Abstract
Study design
Narrative review.
Objectives
The objective was to summarize the literature on nanoplatforms in spinal cord injury (SCI) and describe their effect in facilitating experiments for SCI. Currently, the primary clinical treatment for neuropathic pain (NP) is drug therapy, but these traditional drugs have many disadvantages, such as high dose, rapid clearance from the circulatory system, off-target side effects, and cytotoxicity. Moreover, the treatment for NP is complicated by the existence of blood–brain barrier. In recent years, nanomedicine has been receiving increased attention; this novel modality could help deliver drugs to treat NP via nanoplatforms, making it a promising alternative therapy. The use of nanoplatforms can enhance pharmaceutic effectiveness by either avoiding rapid clearance from the blood or ensuring adequate concentration in the lesion.
Methods
A literature review was conducted, with a focus on nanoplatforms that have been described in the experimental studies of neuropathic pain.
Results
We provide a brief description of the roles of liposomes, polymeric nanoparticles, metal nanoparticles, micelles, and dendrimers in the treatment of NP and discuss the prospective development of the nanoplatform system for NP.
Conclusion
The emergence of various nanoplatform drug delivery systems can provide an advantageous resource tool for real-time diagnosis and effective treatment of SCI-related NP.
Similar content being viewed by others
Introduction
Neuropathic pain (NP), results from a lesion or disease affecting the somatosensory component of the nervous system, with a prevalence rate as high as ~7–8% in the population, and is characterized by either positive (increased somatosensory function) or negative (decreased somatosensory function) sensory signs and symptoms, including burning sensation, induced pain, and abnormal temporal summation [1]. In addition, ~80% of patients with spinal cord injury (SCI) have NP, of which 40% was reported as intense NP, which is a common chronic pain condition that greatly reduces the overall quality of life [2]. SCI occurs usually because of local spine deformation caused by mechanical damage and direct compression and injury of nerve elements and blood vessels from fracture and displaced bone fragments or disc material. SCI includes at-and below-level SCI-based NP, while at-level pain may consist of both peripheral and central NP, below-level pain is a central NP condition [3]. SCI can incur hyperactivity of neurons and glial cell activation, increasing intracellular and extracellular glutamic acid neuropeptide adenosine triphosphate proinflammatory cytokines and concentration of reactive oxygen species (ROS). These factors lead to maladjustment in the injured spinal cord and damage synaptic circuits, thus increasing spinal dorsal horn pain, and permanently causing NP Notwithstanding various therapeutic regimens that have been developed for NP, the treatment outcome of patients with SCI-based NP remains poor, and such patients continue to be affected by sleep disturbances, anxiety, and depression caused by pain.
Current traditional pharmacological treatments for NP are anticonvulsants such as lamotrigine, pregabalin, gabapentin, tricyclic antidepressants, selective serotonin–norepinephrine reuptake inhibitors, lidocaine, capsaicin, tramadol, strong opioids (morphine and oxycodone), and botulinum toxin A [4]. These conventional analgesics have limited therapeutic efficacy and some of these analgesics, such as anticonvulsants, tricyclic antidepressants, and strong opioids could produce typical central side effects such as drowsiness and dizziness, resulting in potential fall risk. The use of lidocaine and capsaicin 8% patch has only short-term analgesic effects in patients with peripheral local NP, and owing to the distinctive features of the neurovascular system, most non-invasive routes of drug delivery cannot access the central nervous system (CNS) [5]. Furthermore, most of these medicines show poor solubility in water. Thus, it is necessary to explore novel effective alternative therapeutic options to overcome these problems and develop anti-NP targeted therapies [6].
With the advancement of nanotechnology and biochemistry, it is possible to establish a nanoplatform drug delivery system for NP treatment. Research has shown that drugs can be lysed, implanted, encapsulated, or attached to nanoparticles, serving as a nanoplatform drug delivery system [7]. Nanoparticles, which comprise natural or artificial polymers with dimensions ranging from 1 to 1000 nm, can target central inflammatory sites [8]. Furthermore, modification of nanoparticles by active targeted molecules also contributes to effective drug delivery, transport through the blood–brain barrier, elimination of undesired off-target effects, and improvement in therapeutic efficacy.
The recent development and advancement in nanoplatforms for drug delivery in NP treatment is introduced and described in Table 1. Diverse types of nanocarriers, including poly lactic-co-glycolic acid (PLGA) nanoparticles, liposome nanoparticles, metal nanoparticles, nanomicelles, nanocapsules, and dendrimers, are being studied and developed for the treatment of NP.
Therefore, in this review, we provide a brief description of the roles of liposomes, polymeric nanoparticles, metal nanoparticles, micelles, and dendrimers in the treatment of NP and discuss the prospective development of the nanoplatform system for NP, especially SCI-based NP in the future.
Research advances in nanoplatforms for the treatment of NP
Polymer nanoparticles—poly (lactic-co-glycolic acid) (PLGA)
PLGA has attracted considerable attention because of its biodegradability and biocompatibility, controlled release of the drug, protection of the drug or gene from decomposition, and ability to modify the surface for diagnosis and treatment of diseases [9].
PLGA is the most widely used drug delivery system and is effective in infiltrating tissues owing to its nano size [10]. Therefore, PLGA has been a promising nanoplatform for the treatment of NP, owing to its advantages such as good biocompatibility, safety and controllable degradation rate, continuous intracellular drug release, low cost, and ease of production.
The long-term use of oxaliplatin can inhibit the expression of Thioredoxin Interacting Protein (TXNIP) in sciatic nerve cells, which regulate autophagy, resulting in NP [11]. TXNIP induces NP in microglia by producing an inflammatory response through the inflammatory body axis. Liu et al. manufactured a PLGA-metformin nanoemulsion using double emulsion solvent diffusion method, which is used as a carrier and metformin as a cargo to target TXNIP [12]. Additionally, the nanoparticle can induce significant upregulation of TXNIP and autophagy-related indicators, increase the threshold of thermal sensitivity and mechanical prickling, inhibit neuropathic sensitivity, and alleviate NP.
