Abstract
Gut-liver-brain axis is a three-way highway of information interaction system among the gastrointestinal tract, liver, and nervous systems. In the past few decades, breakthrough progress has been made in the gut liver brain axis, mainly through understanding its formation mechanism and increasing treatment strategies. In this review, we discuss various complex networks including barrier permeability, gut hormones, gut microbial metabolites, vagus nerve, neurotransmitters, immunity, brain toxic metabolites, β-amyloid (Aβ) metabolism, and epigenetic regulation in the gut-liver-brain axis. Some therapies containing antibiotics, probiotics, prebiotics, synbiotics, fecal microbiota transplantation (FMT), polyphenols, low FODMAP diet and nanotechnology application regulate the gut liver brain axis. Besides, some special treatments targeting gut-liver axis include farnesoid X receptor (FXR) agonists, takeda G protein-coupled receptor 5 (TGR5) agonists, glucagon-like peptide-1 (GLP-1) receptor antagonists and fibroblast growth factor 19 (FGF19) analogs. Targeting gut-brain axis embraces cognitive behavioral therapy (CBT), antidepressants and tryptophan metabolism-related therapies. Targeting liver-brain axis contains epigenetic regulation and Aβ metabolism-related therapies. In the future, a better understanding of gut-liver-brain axis interactions will promote the development of novel preventative strategies and the discovery of precise therapeutic targets in multiple diseases.
Similar content being viewed by others
Introduction
In recent years, the importance of the liver brain axis in maintaining human health has received attention.1 Scientific investigations show that ties among gut dysbiosis or disruption, brain2 and liver3 diseases mean the pathophysiology of liver and brain diseases is frequently linked to gastrointestinal problems.4,5 For example, a leaky gut is described in nonalcoholic fatty liver disease (NAFLD),6 alcoholic liver disease (ALD),7 non-alcoholic steatohepatitis (NASH),8 alcoholic steatohepatitis (ASH),9 hepatocellular carcinoma (HCC),10 and so forth.11 Besides, gut dysbiosis is discovered in multiple gut brain axis-related diseases including Parkinson’s disease (PD),12 Alzheimer’s disease (AD),13 amyotrophic lateral sclerosis,14 autism,15 stroke,16 depression,17 and drug addiction.18 Some liver diseases are closely related to neurological disorders through liver-brain axis, such as hepatic encephalopathy (HE), cirrhosis, and so on. There are many milestone events for gut-liver-brain axis-related theory in the past few centuries. In AD 300–400, Ge Hong collected folk remedies and published “Emergency Prescriptions for Elbow Reserve” which first recorded fecal liquid treating food poisoning and severe diarrhea. In 1998, Marshall put forward the concept of the “gut liver axis”.19 After ten years, the influence of the gut liver brain axis in human health was first revealed.20 Therefore, the crosstalk among the gut, liver and brain is being increasingly recognized and delineated piece by piece (Fig. 1).21
Timeline of the milestone events for the gut liver brain axis. In AD 300–400, Ge Hong collected folk remedies and published “Emergency Prescriptions for Elbow Reserve”, which firstly used fecal liquid to treat food poisoning and severe diarrhea. In 1899, Henry Tissier in France isolated the first strain of Bacillus bifidus from the feces of healthy breastfed infants. In 1900, German bacteriologist Paul Ehrlich discovered the blood–brain barrier. Metchnikoff proposed the famous “May hypothesis” in 1907, pointing out that the gut microbiota and its interactions with the host were crucial for health. In 1921, the concept of enteric nervous system (ENS) was first proposed, which focused on the neuroanatomy, function, and pathophysiology of gut-brain interactions. After six years, Wieland won the Nobel Prize in Chemistry for his discovery of bile acids and their chemical structures. In 1929, the George Burr couple discovered