Abstract
Study design
Secondary data analysis.
Objective
To characterize heart rate (HR) changes during autonomic dysreflexia (AD) in daily life for individuals with chronic spinal cord injury (SCI).
Setting
University-based laboratory/community-based outpatient.
Methods
Cardiovascular data, previously collected during a 24-h ambulatory surveillance period in individuals with chronic SCI, were assessed. Any systolic blood pressure (SBP) increase ≥20 mmHg from baseline was identified and categorized into confirmed AD (i.e., diarized trigger), unknown (i.e., no diary entry), or unlikely AD (i.e., potential exertion driven SBP increase) groups. SBP-associated HR changes were categorized as unchanged, increased or decreased compared to baseline.
Results
Forty-five individuals [8 females, median age and time since injury of 43 years (lower and upper quartiles 36–50) and 17 years (6–23), respectively], were included for analysis. Overall, 797 episodes of SBP increase above AD threshold were identified and classified as confirmed (n = 250, 31.4%), unknown (n = 472, 59.2%) or unlikely (n = 75, 9.4%). The median number of episodes per individual within the 24-h period was 13 (8–28). HR-decrease/increase ratio was 3:1 for confirmed and unknown, and 1.5:1 for unlikely episodes. HR changes resulting in brady-/tachycardia were 34.4%/2.8% for confirmed, 39.6%/3.4% unknown, and 26.7%/9.3% for unlikely episodes, respectively.
Conclusions
Our findings suggest that the majority of confirmed AD episodes are associated with a HR decrease. Using wearable-sensors-derived measures of physical activity in future studies could provide a more detailed characterization of HR changes during AD and improve AD identification.
Similar content being viewed by others
Introduction
There are currently 2.5 million individuals living with spinal cord injury (SCI) worldwide who face a plethora of challenges that can drastically affect their day-to-day quality of life [1]. Changes in certain physiological functions result in challenges such as overcoming mobility limitations and regaining control of autonomic functions such as bowel, bladder and cardiovascular regulation [2]. Cardiovascular dysfunction remains one of the leading causes of mortality and morbidity for individuals living with SCI [3,4,5]. Autonomic dysreflexia (AD) is a potentially life-threatening form of cardiovascular dysfunction following SCI, which can occur >40 times a day [6]. Typically, this condition occurs in individuals with SCI at the 6th thoracic spinal segment (T6) or above [6, 7]. AD is characterized by an increase in systolic blood pressure (SBP) of ≥20 mmHg from the individual’s baseline SBP and is elicited by noxious (e.g., soft tissue laceration or bone fracture) or innocuous (e.g., tight clothing, constipation, and full bladder) stimuli below the level of SCI [6, 7]. Consequently, it is not uncommon for individuals with SCI to experience episodes of AD during daily bowel and bladder-related care routines, with the latter being the leading cause of AD in most individuals with SCI [6, 8]. Moreover, individuals experiencing a refractory episode of AD are at a high risk for immediate life-threatening cardio- and cerebrovascular events (e.g., stroke, brain hemorrhage, and heart attack) due to sudden spikes in SBP that can sometimes rise above 200 mmHg [5]. Although changes in SBP during AD are well defined, studies in the literature show contradicting observations of heart rate (HR) changes during AD, with some studies demonstrating AD to be more commonly associated with bradycardia while others demonstrate tachycardia and HR increase to be more predominant during AD [7, 9, 10]. Several studies have also alluded to the possibility of the directionality of HR change during AD to be associated with the level of injury but this has not yet been formally investigated [7].
Moreover, previous studies were done exclusively on inpatient cohorts where data on the chronicity of injury, concurrent treatment modalities, qualitative reporting of patient symptoms or activities, burden of comorbidities, and method of HR measurements were limited or inconsistent [7, 9, 10]. Due to such methodological limitations, there were many confounding variables that could have contributed to misidentification of AD and subsequent interpretation of HR changes. Therefore, a reliable cardiovascular profile for individuals living with chronic SCI and experiencing AD in the community during habitual activities of daily living remains elusive.