In recent years, gene therapy based on siRNA silencing has attracted attention owing to its therapeutic potential in multiple diseases, which can degrade mRNA molecules by forming RNA-induced silencing complexes to interfere with the expression of specific protein-coding genes [13]. However, these molecules cannot reach their CNS targets as they are unable to pass the blood–brain barrier and, as a result, can be completely eliminated. Thus, Shin et al. designed P38 siRNA-loaded PLGA nanoparticles using the typical double emulsion method (W/O/W) so that hydrophilic P38 siRNA could be effectively embedded in PLGA nanoparticles [14]. After the application of p38 siRNA-PLGA nanoplatforms in a solid lipid nanoparticles (SLN) mouse model, the expression of p38-related inflammatory genes such as TNF-a was modulated, resulting in the loss of p38 phosphorylation in microglia of the spinal dorsal horn, thereby inhibiting microglia activation and alleviating NP [15]. The siRNA-PLGA nanoplatform could cross the blood–brain barrier and enter the CNS, concentrate p38 siRNA in spinal cord microglia, and target microglia-specific genes, which play crucial roles in NP.
Another study showed that CX3CL1 and its receptor CX3CR1 can also play important roles as promising novel targets in the treatment of NP. Similarly, a drug delivery system that can effectively deliver CX3CR1 siRNA to spinal microglia cells is needed [16]. Noh et al. synthesized PLGA-coated CX3CR1 siRNA nanoparticles with the conventional double emulsion method (W/O/W) using ultrasound and injected them intrathecally into mice (Fig. 1). In the spinal dorsal horn, the microglial activation in CX3CR1 siRNA-PLGA nanoplatform-treated mice was significantly reduced, the proinflammatory mediators were significantly downregulated, and mechanical tenderness was significantly alleviated. These studies suggest that PLGA-encapsulated CX3CR1 siRNA nanoparticles can effectively alleviate SNL-induced NP in mouse models through reduction in the microglial activity and the expression of proinflammatory mediators [17].
A Rat model of neuropathic pain was induced by spinal nerve ligation at the L5 vertebra. B PLGA-encapsulated CX3Cr1 siRNA nanoparticles increased the mechanical pain threshold in rats, SNL means spinal nerve ligation; Ipsi means ipsilateral. Data are presented as the mean ± SEM (t-test, one-way ANOVA, **p < 0.01 versus VS siRNA NPs). Reproduced from Noh et al. [17] with permission from International Journal of Molecular Sciences.
PLGA nanoparticles have shown unique advantages in enhancing the targeting of various drugs to the CNS, especially microglia cells. It can be embedded by highly specific microglial markers and a variety of therapeutic agents, facilitating absolute targeted therapy without altering other critical processes. The limitations of PLGA nanoparticles are that its encapsulation efficiency and siRNA delivery capability are lower than those of recombinant viruses. Hering et al. used fish embryo toxicity test to test the toxicity of polymer nanomaterials, and found that PLGA still had some toxicity, although it was very low [18]. Moreover, Lagreca et al. found that many parameters affect the drug loading of PLGA; of these the PLGA terminal cap had a significant effect on the drug loading rate, the ester-capped had a higher encapsulation, while the acid end group had a lower drug packaging efficiency. Therefore, the requirements for manufacture are strict [19]. Nevertheless, PLGA can be a promising effective drug delivery nanoplatform in precise treatment of NP.
Lipid-based nanoparticles
Lipid-based NPs can be categorized as SLN, NLCS, and liposomes [20]. SLN is a colloidal nanoparticle delivery system consisting of a biodegradable solid lipid core and stabilizer. It is a relatively new class of submicron-sized (50–1000 nm) nanoparticles acting as a safe, nontoxic, biocompatible, and temperature-sensitive lipid nanocarrier. Additionally, SLN is physically stable, can protect drug stability in vivo, slowly releases the drug, and has great biological endurance. Lastly, it can serve as an alternative drug carrier with various advantages such as increased bioavailability, drug delivery capacity of lipophilic and hydrophilic drugs, low cost, and high feasibility for mass production [21].
In a preclinical study, Sharma et al. designed an SLN system with a small particle size of 50–80 nm which could target the crucial gene trpv1 in NP [22]. The TRPV1-targeting siRNA (TRPsiRNA) SLNs nanoplatform demonstrates protection against physiological and nuclease-mediated degradation of encapsulated biomolecules, controlling and prolonging the release of their cargo and effectively transfecting cells. TRPsiRNA-SLNs was found to significantly reduce acute heat pain, capsaicin-induced pain, and injurious behavior, confirming the effective downregulation of TRPV1 [23]. The trpsiRNA-SLN system can maintain the biological activity and high transfection rate of trpsiRNA, ensuring the release of the coated trpsiRNA in a biologically active state to trigger a highly effective physiological effect, effectively silencing TRPV1 and significantly reducing the intensity and duration of capsaicin-induced harmful behavior. Sharma et al. also found that TrpsiRNA-SLNs have better therapeutic effect than trpsiRNA alone, even though trpsiRNA has ten times the amount of siRNA used in TrpsiRNA-SLNs nanoplatform [22].
Like most other nanoplatforms, SLNs also possess some disadvantages. High-pressure homogenization is a common method to prepare SLNs, but the high temperatures achieved during the process accelerate the release rate of drug and lipid degradation; in addition, coexistence of colloidal structures such as gels, drug sediment, particle size instability, and kinetic phenomenon are all shortcomings of SLNs [24]. A new generation of NLC NPs lipids can be prepared by mixing solid lipids with liquid lipids of various shapes. The adjunction of liquid and solid lipids destroys the conventional lattice structure, increases the proportional structure of nanoparticle irregular crystal forms (which are essential for enhancing the spatial capability of nanoparticles), and improves the drug delivery ability; liquid lipids are enclosed within the ambient solid lipid barrier. This particular structure allows NLCs to maintain a rigid framework at room temperature, thus controlling drug release in vivo [25].