fatty acids were crucial for health. In 1950, gamma-aminobutyric acid (GABA) was discovered in the mammalian brain. In 1995, the concept of prebiotics was proposed by the international “father of prebiotics”, Glenn R. Gibson, and farnesoid X receptor (FXR) was first discovered by Forman et al. In 1998, Marshall proposed the concept of the “gut-liver axis”. Meanwhile, Rorberfroid further blended probiotics and prebiotics into products called synbiotics. In 2003, takeda G protein-coupled receptor 5 (TGR5) was first discovered as a cell surface receptor for bile acid reactions. In 2008, Wang first revealed the influence of the gut-brain-liver axis in human health. In 2012, the first gut-brain axis-related drug linaclotide was approved for the treatment of irritable bowel syndrome (IBS) by FDA. In 2016, the first gut-liver axis-related drug obeticholic acid was approved for the treatment of primary biliary cirrhosis by FDA. In 2022, the gut-brain axis-related drug vibrating capsule was approved for the treatment of functional constipation by FDA. Created with BioRender.com
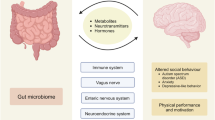
Gut-liver brain axis is a three-way highway of communication.22,23 The connection between the gut and the liver lays on the gut barrier, whose disruption leads to more bacteria or their metabolites entering the liver24,25 and contributes to or worsens a variety of hepatic disorders.26 Various peptides or hormones produced by the intestines in response to nutrition influence neural signaling from the gut to the brain. They enter the blood, act on the local vagal, spinal afferent neurons and brain, then feeds back to the liver vagal parasympathetic nerves and innervates the gut and paracrine.27
There are multiple marketed drugs involved in the regulation of the gut-liver-brain axis (Table 1). For example, odevixibat is a pharmaceutical option for interfering with the enterohepatic circulation in individuals with progressive familial intrahepatic cholestasis.28 Vibrating capsule is a potential alternative physical treatment for functional constipation. It relieves gut burden, mental and physical stress.29 Besides, sodium oligomannate therapeutically remodels gut microbiota and neurological inflammation in AD development and is regarded as a unique technique for AD therapy via remodeling the gut-brain axis.30 In addition, some ongoing research on the gut-liver-brain axis is also constantly emerging. For example, because the microbiome controls intestinal permeability, and changes blood–brain barrier (BBB), vagus nerve, and neurotransmitters, the supplement of probiotics, prebiotics or synbiotics such as VSL#3,31 multistrain probiotics32 and galactooligosaccharides33 is regarded as an effective therapy strategy for the treatment of AFLD, NASH, autism spectrum disorder (ASD), depression, PD, schizophrenia, epilepsy, migraine, and so on.
In this review, we discuss the comprehensive pathophysiology of the gut-liver-brain axis in several chronic liver diseases, nervous and gut disorders, and introduce the candidates now being explored in this axis. These findings have significant implications for society as well as broad health issues throughout the world, which urgently need to be addressed. It is expected to further develop more clinical candidates to regulate gut-liver-brain axis.
Mechanisms linking the gut-liver axis
Gut dysbiosis is a medical condition that happens when there is a microbial imbalance in a person’s intestines. Gut microbiota or their metabolites improve or aggravate the progression of multiple liver diseases such as chronic hepatitis B virus (HBV),34 chronic hepatitis C virus (HCV),35 NAFLD,36 ALD,37 other-induced liver disease38 and HCC39 (Fig. 2) through several mechanisms,40 including changes in the intestinal permeability,41 short-chain fatty acids (SCFAs),42 long-chain fatty acids (LCFAs),43 fasting-induced adipocyte factor (FIAF), choline metabolism,44,45 ethanol production,46 and BAs metabolism47,48 (Fig. 3).