The aim of our study was to investigate the changes in HR that occur during AD by analyzing quantitative cardiovascular parameters (e.g., SBP and HR) linked to qualitative parameters (e.g., symptoms experienced and activity/trigger diary entries) collected from individuals with SCI at or above the T6 level during a 24-h ambulatory surveillance period. This was to identify AD episodes and to determine if a HR increase or decrease was more predominant during AD, and to determine if the directionality of HR change may be associated with the level of injury. Our study will also be one of the first to use the methodology of combining 24-h cardiovascular surveillance data, and subjective data reported by study participants to optimize AD identification in order to investigate HR changes associated with AD experienced in an outpatient population during habitual activities of daily living.
We hope our results will contribute to building a more comprehensive cardiovascular profile for individuals suffering from AD that can guide the refinement of assessment tools and development of diagnostic tools for AD. This will ultimately help improve quality of life in this population by reducing the risks for adverse life-threatening events related to AD and complications experienced during routine bowel and bladder management protocols.
Methods
Study design and ethics
This is a secondary analysis of baseline data collected for screening purposes of two prospective clinical studies (identifier NCT02298660 and NCT02676154) and one pilot cross-sectional study, previously approved by the local ethics board. Details regarding the aims of the three studies are described in detail in Supplementary 1. The first clinical trial aimed to investigate the impact of intradetrusor onabotulinumtoxinA injections (200 U) on AD and the second clinical trial aimed to investigate the effects of fesoterodine on AD. The aim of the cross-sectional study was to investigate the impact of blood pressure instability on cerebrovascular health. In summary, all participants in the two clinical studies underwent a pre-treatment and post- or on-treatment 24-h ambulatory surveillance period. Only pre-treatment 24-h surveillance data and diary entries were included for analysis in our current study. As such, data used for this study’s analysis was collected from participants before they were exposed to any treatment studied in the two clinical trials.
Participants
This cohort represents a convenience sample (Fig. 1). The inclusion and exclusion criteria for all three studies were very similar (please see Supplementary 1 for details). The main inclusion criteria included: female or male individuals over the age of 18 years, or older with a chronic traumatic SCI (≥1-year post injury) at or above the T6 spinal segment with a documented history of AD. Furthermore, an individual had to have at least one recorded episode of SBP increase (from baseline) of 20 mmHg or more during a 24-h ambulatory surveillance period to be included in our analysis. The main exclusion criteria included: presence of acute comorbidities, pregnancy, previous history of systemic illness (such as cardiovascular diseases including hypertension and myocardial infarction, cerebrovascular accident, diabetes, obesity etc.), and confounders of cognition (such as documented traumatic brain injury and depression). Demographics, such as age, sex and time since injury were obtained from the respected case report forms.
Neurological level (NLI) and severity of injury were classified in accordance with the International Standards for Neurological Classification of Spinal Cord Injury by a physician [11].
Outcome objective
Characterization of HR changes during AD over a 24-h ambulatory surveillance period.
24-h ambulatory surveillance
All participants underwent a 24-h ambulatory surveillance period, i.e., blood pressure and HR monitoring (24-h ABPM) using the Meditech Card(X)plore device (Meditech Ltd., Budapest, Hungary) (Supplier), a reliable and well-validated tool for measuring cardiovascular profiles in individuals with SCI [12]. Both SBP and HR were programmed to be automatically recorded at 15-min intervals during daytime (7:00–23:00) and at 1-h intervals at night (23:01–6:59). Participants also had the option to manually record their SBP and HR at any time if they were experiencing potentially AD-related signs and symptoms or were to partake in an activity that could potentially cause changes in SBP (e.g., bladder and bowel routine, physical activity). Baseline SBP and HR values, which the 24-h ABPM values were compared to, were established as the average of three measurements taken from the participants’ nondominant arm while seated in their own wheelchairs. In addition, participants were asked to diarize any activities that could result in AD or non-AD-related changes in SBP as well as potential AD signs and symptoms experienced during the 24-h surveillance period.