Carbamazepine (CMZ) is another effective drug for treatment of NP in murine models, while poor bioavailability, continuous dose adjustment, and toxic effects of long-term use are major barriers to CMZ use [26]. To eliminate these obstacles, Elmowafy et al. successfully manufactured an NLC based on stearic acid and beeswax using a high shear homogenization/ultrasound technique. They integrated it with CMZ, which has a nanometer size range; this resulted in controlled-release ability and excellent physical stability [27]. CMZ-NLC improved the oral bioavailability and analgesic properties of CMZ, while reducing toxic metabolites and alleviating the adverse effects of CMZ.
Liposomes are spherical vesicles composed of one or more phospholipid layers that range in size from nanometers to microns; these have fully biodegradable properties and can encapsulate hydrophilic drugs in the inner aqueous phase and lipophilic drugs within the phospholipid bilayer to generate microcapsules of different sizes [28]. Liposomes have the advantages of fine particle synthesis, drug carriers, and improved therapeutic effect, which allows loading of some hydrophobic drugs. Compared with single drugs, liposome drug-loaded nanoplatforms release drugs slowly, reduce the toxicity of drugs to cells, and prolong the action time of drugs to show better drug activity [29].
Zhu et al. successfully developed a highly stable oral liposome containing capsaicin using cholesterol, sodium cholic acid, and isopropyl myristic acid as raw materials for the first time [30]. In gastric mucosa stimulation studies, liposome-encapsulated capsaicin has been shown to be safe for oral administration, significantly reducing gastric mucosal irritation, with increased relative bioavailability that is 3.34 times higher than that of free capsaicin. Consequently, liposome nanomaterials are expected to be used as a carrier system for hydrophobic drugs, improve the oral bioavailability of capsaicin, and become a new choice for NP treatment.
Recent studies highlighted that the clinical application of novel cannabinoid receptor agonist CB13, an excellent drug for NP treatment, is severely restricted by the influence of systemic metabolism, lymphatic transport, enterohepatic recirculation, and specific lipoprotein binding [31]. Thus, the use of novel carrier systems becomes an attractive strategy for the development of such compounds as a valuable therapeutic approach for NP. Durán-Lobato et al. used liposome nanoparticles as a nanoplatform for cannabinoid delivery system; these combine the best features of other colloidal carriers and can be made from physiological lipids in a variety of ways [32].
Cannabinoid liposome nanoplatforms, as lipophilic compounds, can reduce the initial metabolism and lymphatic absorption of cannabinoids, improve the gastrointestinal absorption of cannabinoids, and increase the oral bioavailability of cannabinoids to enhance the effect of cannabinoids on NP. It demonstrated that the cannabinoid-loaded liposome nanoplatform has a potential clinical application for the treatment of NP [33].
However, there are still some limitations for the use of liposomes in the clinical treatment of NP. Despres et al. found that the surface charge of liposome nanomaterials could affect immune reactions [34]. Villegas et al. also reported that liposome nanomaterials were characterized by low stability and poor in vivo stability in the presence of serum proteins and could produce certain human toxicity [35].
Metal nanoparticles
Metal NPs are a promising delivery vector owing to their persistent existence in cells and ability to escape enzymatic degradation. They have a large body surface area, which can make the embellished metal nanoparticles carry drugs, thereby improving the solubility, and biological efficacy of the drugs, as well as having unique photonic properties [36]. In current studies, metal nanoparticles have been proved to have significant effects for NP in in vitro and in vivo experiments as they can enable antibodies or drugs to actively target disease sites, and can simultaneously play targeting, diagnostic, and therapeutic functions in the treatment process [35,36,37,38,39,40,41].
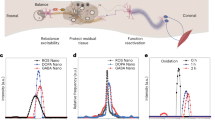
Neurological damage caused by abnormal ROS in the spinal cord leads to the activation of proinflammatory spinal microglia, which play a key role in subsequent pain sensitization [38]. Therefore, it is important to reduce the ROS level of microglia for preventing the occurrence of NP. In recent years, compared with natural antioxidants, nano-antioxidants have attracted extensive attention owing to their advantages such as easy mass production, high thermal and biological stabilities, multi-functionality, and adjustable performance [39]. Choi et al. used the oxidation of microglia-specific antibody parcel ceria nanoparticles (CZ nanoparticles) to treat NP. These can help microglia-specific antibodies to pass through any barrier so that targeted drug delivery can help reduce the release of inflammatory factors of microglia such as IL-1β、IL-6 and NO and ROS and allow it to quickly and efficiently suppress the activation of microglia (Fig. 2) [40]. At the same time of treatment, the appropriate delivery strategy of cerium dioxide nanoparticles allows it to be specifically delivered to target cells or tissues and is limited only to the central region of the disease. CZ nanoparticles inhibit the expression of pain-mediated genes in microglia and significantly reduce the mRNA expression levels of inflammatory mediator, thereby genetically reducing the occurrence of pain. CZ nanoparticles significantly improved mechanical tenderness in a spinal nerve transection-induced NP mouse model demonstrating its analgesic effect.
Reproduced from Choi et al. [40] with permission from Nanoscale.
As previously stated, proinflammatory TNF is a key mediator in the pathogenesis of NP; thus, drugs or genetic manipulation that inhibit TNF can reduce hyperalgesia [41]. Small interfering RNAs can be used to block the expression of specific genes in the brain, but their effectiveness is short lived (1–3 days) and they need to be used in large quantities. Thus, a suitable vector is needed when using siRNA to treat NP [42]. Gerard et al. used inert gold nanorods (GNRs) as nanometer carrier which provides stability to siRNA and gumming point control release, and found that the TNF-siRNA nanometer platform can selectively inhibit the expression of TNF in the CA1 region of hippocampus. Selectively inhibiting the production of TNF can block the induction of related pain cytokines and enhance the release of monoamines, thereby disrupting the malignant NP cycle and significantly reducing the hyperalgesia and mechanical ectopic pain in NP [43].