Gut dysbiosis influences liver disease progression. (1) Gut dysbiosis increases the number of pathogens and the release of their metabolites like lipopolysaccharide (LPS) and destroys tight junctions (TJs) and gut permeability. (2) Gut dysbiosis changes SCFAs and FIAF production. (3) Gut dysbiosis increases intestinal choline and ethanol production. (4) Gut dysbiosis influences BAs metabolism. These factors and metabolites together with dietary lipids result in liver steatosis, inflammation, and eventually, HCC. Created with BioRender.com
Changing intestinal permeability in the gut-liver axis
Microbiome controls intestinal permeability in the gut-liver axis
Gut microbiota communities are highly flexible, with their composition influenced by a variety of external and host factors such as high-fat diet, age, physiological condition, and genetic background.49 When gut dysbiosis happens, the amount of some gut microbiota including Lactobacillus, Bacteroides, and Bifidobacterium decreases, and leads to the damage of TJs protein50 and the change of intestinal permeability, which promotes the pathogens and their metabolites entering vessel circulation, and subsequently activates the proinflammatory pathways (Fig. 4).51,52 For example, intestinal microbial metabolites alter host gut mucosal proteins and lead to liver injury.53 Endogenous changes in the gut affect the intestinal barrier and promote intestinal inflammation.54 Besides, intestinal-associated lymphoid tissue participates in intestinal barrier function and prevents intestinal inflammation.55
Gut microbiota and its metabolites in the gut-liver axis. (1) Microbial metabolites such as pathogen-associated molecular patterns (PAMP) and LPS bind to TLRs on the membrane of intestinal epithelial cells. Activation of these TLR/MYD88-dependent signaling pathways leads to the translocation of nuclear factor-kappa B (NF-κB) into the nucleus, and promotes the transcription of numerous cytokines. (2) Gut dysbiosis inhibits the secretion of FIAF, and then inhibits the release of endothelial LPL, which is responsible for the release of triglycerides from circulating chylomicrons and VLDL. (3) The intestinal microbiota converts dietary phosphatidylcholine to choline or hepatotoxic TMA. These metabolites increase intestinal permeability with disruption of TJs proteins such as claudins, TAMPs, and JAMs. (4) Gut dysbiosis also results in increased endogenous alcohol production, which allows endotoxins and ethanol directly into the liver. (5) Gut dysbiosis inhibits the secretion of SCFAs. It has effects on G-protein coupled receptors, such as GPR41 and GPR43, causing the release of PYY and GLP-1, respectively, from neuroendocrine L cells. (6) Endotoxins released from intestinal microbiota stimulate the secretion of inflammatory factors by immune cells. Created with BioRender.com
Cytokines control intestinal permeability in the gut-liver axis
In general, the intestinal barrier consists of TJs proteins including transmembrane proteins such as claudins, TJs-associated marvel proteins (TAMPs), and junctional adhesion molecules (JAMs),56,57 and the scaffolding protein containing zona occludens (ZO)-1, ZO-2, and ZO-3.58 Cytokines control intestinal barrier function, especially tumor necrosis factor (TNF), interferon-gamma (IFN-γ), interleukin (IL)-1β, endotoxins and chemokines, which become the key mediators to destroy the intestinal barrier.59 Macrophages and T cells as the important immune cells maintain the balance of the barrier, whose position is close to the blood vessels.60 The activation of macrophages releases some inflammatory substances, which promote the development of steatosis, inflammation, and fibrosis.61
LPS as one of the pathogen-associated molecular patterns contains lipid A, crosses the intestinal mucosa via TJs or with the aid of chylomicrons, binds to the LPS binding protein (LBP), and interacts with toll-like receptor (TLR)/myeloid differentiation primary response 88 in the liver and gut mucosal tissues. It promotes the transcription of numerous cytokines in the liver and adipose tissues,62 the activation of liver inflammasomes via NACHT, LRR, and PYD domains-containing protein 3,63 and the development of fibrosis in the livers (Fig. 4).64,65 This may be linked to the increase of certain gram-negative bacterial genera, including Bacteroides, Enterobacteria, Escherichia, and Proteus, which is discovered in patients with NAFLD and NASH.66
Orchestrating intestinal SCFAs, LCFAs, and FIAF in the gut-liver axis
SCFAs and LCFAs in the gut-liver axis
Normal gut microbiome everyday produces 50–100 mM of SCFAs such as acetic acid, propionic acid, butyric acid, and so forth.67 For example, butyric acid as the most important SCFAs is mainly generated by Coprococcus, Faecalibacterium prausnitzii, Eubacterium rectale, Eubacterium hallii, and Roseburia bromii.68 Acetic acid as the most productive SCFAs is mainly generated by gut bacteria, including Prevotella, Ruminococcus, Bifidobacterium, Bacteroides, Clostridium, Streptococcus, Akkermansia muciniphila, and Hydrobacillus.69 In addition, propionic acid as the third most important SCFAs is mainly synthesized by Akkermansia municiphilla, Salmonella enterica serovar Typhimurium, and Roseburia inulinivorans.70