Data extraction
From the 24-h ABPM records for each participant, we identified any recording with an SBP increase ≥20 mmHg from baseline, which is based on the clinical definition of AD as outlined by the International Standards for the Assessment of Autonomic Function after Spinal Cord Injury (ISAFSCI) [13]. We then used diarized information from the participant to categorize each episode into one of three groups: confirmed AD (known trigger was diarized, e.g., bowel routine), unknown AD (no trigger diarized), and potentially not AD (activity was diarized that could potentially cause a physiological increase in SBP, e.g., physical exercise).
Statistical analysis
Data are presented as raw values and percentages. Furthermore, data were assessed for normal distribution using the Kolmogorov–Smirnov test. Thus, non-parametric descriptive statistics were applied on continuous variables, such as SBP and HR (i.e., at baseline and maximum change (Δ) across all episodes and those with either confirmed AD trigger, unknown AD trigger or unlikely AD) as well as age and time since injury across all participants and those with either cervical or thoracic NLI. Non-parametric comparative statistics (i.e., Mann–Whitney U test) were conducted to assess for differences between those with cervical vs. thoracic NLI with respect to baseline SBP and HR, maximum change (Δ) in SBP and HR within each of the three categories of episodes (i.e., confirmed AD trigger, unknown AD trigger, and AD unlikely), as well as age and time since injury. Results are presented as median with lower and upper quartiles. In addition, range (i.e., min–max) is presented for age, time since injury, and number of eligible episodes [i.e., SBP increase above the AD threshold (≥20 mmHg)] per individual within the 24-h surveillance period. Statistical analyses were conducted using R Statistical Software Version 4.0.5 for Mac OS (Supplier).
Results
Participants
Data from 45 participants who completed diary entries, including eight females (17.8%), were included for analysis (Fig. 1). There were eight study participants who were excluded from analysis due to insufficient data secondary to failure to document any diary entries within the 24-h surveillance period or failure to record any measurements with SBP increase ≥20 mmHg from baseline. Median age and time since injury were 43 years (36–50 years, range 22–63 years) and 17 years (6–23 years, range 1–45 years), respectively.
The distribution of participants according to the injury severity and NLI was as follows: sensorimotor complete (AIS A = 23, 51%), sensory incomplete/motor complete (AIS B = 16, 36%), and sensorimotor incomplete (AIS C = 4, 9%; AIS D = 2, 4%); cervical (33/45, 73%) or thoracic SCI (12/45, 27%). Median baseline SBP across all participants was 105 mmHg (94–114). Median baseline SBP was significantly lower in the cervical (n = 33) compared to the upper-thoracic (n = 12) group (103 mmHg (92–108) vs. 114 mmHg (105–117), p = 0.027). Median baseline HR across all participants was 74 bpm (66–85). Median baseline HR was significantly lower in the cervical compared to the thoracic group (70 bpm (63–80) vs. 82 bpm (77–90), p = 0.025). Participant demographics and SCI characteristics are summarized in Table 1.
Characteristics of systolic blood pressure and heart rate during autonomic dysreflexia in daily life
Median max. SBP during AD across all participants was 172 mmHg (147–187). Median max. SBP during AD was not significantly different between cervical and thoracic groups (175 mmHg (151–187) vs. 159 mmHg (145–178), p = 0.397). A total of 797 episodes where SBP increased above the AD threshold (≥20 mmHg) were identified and further categorized as AD trigger known (n = 250, 31.4%), AD trigger unknown (n = 472, 59.2%), or AD unlikely (n = 75, 9.4%). The median number of eligible episodes per individual within the 24-h surveillance period was 13 (8–28, range 3–41) across all participants, and not significantly different (p = 0.8) between individuals with cervical (15, 8–28, range 3–41) and thoracic lesions (11, 5.75–19.5, range 4–40).