Metal nanoparticles also have inevitable limitations. In animal experiments, silver nanoparticles were reported to cause some dose-dependent toxic reactions through the release of silver ions from the particle surface, such as changes in neurotransmitter levels, liver enzymes, heart expansion, and immune response [44] Similarly, Sharma and other researchers also observed in the experiment that the nanoparticles of silver and Cu nanoparticles could induce neurotoxicity in rats or mice after administration through systemic or intracerebral pathways, in which the glial axons and endothelial cells were most obviously damaged, thus changing the sensorimotor and cognitive functions of experimental animals [45].
Micelles
Micelles are nanostructured material formed by self-aggregation of amphiphilic molecules in water above a certain critical micelle concentration; it is usually composed of a core-shell and amphiphilic block copolymer [46]. It is a unique and versatile material that can encapsulate hydrophobic drugs and can prolong their circulation in the blood by their hydrophilic shell; thus, it can be a very promising nanocarrier system with a fairly high capacity to carry drugs [47]. The micelle size ranges from 10 to 100 nm and has many unique properties such as stability, excellent biocompatibility in vivo, continuous circulation in the blood with high permeability to nerve inflammation tissues, and integration of a large number of hydrophobic drugs, which can contribute to drug targeting of NP and reduce nonspecific targeting of normal cells.
It was found that the phospholipase A2 (PLA2) family is one of the powerful inflammatory mediators involved in the immune regulation of spinal nerve pain. It recognizes and catalyzes the hydrolysis of the SN-2 ester bond of glycerides, releasing free fatty acids involved in tissue damage that leads to the development of NP [48]. Therefore, SPLA2 is a sensitive marker of spinal cord neuroinflammation and painful neurologic injury, and it can be used as a therapeutic target for NP. Kartha et al. prepared therapeutic nanoparticles based on phospholipid micelles by adding the SPLA2 inhibitor TEA-PC to hydrophobic superparamagnetic iron oxide nanoparticles, a nanoplatform capable of releasing a payload based on SPLA2 activity (Fig. 3) [49]. This delivery system increases the effective delivery concentration of the drug to the inflammatory target tissue and reduces the total dose required. Thus, it increases the effective concentration of the drug administered to the target tissue, thereby requiring less total dose, and reduces the toxic side effects on the surrounding nerve tissues and can release their loads according to the pathology of the pain itself.
A The phospholipid and hydrophobic SPIO nanoparticles were assembled and then sPLA2 inhibitors were inserted into them to form sPLA2 Inhibitor-Loaded phosphoolipid Micelles. B sPLA2 inhibitor-loaded nanomicelles were used to treat neuropathic pain in a rat model. Reproduced from Kartha et al. [49] with permission from ACS Nano.
Synthetic cannabinoids have previously been shown to alleviate hyperalgesia and tactile pain in rat models of NP, but its psychiatric side effects and low blood–brain barrier penetration severely restrict its clinical use [50]. Linsell et al. successfully synthesized the water-soluble nano-micellar platform SMA-WIN based on styrene maleic acid (SMA) and the potent CB1/CB2 receptor agonist cannabinoid WIN 55,212-2 (which is a chemical described as an aminoalkylindole derivative, which produces effects similar to those of cannabinoids such as tetrahydrocannabinol). It was loaded with water-soluble cannabinoids such as cannabinoid esters [51]. Highly loaded nano-micelle SMA-WIN can encapsulate hydrophobic molecules in hydrophilic structures so that a more stable structure can be formed between styrene groups and WIN. Further, it has ideal targeting delivery conditions and can selectively deliver cannabinoids to inflammatory tissues in NP. SMA-WIN can reduce the CNS’ effects of embedded WIN by restricting its transport across the blood–brain barrier. The high permeability of SMA-WIN can directly target the site of neuroinflammation, reducing the inflammatory response and subsequent NP, and producing more effective and lasting analgesia.
The stability of polymer nanomicelles is closely related to the matrix material. Miller found that peG-PVPY micelles have lower serum stability than polyester based micelles and peG-PVPY micelles rapidly become unstable after 3 h using a assay based on Foerster Resonance Energy Transfer [52]. In addition, Horn et al. found that nanomicelles may not be stabilized without certain temperature conditions and ionic strength [53]. The above reasons limit the utility of micelles to practical clinical applications.
Nanocapsules
Nanocapsules are nanoscale sac-like systems with a central cavity in which drugs can be encapsulated. Its central nucleus is encased in an envelope polymer membrane for surface binding of antibodies or targeted drugs [54]. In the past decade, nanocapsules have become the focus of research because of their protective coating functions, such as protection from spontaneous combustion, easy oxidation, and slow release of active ingredients [55]. Interfacial polymerization and nano-deposition are the best methods to prepare nanocapsules, and extremely high reproducibility makes nanocapsules a potential product candidate for biomedical applications, such as genetic engineering, radiation therapy, controlled drug delivery, and nanocapsules for anti-infection bandages [56].
Several studies indicate that neurokinin 1 receptor (NK1R) in the endosome is an important target for the relief of NP [57]. Endocytic inhibitors or antagonists targeting these receptors may provide an effective anti-NP effect. Thus, Ramirez-Garcia et al. assembled a nanocapsule with the hydrophobic NK1R antagonist aprepitant (MK-869) to synthesize diblock copolymers with the same hydrophilic shell to form pH-responsive BMA-Cy5 nanoparticles [58]. After being injected into the peritoneal cavity of rodents, BMA-Cy5 nanoparticles enter cells through dynamically dependent endocytosis, triggering drug release according to changes in endosomal and lysosomal pH values. This inhibits SP-induced neuronal activation in the spinal cord, accurately suppressing endosomal signaling events and leading to NP. Compared with aprepitant alone, the reactive BMA-CY5 nano-encapsulation platform can improve the stability and efficacy of the drug in the diseased tissue, tolerance, and transfer and retention; enhance the targeted drug delivery; and avoid side effect [59].
