensive analysis of the autonomic, movement, and stimulation-related safety outcomes is included. However, the results remain limited by the number of participants enrolled thus far who have completed a sufficient period of follow-up. Therefore, these findings are preliminary and add to the growing literature of eSCS for chronic SCI. Higher statistical power is needed to definitively conclude that the implantation of SCS in SCI patients has an equivalent safety profile to SCS used in chronic pain patients.
Conclusion
Epidural stimulators can be safely implanted in SCI patients and exploring large stimulation ranges does not increase the risk of motor, CV, and bladder-related AEs. The choice of IPG should be carefully considered to allow greater freedom in stimulation use. Further research assessing the efficacy of eSCS for improving motor and autonomic functions in SCI patients is warranted. The safety of this intervention needs to be established in order to support larger and multi-institutional studies.
Data availability
Data will be made available upon reasonable request.
References
Darrow D, Balser D, Netoff TI, Krassioukov A, Phillips A, Parr A, et al. Epidural spinal cord stimulation facilitates immediate restoration of dormant motor and autonomic supraspinal pathways after chronic neurologically complete spinal cord injury. J Neurotrauma. 2019;36:2325–36.
Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med. 2018;379:1244–50.
Wagner FB, Mignardot J-B, Le Goff-Mignardot CG. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563:65–71.
Phillips AA, Squair JW, Sayenko DG, Edgerton VR, Gerasimenko Y, Krassioukov AV, et al. An autonomic neuroprosthesis: noninvasive electrical spinal cord stimulation restores autonomic cardiovascular function in individuals with spinal cord injury. J Neurotrauma. 2018;35:446–51.
Nightingale TE, Walter M, Williams AMM, Lam T, Krassioukov AV. Ergogenic effects of an epidural neuroprosthesis in one individual with spinal cord injury. Neurology. 2019;92:338–40.
Brinzeu A, Cuny E, Fontaine D, Mertens P, Luyet P-P, Van den Abeele C, et al. Spinal cord stimulation for chronic refractory pain: Long-term effectiveness and safety data from a multicentre registry. Eur J Pain. 2019;23:1031–44.
Verrills P, Sinclair C, Barnard A. A review of spinal cord stimulation systems for chronic pain. J Pain Res. 2016;9:481–92.
Bendersky D, Yampolsky C. Is spinal cord stimulation safe? A review of its complications. World Neurosurg. 2014;82:1359–68.
Franzini A, Ferroli P, Marras C, Broggi G. Huge epidural hematoma after surgery for spinal cord stimulation. Acta Neurochir. 2005;147:565–7.
Dolbow DR, Gorgey AS, Daniels JA, Adler RA, Moore JR, Gater DR Jr., et al. The effects of spinal cord injury and exercise on bone mass: a literature review. NeuroRehabilitation. 2011;29:261–9.
Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, et al. Traumatic spinal cord injury. Nat Rev Dis Prim. 2017;3:17018.
Bjelakovic B, Dimitrijevic L, Lukic S, Golubovic E. Hypertensive encephalopathy as a late complication of autonomic dysreflexia in a 12-year-old boy with a previous spinal cord injury. Eur J Pediatr. 2014;173:1683–4.
Duvall JR, Mathew PG, Robertson CE. Headache attributed to autonomic dysreflexia: clinical presentation, pathophysiology, and treatment. Curr Pain Headache Rep. 2019;23:80.
Eldahan KC, Rabchevsky AG. Autonomic dysreflexia after spinal cord injury: Systemic pathophysiology and methods of management. Auton Neurosci. 2018;209:59–70.
Ashley EA, Laskin JJ, Olenik LM, Burnham R, Steadward RD, Cumming DC, et al. Evidence of autonomic dysreflexia during functional electrical stimulation in individuals with spinal cord injuries. Paraplegia. 1993;31:593–605.
DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a National Institutes of Health-sponsored clinical trial. Part I: methodology and effectiveness of expiratory muscle activation. Arch Phys Med Rehabil. 2009;90:717–25.
Ravensbergen HJC, Walsh ML, Krassioukov AV, Claydon VE. Electrocardiogram-based predictors for arrhythmia after spinal cord injury. Clin Auton Res. 2012;22:265–73.
Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Aslan SC, Legg Ditterline BE, Park MC, Angeli CA, Rejc E, Chen Y, et al. Epidural spinal cord stimulation of lumbosacral networks modulates arterial blood pressure in individuals with spinal cord injury-induced cardiovascular deficits. Front Physiol. 2018;9:565.
Team R, Others. RStudio: integrated development for R. RStudio, Inc, Boston, MA; 2015. http://www.rstudio.com.
Lagauche D, Facione J, Albert T, Fattal C. The chronic neuropathic pain of spinal cord injury: which efficiency of neuropathic stimulation? Ann Phys Rehabil Med. 2009;52:180–7.