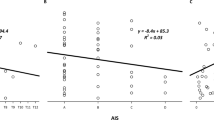
Median change in SBP from baseline within a 24-h surveillance period across all participants was 30 mmHg (24–40), while SBP-associated median HR change from baseline was −7 bpm (−18–1). Median changes in SBP from baseline and SBP-associated HR by category are illustrated in Figs. 2 and 3, and furthermore dichotomized by NLI in Table 2, respectively.
Median systolic blood pressure (SBP) change was greatest in episodes with autonomic dysreflexia (AD) trigger known (left, in black: 37 mmHg, 26–47) compared to AD trigger unknown (middle, in gray: 28 mmHg, 23–35) and AD unlikely (right, in white: 30 mmHg, 24–36). Dotted line represents AD cut-off, i.e., 20 mmHg SBP increase from baseline.
The HR decrease/increase ratio highlights how many episodes of SBP increase were associated with a HR decrease vs. a HR increase compared to the participant’s baseline HR in each of the three groups. Episodes with AD trigger known and AD trigger unknown both had a HR decrease/increase ratio of 3:1, i.e., known (n = 184, 74% vs. n = 59, 24%) and unknown (n = 346, 73% vs. n = 114, 24%,). However, AD unlikely episodes were more balanced between HR decrease and increase (n = 44, 59% vs. n = 29, 39%). Only about 3% of all episodes in each group were associated with unchanged HR from baseline, i.e., AD trigger known (n = 7), AD trigger unknown (n = 12), and AD unlikely (n = 2).
Rate of bradycardia and tachycardia at baseline and during episodes of elevated systolic blood pressure
Sixteen percent of participants (7/45) had baseline HR below the threshold for bradycardia (HR < 60 bpm) and 7% (3/45) above the threshold for tachycardia (HR > 100 bpm). Percentage of episodes associated with bradycardia vs. tachycardia was 34.4% vs. 2.8% (86/250 vs. 7/250, ratio 12:1) for AD trigger known group, 39.6% vs. 3.4% (187/472 vs. 16/472, ratio 12:1) for AD trigger unknown group, and 26% vs. 9.3% (20/75 vs. 7/75, ratio 3:1) for unlikely AD group.
Frequency of diarized triggers associated with autonomic dysreflexia
The majority of diarized triggers associated with confirmed episodes of AD (n = 250) were bladder-related (n = 183, 73%), with the remainder being bowel-related (n = 57, 23%) or related to other events (n = 10, 4%). Bladder-related items included “intermittent catheterization” (n = 148), i.e., “before” (n = 50), “during” (n = 68) or “after” (n = 30); “bladder distension” (n = 8), “bladder-related discomfort/spasm” (n = 25), or while “draining the leg urine bag” (n = 2). Bowel-related triggers comprised “during bowel routine” (n = 42), “bowel stimulation” (n = 12), i.e., “before” (n = 1) or “during” (n = 11), “insertion of suppository” (n = 2), or “impacted bowel” (n = 1). Other triggers were “unspecified spasm” (n = 5), “sexual intercourse” (n = 3), and “restricted clothing” (n = 2).
Frequency of diarized activities associated with unlikely autonomic dysreflexia episodes
For the episodes identified in the unlikely AD group (n = 75), the majority of diarized activities was “transfer to or from wheelchair” (n = 46, 61%). Furthermore, participants diarized “wheeling” (n = 17), “position change (lie down or sit up)” (n = 7) or “workout/stretching” (n = 2). In addition, “transferring laundry”, “getting dressed” and “anxiety” were each diarized once.
Frequency of diarized autonomic dysreflexia specific signs and symptoms and non-specific bodily symptoms
Of the 45 participants, only 12 diarized their bodily signs and symptoms, which are highlighted in Table 3.
Discussion
The main findings from our data analysis showed that most confirmed (74%) and unknown (73%) AD episodes were associated with a HR decrease while unlikely AD episodes were more balanced with respect to HR decreases (59%) and increases (39%). Moreover, the overall rate of bradycardia associated with identified episodes of elevated SBP was much higher than that of tachycardia regardless of whether an AD trigger was identified. Confirmed episodes of AD were most frequently associated with bladder-related events.