Organic selenium compound 2 (OMEPHSE2) is effective in reducing mechanical injurious behavior as well as PSNL-altered levels of inflammatory and apoptotic proteins (PSNL is a mouse model of NP that mimics some of the changes associated with the pathology of NP in humans). Therefore, OMEPHSE2, as a new synthetic molecule with pharmacological activity, has great potential in the treatment of NP. However, its properties such as poor water solubility of the organic selenium compounds may affect its overall bioavailability, thus affecting the biological effects. These limitations have delayed the progression to its usage in the clinical setting [60]. Henrique et al. recently found that compared with free OMEPHSE2, OMEPHSE2-nanocapsules extended their pharmacological activity, improved the biodistribution characteristics of OMEPHSE2 without any toxic effects, and enhanced pain resistance [61]. These can be attributed to its superior bioavailability and continuous controlled release of the drug.
Nanocapsule parameters such as capsule radius distribution, capsule surface, thickness, permeability of the capsule membrane, and its thermal or chemical decomposition determine whether it can be used to achieve effective controlled release and drug targeted therapy [62]. However, the excessively harsh preparation process and preservation condition limits its clinical application in the current NP therapy.
Dendrimers
Dendrimers are nano-sized and three-dimensional globular polymeric structures. Drug molecules can be attached on the functional groups on the surface or inside the dendritic macromolecules [63]. Dendrimers are viewed as excellent drug delivery nanoplatforms owing to their size monodispersity, high drug payloads, diverse surface functionalization capability, molecular stability, degradability, and the ability to transport hydrophobic and hydrophilic drug molecules [64]. Additionally, the size, shape, and composition of dendrimers can be accurately controlled. Owing to its slow-release properties, drug-bound dendritic macromolecular nanoplatforms have lower cytotoxic effects than free drugs, and the combined drug therapy has a strong synergistic effect.
It is generally acknowledged that oral morphine sustained-release formulations require continuous administration and complex dosing mechanisms, thus limiting their clinical use [65]. The multifunctional dendrimers are ideal “carriers” for hydrophobic drug encapsulation or complexation, which is particularly attractive in clinical therapeutic applications. Brent et al. developed morphine prodrugs complexed with PAMAM dendrimer by combining esterase-activated morphine prodrugs with surface-modified fifth generation (G5) PAMAM dendrimers [66]. Complexation with dendritic macromolecular nanopolymers allows the solubility to increase and slow controlled release of morphine proagents in vivo without altering the pharmacokinetics of morphine, thereby enhancing and prolonging the analgesic action and duration of morphine in animal neuropain models.
However, transfection efficiency of dendrimers depends on high charge density. Nevertheless, increased charge density leads to cell membrane destruction, which leads to cytotoxicity [67], and they can be quickly cleared by the reticuloendothelial system in vivo circulation, which reduces their therapeutic effectiveness in the NP model [68].
Conclusion
We summarized various nanoplatforms for the treatment of NP that play a crucial role in solving conundrums in the current single-drug treatment for NP, especially SCI-related NP. These can deliver drugs to the CNS target more efficiently and reduce the accumulation of drugs in non-target nerve tissues, thus reducing various toxic side effects. However, there remain some concerns that must be addressed to better utilize nanoplatforms for NP, such as nanoparticle instability, insufficient drug circulation time in the body, lower blood–brain barrier crossing rate, and neurotoxicity. Furthermore, silver nanoparticles can reduce the value of transendothelial resistance, resulting in the destruction of the blood–brain barrier [69], while polymeric PLGA have drawbacks such as poor drug loading and potential chronic-systemic toxicities. Lipid-based nanoparticles face similar limitations as those of PLGA including insufficient biodistribution, the risk of stimulating an immune response, and potential toxicity. Metal NPs with surface modifications easily cross the blood–brain barrier. Thus, these tend to accumulate in the brain, especially in the cortex and hippocampus, resulting in reduced glutamate uptake by astrocytes; subsequently, this leads to a potentially damaging effect on brain function and an increased risk of neurodegenerative processes. The use of micelles is limited by its low stability, short shelf life, and interbranch reproducibility. Nanocapsules overcome the drawbacks of lipid-based nanoparticles such as low loading capacity of lipophilic drugs and unstable characteristics. However, nanocapsules have a complex manufacturing process and low mechanical system stability. Dendrimers have some disadvantages such as rapid clearance by the reticuloendothelial system, toxicity due to the interaction of the amine terminated group with the cell membrane, low transfection efficiency, and lack of controlled-release behavior, which reduce their therapeutic efficiency [70].
Variations in the size of nanoplatforms may affect their physiological stability; thus, careful consideration of the nanoparticle design, mass production capacity, and standardized characterization are necessary to ensure complete conversion of nanomaterials into clinical products for the treatment of NP. Furthermore, toxicity, degradation products, metabolic pathways, and system performance evaluation of nano-drug delivery platforms in vivo need to be studied further. Designing the right clinical trials is critical to ensure the use of nanoplatforms in clinical settings; individual, personalized, and patient-centered nanoplatform drug delivery system selection should be followed, and nanoparticles alone, which is the foundation of nanoplatforms, should be established as a control group to demonstrate its impact in clinical trials. In conclusion, the nanoplatform drug delivery system is a promising prospective clinical application for patients with NP, especially SCI-based NP. We hope that therapeutic strategies combined with nanoplatforms will not only provide a better diagnosis and effective treatment for NP in patients with SCI, but that they will also help provide a novel research focus on pain.
Data availability
Previously reported data were used to support this study and are available at DOI. These prior studies (and datasets) are cited at relevant places within the text as references.
References
Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–19. https://doi.org/10.1016/s1474-4422(10)70143-5.
Hatch MN, Cushing TR, Carlson GD, Chang EY. Neuropathic pain and SCI: identification and treatment strategies in the 21st century. J Neurol Sci. 2018;384:75–83. https://doi.org/10.1016/j.jns.2017.11.018.