Arnold FW, Bishop S, Johnson D, Scott L, Heishman C, Oppy L, et al. Root cause analysis of epidural spinal cord stimulator implant infections with resolution after implementation of an improved protocol for surgical placement. J Infect Prev. 2019;20:185–90.
Clark JM, Findlay DM. Musculoskeletal health in the context of spinal cord injury. Curr Osteoporos Rep. 2017;15:433–42.
Dolbow JD, Dolbow DR, Gorgey AS, Adler RA, Gater DR. The effects of aging and electrical stimulation exercise on bone after spinal cord injury. Aging Dis. 2013;4:141–53.
Walter M, Lee AHX, Kavanagh A, Phillips AA, Krassioukov AV. Epidural spinal cord stimulation acutely modulates lower urinary tract and bowel function following spinal cord injury: a case report. Front Physiol. 2018;9:1816.
Beck L, Veith D, Linde M, Gill M, Calvert J, Grahn P et al. Impact of long-term epidural electrical stimulation enabled task-specific training on secondary conditions of chronic paraplegia in two humans. J Spinal Cord Med. 2021;44:800–5.
Phillips AA, Krassioukov AV. Cardiovascular Dysfunction Following Spinal Cord Injury. In: Weidner N, Rupp R, Tansey KE, editors. Neurological Aspects of Spinal Cord Injury. Cham: Springer International Publishing; 2017, pp 325–361.
Krassioukov A, Warburton DE, Teasell R, Eng JJ, Spinal Cord Injury Rehabilitation Evidence Research Team. A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil. 2009;90:682–95.
Lindan R, Joiner E, Freehafer AA, Hazel C. Incidence and clinical features of autonomic dysreflexia in patients with spinal cord injury. Paraplegia. 1980;18:285–92.
Harkema SJ, Wang S, Angeli CA, Chen Y, Boakye M, Ugiliweneza B, et al. Normalization of blood pressure with spinal cord epidural stimulation after severe spinal cord injury. Front Hum Neurosci. 2018;12:83.
Issa ZF, Zhou X, Ujhelyi MR, Rosenberger J, Bhakta D, Groh WJ, et al. Thoracic spinal cord stimulation reduces the risk of ischemic ventricular arrhythmias in a postinfarction heart failure canine model. Circulation. 2005;111:3217–20.
Ardell JL, Cardinal R, Beaumont E, Vermeulen M, Smith FM, Andrew Armour J, et al. Chronic spinal cord stimulation modifies intrinsic cardiac synaptic efficacy in the suppression of atrial fibrillation. Auton Neurosci. 2014;186:38–44.
Calvert JS, Grahn PJ, Strommen JA. Electrophysiological guidance of epidural electrode array implantation over the human lumbosacral spinal cord to enable motor function after chronic paralysis. J Neurotrauma. 2019;36:1451–60.
Harkema S, Gerasimenko Y, Hodes J. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–47.
Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–83.
Acknowledgements
We would like to thank the Minnesota Office of Higher Education SCI/TBI Grant Program for the funding to carry out this study and St. Jude/Abbott for a generous device donation. We would like to acknowledge the E-STAND study group in both Minnesota and Canada for carrying out study procedures. The authors would like to thank the participants of this study and study coordinators who were crucial for the success of the present study.
Funding
This study is funded by a MN State SCI/TBI grant from the Minnesota Office of Higher Education. Devices are donated by Abbott/St. Jude. AVK holds the Endowed Chair in Rehabilitation Medicine, UBC. TEN (grant number: 17767) and MW (grant number: 17110) were recipients of Michael Smith Foundation for Health Research Trainee Awards in conjunction with the International Collaboration on Repair Discoveries and Rick Hansen Foundation, respectively.
Author information
Authors and Affiliations
Contributions
The study was conceived and designed by DPD, AP, and AVK. TIN, AP, US provided conceptual and technical guidance for all aspects of the study. IPP, TEN, CH, ZZ, JES, MW collected the data. IPP, TEN, ZZ, MC, TWD, MW, JES, and TIN performed data analysis and interpretation. IPP, TEN, MC, TWD, MW, TIN drafted the article. All authors provided critical revision of the manuscript and final approval of the version to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Approval for this study was provided by the institutional review boards of both Hennepin County Medical Center and the Minneapolis Veterans Affairs Medical Center. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pino, I.P., Nightingale, T.E., Hoover, C. et al. The safety of epidural spinal cord stimulation to restore function after spinal cord injury: post-surgical complications and incidence of cardiovascular events. Spinal Cord 60, 903–910 (2022). https://doi.org/10.1038/s41393-022-00822-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-022-00822-w
This article is cited by
-
Epidural stimulation restores muscle synergies by modulating neural drives in participants with sensorimotor complete spinal cord injuries
Journal of NeuroEngineering and Rehabilitation (2023)
-
Recent updates in autonomic research: advances in the understanding of autonomic dysfunction after spinal cord injury
Clinical Autonomic Research (2023)