The high rate of bradycardia and HR decreases associated with confirmed AD cases in our analysis is in line with the previous understanding of the parasympathetically mediated HR changes that are believed to occur during AD, which suggest bradycardia as a pathognomonic feature of AD [14]. In cervical and upper-thoracic SCI, the sympathetic innervation of the heart (T1–T5) may be compromised, but parasympathetic innervation of the heart by the vagus nerve remains intact as it is located external to the spinal system and more or less functions independently from it [15]. Thus, bradycardia may be a compensatory response by the parasympathetic nervous system to counteract the significantly elevated SBP detected by baroreceptors during episodes of AD [3, 15]. Although we applied the clinical definition of bradycardia in our statistical analysis, it may not be applicable as it does not take into account the new baseline cardiovascular changes that are established in individuals with chronic SCI [16]. The level of neurological lesion associated with chronic SCI may have an impact on baseline resting HR as one meta-analysis depicted resting HR to be significantly lower in individuals with chronic SCI originating at the cervical level compared to those below T6 [16]. As our cohort consisted of mostly individuals with lesions of cervical origin (n = 33), this may appear to account for the high rates of bradycardia seen. Rather than applying classic clinical definitions or setting arbitrary thresholds to describe HR changes, alternative methods to describe cardiovascular parameters may more appropriately reflect the physiological baseline changes associated with chronic SCI. Analyzing the change in HR from baseline may be a more appropriate method as some of the participants in our study had baseline HR values in the bradycardic and tachycardic range.
Contrary to our results, a few studies have indicated tachycardia as a predominant feature of AD with bradycardia occurring much less frequently. Kewalramani’s combined retrospective and prospective review of AD episodes in patients with traumatic myelopathy showed bradycardia in 10% of participants and tachycardia in 38%, which was the opposite of our findings [10]. Solinsky et al.’s analysis of a prospectively acquired AD dataset showed a significantly higher prevalence of tachycardia during AD as 68% of AD episodes were associated with tachycardia while 0.3% were associated with bradycardia [9]. Lindan et al.’s study found bradycardia and tachycardia to be equally prevalent during AD [17]. Karlsson speculated that the presence of tachycardia in AD may be possible if the spinal reflex arc responsible for vasoconstriction below the level of lesion also involves the sympathetic innervation of the heart (T1–T5), there by resulting in a higher HR in those with an intact connection between the brain and sympathetic innervation of the heart compared to those with cervical lesions where that connections is disrupted [14]. In our analysis, mean HR change between those with upper-thoracic and cervical SCI were not significantly different (Table 2), which is not in line with this proposed theory.
Potential explanations for the relative tachycardia seen predominantly in other studies could include misidentification of AD due to lack of contextual information, confounding variables such as systemic comorbidities, medications affecting the cardiovascular system, and lack of true baseline measurements etc. As the core strength of our study lies within its methodology, these factors were accounted for through the design, as well as the exclusion and inclusion criteria for the clinical trials included in our analysis. Specifically, major exclusion criteria included previous history of systemic illness and any acute comorbidities including cardiovascular diseases. Therefore, both comorbidity burden and medication burden would have been unlikely confounding variables in our analysis.