Shiao R, Lee-Kubli CA. Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics. 2018;15:635–53. https://doi.org/10.1007/s13311-018-0633-4.
Liu M, Li K, Wang Y, Zhao G, Jiang J. Stem cells in the treatment of neuropathic pain: research progress of mechanism. Stem Cells Int. 2020;2020:8861251. https://doi.org/10.1155/2020/8861251.
Umlauf BJ, Shusta EV. Exploiting BBB disruption for the delivery of nanocarriers to the diseased CNS. Curr Opin Biotechnol. 2019;60:146–52. https://doi.org/10.1016/j.copbio.2019.01.013.
Gupta J, Fatima MT, Islam Z, Khan RH, Uversky VN, Salahuddin P. Nanoparticle formulations in the diagnosis and therapy of Alzheimer’s disease. Int J Biol Macromol. 2019;130:515–26. https://doi.org/10.1016/j.ijbiomac.2019.02.156.
Cerqueira SR, Ayad NG, Lee JK. Neuroinflammation treatment via targeted delivery of nanoparticles. Front Cell Neurosci. 2020;14:576037. https://doi.org/10.3389/fncel.2020.576037.
Tomitaka A, Kaushik A, Kevadiya BD, Mukadam I, Gendelman HE, Khalili K, et al. Surface-engineered multimodal magnetic nanoparticles to manage CNS diseases. Drug Disco Today. 2019;24:873–82. https://doi.org/10.1016/j.drudis.2019.01.006.
Mir M, Ahmed N, Rehman AU. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf B Biointerfaces. 2017;159:217–31. https://doi.org/10.1016/j.colsurfb.2017.07.038.
Durán V, Yasar H, Becker J, Thiyagarajan D, Loretz B, Kalinke U, et al. Preferential uptake of chitosan-coated PLGA nanoparticles by primary human antigen presenting cells. Nanomedicine. 2019;21:102073. https://doi.org/10.1016/j.nano.2019.102073.
Xu Q, Xing H, Wu J, Chen W, Zhang N. miRNA-141 induced pyroptosis in intervertebral disk degeneration by targeting ROS generation and activating TXNIP/NLRP3 signaling in nucleus pulpous cells. Front Cell Dev Biol. 2020;8:871. https://doi.org/10.3389/fcell.2020.00871.
Liu Y, Zhou G, Xu N. Effect of metformin nanoparticle-mediated thioredoxin interacting protein expression on oxaliplatin-induced peripheral neuralgia. J Nanosci Nanotechnol. 2020;20:6123–32. https://doi.org/10.1166/jnn.2020.18508.
Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–77. https://doi.org/10.1038/nmat3765.
Shin J, Yin Y, Park H, Park S, Triantafillu UL, Kim Y, et al. p38 siRNA-encapsulated PLGA nanoparticles alleviate neuropathic pain behavior in rats by inhibiting microglia activation. Nanomedicine. 2018;13:1607–21. https://doi.org/10.2217/nnm-2018-0054.
Thangaraj A, Periyasamy P, Liao K, Bendi VS, Callen S, Pendyala G, et al. HIV-1 TAT-mediated microglial activation: role of mitochondrial dysfunction and defective mitophagy. Autophagy. 2018;14:1596–619. https://doi.org/10.1080/15548627.2018.1476810.
Zhang ZJ, Jiang BC, Gao YJ. Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci. 2017;74:3275–91. https://doi.org/10.1007/s00018-017-2513-1.
Noh C, Shin HJ, Lee S, Kim SI, Kim YH, Lee WH, et al. CX3CR1-targeted PLGA nanoparticles reduce microglia activation and pain behavior in rats with spinal nerve ligation. Int J Mol Sci. 2020;21. https://doi.org/10.3390/ijms21103469.
Hering I, Eilebrecht E, Parnham MJ, Günday-Türeli N, Türeli AE, Weiler M, et al. Evaluation of potential environmental toxicity of polymeric nanomaterials and surfactants. Environ Toxicol Pharmacol. 2020;76:103353. https://doi.org/10.1016/j.etap.2020.103353.
Lagreca E, Onesto V, Di Natale C, La Manna S, Netti PA, Vecchione R. Recent advances in the formulation of PLGA microparticles for controlled drug delivery. Prog Biomater. 2020;9:153–74. https://doi.org/10.1007/s40204-020-00139-y.
Zhao Z, Li D, Wu Z, Wang Q, Ma Z, Zhang C. Research progress and prospect of nanoplatforms for treatment of oral cancer. Front Pharmacol. 2020;11:616101. https://doi.org/10.3389/fphar.2020.616101.
Souto EB, Baldim I, Oliveira WP, Rao R, Yadav N, Gama FM, et al. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin Drug Deliv. 2020;17:357–77. https://doi.org/10.1080/17425247.2020.1727883.
Sharma G, Chopra K, Puri S, Bishnoi M, Rishi P, Kaur IP. Topical delivery of TRPsiRNA-loaded solid lipid nanoparticles confer reduced pain sensation via TRPV1 silencing, in rats. J Drug Target. 2018;26:135–49. https://doi.org/10.1080/1061186x.2017.1350857.
Xu Y, Tan HY, Li S, Wang N, Feng Y. Panax notoginseng for inflammation-related chronic diseases: a review on the modulations of multiple pathways. Am J Chin Med. 2018;46:971–96. https://doi.org/10.1142/s0192415x18500519.
Estella-Hermoso de Mendoza A, Campanero MA, Mollinedo F, Blanco-Prieto MJ. Lipid nanomedicines for anticancer drug therapy. J Biomed Nanotechnol. 2009;5:323–43. https://doi.org/10.1166/jbn.2009.1042.
Fonseca-Santos B, Silva PB, Rigon RB, Sato MR, Chorilli M. Formulating SLN and NLC as innovative drug delivery systems for non-invasive routes of drug administration. Curr Med Chem. 2020;27:3623–56. https://doi.org/10.2174/0929867326666190624155938.