While past studies have relied on SBP thresholds (e.g., 150 mmHg) that differ from the clinical definition of AD or AD symptomology to identify episodes of AD [9, 10, 17], our study used the ISAFSCI’s definition of AD for identification [13]. This means our methodology was designed to be more robust in the identification of low-grade AD and asymptomatic AD episodes, as well as reduce the likelihood of misidentifying elevated SBP readings due to essential hypertension as AD. Furthermore, our study utilized 24-h ABPM data in conjunction with diary entries to provide contextualized details, which optimized the validity of AD identification by capturing commonly experienced habitual AD triggers over a prolonged period during each participant’s everyday routine rather than at one point in time. This contrasts with the Kewalramani, Solinsky, and Lindan studies, which analyzed AD episodes in an inpatient setting where the context surrounding their admission and the treatments or procedures they underwent remained largely unknown [9, 10, 17]. This context is important as certain provocations such as laryngoscopy and tracheal intubation have been shown to elicit arrhythmias and tachycardia [18]. In addition, our study determined baseline vitals prior to the initiation of any treatment. Previous studies in the literature analyzed inpatient data and may have used baseline vitals for comparison that were determined after the initiation of treatments or not even used baseline values at all [9, 10, 17]. The analysis of participant diaries allowed for the identification of activities that were known triggers of AD and allowed us to account for confounding activities that mimicked the SBP elevation in AD but are considered false triggers. The most common triggers of confirmed AD episodes noted in participant diary entries were associated with bladder-related events, which is in line with literature reports [8, 17]. Examples of diarized confounding activities that are not considered triggers of AD but concurrently correlated with episodes of elevated SBP (thus categorized into the unlikely AD group) included anxiety and physical activity during exercise, wheeling, or transfers to/from wheelchair.
The misidentification of AD was discussed recently in two case studies where participants scheduled to undergo urodynamic studies had elevated SBP thought to be associated with AD [19]. Further review revealed that the elevation in SBP was likely due to anxiety as a SBP decrease was noted without initiation of AD therapeutic interventions or identification of AD triggers immediately after the participant in case study 1 was informed his case would be rescheduled [19]. Similarly, in case study 2, an SBP elevation was noted after the participant’s bladder was filled and was followed by SBP decrease after the participant was told the procedure would be aborted but before the bladder was drained hence the suspected trigger was still present [19]. Such confusion surrounding AD identification can result in a misunderstanding of its accompanying cardiovascular parameters. These events can be concurrently monitored through a self-report diary or captured by utilizing a combination of wearable devices to optimize the identification of AD episodes and cardiovascular parameters during free-living monitoring. For example, a 24-h ABPM device to capture BP, 24-h Holter electrocardiogram to capture HR and sensors/accelerometers can be used to capture physical activity to rule out a non-AD mediated increase in SBP. In the future, such approaches may help to optimize the provision of healthcare for individuals with SCI by increasing awareness regarding the importance of AD recognition, since a significant lack of knowledge in this domain has been shown in medical staff working at emergency departments [20]. Even more alarming, current literature indicates that up to 41% of individuals with chronic SCI have never heard of AD despite 22% reporting signs of unrecognized AD [21].
Study limitations
One limitation of our study was that the majority of episodes with elevated SBP ≥ 20 mmHg were recorded without associated triggers, which could, at least in part, be seen as asymptomatic AD, i.e., silent AD [22]. However, it could also partially be attributed to the variability in the quality of participant diary records, or the lack thereof. Only 16% of our participants reported experiencing AD specific symptoms and it is unknown whether those who did not record AD symptoms truly experienced no symptoms at all or failed to record them for other reasons (e.g., perceived burden of providing a detailed recall of activities over a 24-h surveillance period). This happened despite participants undergoing an in-depth debriefing with a research coordinator the day after the 24-h surveillance period to clarify diary records and activities. Although silent AD is relatively common in the literature, occurring in more than 50% of individuals who experience AD, educational sessions about AD sign and symptom recognition for participants prior to the 24-h surveillance period could improve the quality of diary reports [22, 23].
Furthermore, although participants were asked to diarize purposeful physical activity, we did not quantitatively measure intensity of physical activity throughout the day and therefore cannot definitively rule out non-AD mediated increases in SBP.
Future studies may want to consider the use of wearable sensors/devices to objectively monitor the frequency and intensity of physical activity [24]. Expanding the 24-h ABPM and diary data collection period to multiple days rather than just a single 24-h surveillance period would allow for a more robust analysis of cardiovascular parameters during AD in daily life and provide more insight on silent AD. However, participant compliance could decline with longer surveillance periods as frequent BP measurements could interfere with daily activities such as wheeling or transferring and could result in sleep disturbances in some participants [12]. Another limitation was that we conducted a secondary data analysis using a convenience sample, i.e., no a priori power analysis to calculate the statistical power. Nevertheless, the size our cohort was sufficient to answer our outcome objective. A further limitation was the unbalanced ratio of cervical to thoracic level lesions (3:1), as well as between motor-complete to motor-incomplete injury (>6:1) in our cohort. Considering this, future studies should aim to achieve a balanced ratio for the level and severity of injury across participants.
Conclusion
Our results suggest that most confirmed AD episodes were associated with a HR decrease. However, the number of unknown episodes, which could potentially be silent AD, was high. Nonetheless, the use of 24-h ABPM data in conjunction with diary records of daily activities and symptoms provides a promising methodology for the valid identification and differentiation of confirmed AD episodes from unlikely AD episodes, which is essential for developing a more accurate cardiovascular profile for individuals with chronic SCI experiencing AD.
Developing a more accurate and comprehensive cardiovascular profile for this population can ultimately help improve quality of life by reducing the risks for adverse life-threatening cardiovascular events and complications related to AD experienced during routine daily activities, such as the initiation of bowel and bladder management protocols. Further improvements, such as more precise documentation in participant diaries and the combined use of a 24-h Holter electrocardiogram and wearable-sensors-derived measures of physical activity could increase the validity of HR changes during an episode of AD.
Supplier
a. Meditech Card(X)plore device (Meditech Ltd., Budapest, Hungary)
b. R Statistical Software Version 4.0.5 for Mac OS
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding authors on request.
References
Inskip JA, Lucci VEM, McGrath MS, Willms R, Claydon VE. A community perspective on bowel management and quality of life after spinal cord injury: the influence of autonomic dysreflexia. J Neurotrauma. 2018;35:1091–105.
Krassioukov AV. Autonomic dysreflexia: current evidence related to unstable arterial blood pressure control among athletes with spinal cord injury. Clin J Sport Med. 2012;22:39–45.
Grigorean VT, Sandu AM, Popescu M, Iacobini MA, Stoian R, Neascu C, et al. Cardiac dysfunctions following spinal cord injury. J Med Life. 2009;2:133–45.
Phillips AA, Krassioukov AV. Contemporary cardiovascular concerns after spinal cord injury: mechanisms, maladaptations, and management. J Neurotrauma. 2015;32:1927–42.
Wan D, Krassioukov AV. Life-threatening outcomes associated with autonomic dysreflexia: a clinical review. J Spinal Cord Med. 2014;37:2–10.
Hubli M, Gee CM, Krassioukov AV. Refined assessment of blood pressure instability after spinal cord injury. Am J Hypertens. 2015;28:173–81.
Eldahan KC, Rabchevsky AG. Autonomic dysreflexia after spinal cord injury: systemic pathophysiology and methods of management. Auton Neurosci. 2019;209:59–70.
Walter M, Kran SL, Ramirez AL, Rapoport D, Nigro MK, Stothers L, et al. Intradetrusor onabotulinumtoxinA injections ameliorate autonomic dysreflexia while improving lower urinary tract function and urinary incontinence-related quality of life in individuals with cervical and upper thoracic spinal cord injury. J Neurotrauma 2020;37:2023–7.
Solinsky R, Kirshblum SC, Burns SP. Exploring detailed characteristics of autonomic dysreflexia. J Spinal Cord Med. 2018;41:549–55.
Kewalramani LS. Autonomic dysreflexia in traumatic myelopathy. Am J Phys Med. 1980;59:1–21.
Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34:535–46.
Hubli M, Krassioukov AV. Ambulatory blood pressure monitoring in spinal cord injury: clinical practicability. J Neurotrauma. 2013;31:789–97.