Bell WL, Crawford IL, Shiu GK. Reduced bioavailability of moisture-exposed carbamazepine resulting in status epilepticus. Epilepsia. 1993;34:1102–4. https://doi.org/10.1111/j.1528-1157.1993.tb02140.x.
Elmowafy M, Shalaby K, Badran MM, Ali HM, Abdel-Bakky MS, Ibrahim HM. Multifunctional carbamazepine loaded nanostructured lipid carrier (NLC) formulation. Int J Pharm. 2018;550:359–71. https://doi.org/10.1016/j.ijpharm.2018.08.062.
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60. https://doi.org/10.1038/nrd1632.
Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. https://doi.org/10.1016/j.addr.2012.09.037.
Zhu Y, Wang M, Zhang J, Peng W, Firempong CK, Deng W, et al. Improved oral bioavailability of capsaicin via liposomal nanoformulation: preparation, in vitro drug release and pharmacokinetics in rats. Arch Pharm Res. 2015;38:512–21. https://doi.org/10.1007/s12272-014-0481-7.
Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3:Cd012182. https://doi.org/10.1002/14651858.CD012182.pub2.
Durán-Lobato M, Martín-Banderas L, Lopes R, Gonçalves LM, Fernández-Arévalo M, Almeida AJ. Lipid nanoparticles as an emerging platform for cannabinoid delivery: physicochemical optimization and biocompatibility. Drug Dev Ind Pharm. 2016;42:190–8. https://doi.org/10.3109/03639045.2015.1038274.
Donvito G, Nass SR, Wilkerson JL, Curry ZA, Schurman LD, Kinsey SG, et al. The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology. 2018;43:52–79. https://doi.org/10.1038/npp.2017.204.
Despres HW, Sabra A, Anderson P, Hemraz UD, Boluk Y, Sunasee R, et al. Mechanisms of the immune response cause by cationic and anionic surface functionalized cellulose nanocrystals using cell-based assays. Toxicol Vitr. 2019;55:124–33. https://doi.org/10.1016/j.tiv.2018.12.009.
Villegas MR, Baeza A, Vallet-Regí M. Nanotechnological strategies for protein delivery. Molecules. 2018;23. https://doi.org/10.3390/molecules23051008.
Navalón S, Álvaro M, Dhakshinamoorthy A, García H. Encapsulation of metal nanoparticles within metal-organic frameworks for the reduction of nitro compounds. Molecules. 2019;24. https://doi.org/10.3390/molecules24173050.
Singh P, Pandit S, Mokkapati V, Garg A, Ravikumar V, Mijakovic I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci. 2018;19. https://doi.org/10.3390/ijms19071979.
Ilari S, Giancotti LA, Lauro F, Dagostino C, Gliozzi M, Malafoglia V, et al. Antioxidant modulation of sirtuin 3 during acute inflammatory pain: the ROS control. Pharm Res. 2020;157:104851. https://doi.org/10.1016/j.phrs.2020.104851.
Janzadeh A, Karami Z, Hosseini M, Zarepour L, Yousefifard M, Nasirinezhad F. The role of CGRP receptor antagonist (CGRP8-37) and Endomorphin-1 combination therapy on neuropathic pain alleviation and expression of Sigma-1 receptors and antioxidants in rats. J Chem Neuroanat. 2020;106:101771. https://doi.org/10.1016/j.jchemneu.2020.101771.
Choi B, Soh M, Manandhar Y, Kim D, Han SI, Baik S, et al. Highly selective microglial uptake of ceria-zirconia nanoparticles for enhanced analgesic treatment of neuropathic pain. Nanoscale. 2019;11:19437–47. https://doi.org/10.1039/c9nr02648g.
Zhao H, Alam A, Chen Q, Eusman MA, Pal A, Eguchi S, et al. The role of microglia in the pathobiology of neuropathic pain development: what do we know? Br J Anaesth 2017;118:504–16. https://doi.org/10.1093/bja/aex006.
O’Mary HL, Hanafy MS, Aldayel AM, Valdes SA, Alzhrani RF, Hufnagel S, et al. Effect of the ratio of betamethasone to TNF-α siRNA coencapsulated in solid lipid nanoparticles on the acute proinflammatory activity of the nanoparticles. Mol Pharm. 2019;16:4496–506. https://doi.org/10.1021/acs.molpharmaceut.9b00629.
Gerard E, Spengler RN, Bonoiu AC, Mahajan SD, Davidson BA, Ding H, et al. Chronic constriction injury-induced nociception is relieved by nanomedicine-mediated decrease of rat hippocampal tumor necrosis factor. Pain. 2015;156:1320–33. https://doi.org/10.1097/j.pain.0000000000000181.
Hadrup N, Lam HR. Oral toxicity of silver ions, silver nanoparticles and colloidal silver_a review. Regul Toxicol Pharmacol. 2014;68:1–7. https://doi.org/10.1016/j.yrtph.2013.11.002.
Sharma HS, Sharma A. Neurotoxicity of engineered nanoparticles from metals. CNS Neurol Disord Drug Targets. 2012;11:65–80. https://doi.org/10.2174/187152712799960817.
Yokoyama M. Polymeric micelles as drug carriers: their lights and shadows. J Drug Target. 2014;22:576–83. https://doi.org/10.3109/1061186x.2014.934688.
Movassaghian S, Merkel OM, Torchilin VP. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:691–707. https://doi.org/10.1002/wnan.1332.
Soubhye J, van Antwerpen P, Dufrasne F. Targeting cytosolic phospholipase A2α for novel anti-inflammatory agents. Curr Med Chem. 2018;25:2418–47. https://doi.org/10.2174/0929867325666180117103919.
Kartha S, Yan L, Ita ME, Amirshaghaghi A, Luo L, Wei Y, et al. Phospholipase A(2) inhibitor-loaded phospholipid micelles abolish neuropathic pain. ACS Nano. 2020;14:8103–15. https://doi.org/10.1021/acsnano.0c00999.