Krassioukov AV, Biering-Sorensen F, Donovan W, Kennelly M, Kirshblum S, Krogh K, et al. International standards to document remaining autonomic function after spinal cord injury (ISAFSCI), first edition 2012. Top Spinal Cord Inj Rehabil. 2012;18:282–96.
Karlsson AK. Autonomic dysreflexia. Spinal Cord. 1999;37:383–91.
Biering-Sørensen F, Biering-Sørensen T, Liu N, Malmqvist L, Wecht JM, Krassioukov AV. Alterations in cardiac autonomic control in spinal cord injury. Auton Neurosci Basic Clin. 2018;209:4–18.
West CR, Mills P, Krassioukov AV. Influence of the neurological level of spinal cord injury on cardiovascular outcomes in humans: a meta-analysis. Spinal Cord. 2012;50:484–92.
Lindan R, Joiner E, Freehafer AA, Hazel C. Incidence and clinical features of autonomic dysreflexia in patients with spinal cord injury. Paraplegia. 1980;18:285–92.
Hector SM, Biering-Srøensen T, Krassioukov AV, Biering-Srøensen F. Cardiac arrhythmias associated with spinal cord injury. J Spinal Cord Med. 2013;36:591–9.
Solinsky R, Linsenmeyer TA. Anxiety masquerading as autonomic dysreflexia. J Spinal Cord Med. 2019;42:639–42.
Jackson CR, Acland R. Knowledge of autonomic dysreflexia in the emergency department. Emerg Med J. 2011;28:866–9.
Mcgillivray CF, Hitzig SL, Craven BC, Tonack MI, Krassioukov AV. Evaluating knowledge of autonomic dysreflexia among individuals with spinal cord injury and their families. J Spinal Cord Med. 2009;32:54–62.
Walter M, Knüpfer SC, Cragg JJ, Leitner L, Schneider MP, Mehnert U, et al. Prediction of autonomic dysreflexia during urodynamics: a prospective cohort study. BMC Med. 2018;16:1–11.
Huang YH, Bih LI, Liao JM, Chen SL, Chou LW, Lin PH. Blood pressure and age associated with silent autonomic dysreflexia during urodynamic examinations in patients with spinal cord injury. Spinal Cord. 2013;51:401–5.
Nightingale TE, Rouse PC, Thompson D, Bilzon JLJ. Measurement of physical activity and energy expenditure in wheelchair users: methods, considerations and future directions. Sport Med Open. 2017;3:10.
Funding
BY is a University of British Columbia—Faculty of Medicine Summer Student Research Program (FoM SSRP) award recipient. TEN (grant number 17767) and MW (grant number 17110) were recipients of Michael Smith Foundation for Health Research Trainee Awards in conjunction with the International Collaboration on Repair Discoveries and Rick Hansen Foundation, respectively. AVK is supported by Endowed Chair, Department of Medicine, Univerty of British Columbia. Funding was provided for NCT02298660 (by Praxis Spinal Cord Institute, i.e., formerly Rick Hansen Institute, grant number G2013-09 and Allergan Inc.), NCT02676154 (by Pfizer Canada investigator-initiated research, grant number WI207218), and cross-sectional study (by Praxis Spinal Cord Institute, grant number G2015-31).
Author information
Authors and Affiliations
Contributions
BY: study conception and design, acquisition of data, statistical analysis and interpretation of data, drafting of the manuscript and critical revisions. TEN and ALR: study conception and design, acquisition of data and interpretation of data, and critical revisions. MW: study conception and design, acquisition of data, statistical analysis and interpretation of data, critical revisions of the manuscript, and supervision. AVK: study conception and design, acquisition of data and interpretation of data, critical revisions of the manuscript, and supervision. All authors approve the final version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yee, B., Nightingale, T.E., Ramirez, A.L. et al. Heart rate changes associated with autonomic dysreflexia in daily life of individuals with chronic spinal cord injury. Spinal Cord 60, 1030–1036 (2022). https://doi.org/10.1038/s41393-022-00820-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-022-00820-y