Castaneto MS, Gorelick DA, Desrosiers NA, Hartman RL, Pirard S, Huestis MA. Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014;144:12–41. https://doi.org/10.1016/j.drugalcdep.2014.08.005.
Linsell O, Brownjohn PW, Nehoff H, Greish K, Ashton JC. Effect of styrene maleic acid WIN55,212-2 micelles on neuropathic pain in a rat model. J Drug Target. 2015;23:353.
Miller T, Rachel R, Besheer A, Uezguen S, Weigandt M, Goepferich A. Comparative investigations on in vitro serum stability of polymeric micelle formulations. Pharm Res. 2012;29:448–59. https://doi.org/10.1007/s11095-011-0555-x.
Van Horn WD, Simorellis AK, Flynn PF. Low-temperature studies of encapsulated proteins. J Am Chem Soc. 2005;127:13553–60. https://doi.org/10.1021/ja052805i.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–51. https://doi.org/10.1038/nbt.3330.
Iaccarino G, Profeta M, Vecchione R, Netti PA. Matrix metalloproteinase-cleavable nanocapsules for tumor-activated drug release. Acta Biomater. 2019;89:265–78. https://doi.org/10.1016/j.actbio.2019.02.043.
Ding D, Xu Y, Zou Y, Chen L, Chen Z, Tan W. Graphitic nanocapsules: design, synthesis and bioanalytical applications. Nanoscale. 2017;9:10529–43. https://doi.org/10.1039/c7nr02587d.
Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron. 2019;101:412–20.e3. https://doi.org/10.1016/j.neuron.2019.01.012.
Ramírez-García PD, Retamal JS, Shenoy P, Imlach W, Sykes M, Truong N, et al. A pH-responsive nanoparticle targets the neurokinin 1 receptor in endosomes to prevent chronic pain. Nat Nanotechnol. 2019;14:1150–9. https://doi.org/10.1038/s41565-019-0568-x.
Chizh BA, Göhring M, Tröster A, Quartey GK, Schmelz M, Koppert W. Effects of oral pregabalin and aprepitant on pain and central sensitization in the electrical hyperalgesia model in human volunteers. Br J Anaesth. 2007;98:246–54. https://doi.org/10.1093/bja/ael344.
Wilhelm EA, Bortolatto CF, Nogueira CW. p-Methoxyl-diphenyl diselenide protects against cisplatin-induced renal toxicity in mice. Food Chem Toxicol. 2012;50:1187–93. https://doi.org/10.1016/j.fct.2012.02.037.
Marcondes Sari MH, Zborowski VA, Ferreira LM, Jardim NDS, Araujo PCO, Brüning CA, et al. Enhanced pharmacological actions of p,p’-methoxyl-diphenyl diselenide-loaded polymeric nanocapsules in a mouse model of neuropathic pain: Behavioral and molecular insights. J Trace Elem Med Biol. 2018;46:17–25. https://doi.org/10.1016/j.jtemb.2017.11.002.
Mayer C. Nanocapsules as drug delivery systems. Int J Artif Organs. 2005;28:1163–71. https://doi.org/10.1177/039139880502801114.
Chauhan AS. Dendrimers for drug delivery. Molecules. 2018;23. https://doi.org/10.3390/molecules23040938.
Sherje AP, Jadhav M, Dravyakar BR, Kadam D. Dendrimers: a versatile nanocarrier for drug delivery and targeting. Int J Pharm. 2018;548:707–20. https://doi.org/10.1016/j.ijpharm.2018.07.030.
Cooper TE, Chen J, Wiffen PJ, Derry S, Carr DB, Aldington D, et al. Morphine for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;5:Cd011669. https://doi.org/10.1002/14651858.CD011669.pub2.
Ward BB, Huang B, Desai A, Cheng XM, Vartanian M, Zong H, et al. Sustained analgesia achieved through esterase-activated morphine prodrugs complexed with PAMAM dendrimer. Pharm Res. 2013;30:247–56. https://doi.org/10.1007/s11095-012-0869-3.
Lee J, Kwon YE, Kim Y, Choi JS. Enhanced transfection efficiency of low generation PAMAM dendrimer conjugated with the nuclear localization signal peptide derived from herpesviridae. J Biomater Sci Polym Ed. 2021;32:22–41. https://doi.org/10.1080/09205063.2020.1815496.
Ghaffari M, Dehghan G, Abedi-Gaballu F, Kashanian S, Baradaran B, Ezzati Nazhad Dolatabadi J, et al. Surface functionalized dendrimers as controlled-release delivery nanosystems for tumor targeting. Eur J Pharm Sci. 2018;122:311–30. https://doi.org/10.1016/j.ejps.2018.07.020.
Chen IC, Hsiao IL, Lin HC, Wu CH, Chuang CY, Huang YJ. Influence of silver and titanium dioxide nanoparticles on in vitro blood-brain barrier permeability. Environ Toxicol Pharmacol. 2016;47:108–18. https://doi.org/10.1016/j.etap.2016.09.009.
Wang Z, Dong X, Sun Y. Mixed carboxyl and hydrophobic dendrimer surface inhibits amyloid-β fibrillation: new insight from the generation number effect. Langmuir. 2019;35:14681–7. https://doi.org/10.1021/acs.langmuir.9b02527.
Funding
This study was financially supported by the Science and Technology Development Plan Projects of Jilin Province (Grant No. 20210101294JC), the Bethune project of Jilin University (Grant No. 2018A03)
Author information
Authors and Affiliations
Contributions
BY was responsible for conducting the search and writing the report; KW was responsible for interpreting results and text checking; XX was responsible for screening potentially eligible studies; JJ and YL contributed to experimental design and provided feedback on the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, B., Wu, K., Xu, X. et al. Recent advances in nanoplatforms for the treatment of neuropathic pain. Spinal Cord 60, 594–603 (2022). https://doi.org/10.1038/s41393-021-00746-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-021-00746-x