SCFAs provide energy sources, and promote hepatic lipogenesis and gluconeogenesis via acting on the G-protein coupled receptors (GPR) such as GPR41 and GPR43.71 Butyrate is the most important SCFAs in sustaining colonic health since it directly provides energy to colonic epithelial cells.72 Almost all butyric acid is absorbed by colonocytes, while a small amount is distributed in peripheral blood.73 Butyrate directly acts on T regulatory cells in the mucosa, suppresses inflammation74 and fatty acid synthesis, and promotes the growth of probiotics.75 Acetate is absorbed by the proximal colon and swiftly transferred to the liver, where it acts as a substrate for cholesterol production.76 In addition, 90% of the third major SCFAs like propionic acid are delivered to the liver and used as a substrate for other pathways such as lipogenesis, gluconeogenesis and protein synthesis.77 All of the above SCFAs acting on GPR41 and GPR43 on L cells release peptide tyrosine tyrosine (PYY), which reduces gastric emptying and intestinal transit, and improves food absorption.78 These L cells also secrete glucagon-like peptide 1 (GLP-1) which promotes glucose-dependent insulin secretion.79,80
However, the impact of SCFAs is controversial.81 At present, the function of SCFAs in bacterial-host interactions is unclear. It is difficult to determine whether SCFAs are beneficial or harmful to the host.82 As a recent study, excessive accumulation of butyrate leads to cholestasis, hepatocyte mortality, and neutrophilic inflammatory reactions in the liver, which finally induces icteric HCC.83
LCFAs belonging to the derivatives of triglycerides are isolated from animal fats and vegetable oils and catalyzed by gut bacteria like Lactobacillus rhamnosus GG.84 For example, LCFAs favorably influence the bacterial population in the gut, which has been demonstrated to improve intestinal barrier function, decrease endotoxemia, and inhibit ALD.85 However, when LCFAs reach high concentration, they become toxic detergents within cells.86
FIAF in the gut-liver axis
In general, SCFAs especially for butyrate87 can further activate the release of FIAF (also named angiopoietin-related protein 4) from L cells, brown fat, white fat and hepatocytes (Fig. 4).88 Meanwhile, FIAF inhibits the expression of lipoprotein lipase (LPL)6 and triglyceride buildup in both adipose tissue and the livers.88,89 Inhibition of FIAF activates carbohydrate-responsive element-binding protein and sterol regulatory element-binding protein 1 in livers,88,90 which boosts lipogenic enzymes and increases fat formation.91
Regulating intestinal production of choline and ethanol in the gut-liver axis
Choline metabolism in the gut-liver axis
Choline, a component of cell membranes, is generated endogenously in the liver92 and decomposed by gut bacteria (Fig. 4). Choline acts in the generation of very low-density lipoprotein (VLDL) because it is required for the formation of the phosphatidyl-choline component of VLDL particles in the liver.93 VLDL particles cannot be released in the deficiency of choline, which increases lipoperoxidation in hepatocytes, and in turn results in an increase in intracellular free radicals linked with DNA damage, apoptosis, and tumorigenesis. In the gut, the increased choline metabolism is closely related to high levels of the taxa Firmicutes Erysipelotrichia.94
Gut microbiota converts choline into dimethylamine and trimethylamine (TMA),95,96 and catalyzes choline or TMA into toxic metabolites like trimethylamine N-oxide (TMAO).95 L-carnitine, choline and betaine are the main substrates for TMA synthesis by gut bacterial strains including Clostridium asparagiforme, Clostridium sporogenes, Clostridium hathewayi, Escherichia fergusonii, Anaerococcus hydrogenalis, and Proteus penneri.97 Higher circulatory distribution of TMAO is associated with decreased levels of host-produced phosphatidylcholine, a sign of intestinal dysbiosis.98 This is related to liver damage as a result of increased triglyceride buildup, which causes hepatic steatosis.
Ethanol production in the gut-liver axis
Gut dysbiosis stimulates intestinal ethanol production, which is implicated in the development of NASH and NAFLD (Fig. 4).99,100 For example, 1 g of Escherichia coli generates 0.8 g of ethanol each hour in anaerobic conditions,101 further increases intestinal permeability and portal levels of LPS, triggers the expression of TLR and inflammasome, and contributes to liver injury. Ethanol significantly alters the composition of the gut microbiota, including decreasing the relative abundance of Bacteroidetes and increasing the relative abundance of Proteobacteria.54 Its metabolites, especially for acetaldehyde, may damage TJs of the gut epithelial tissue, cause a leaky gut, and facilitate bacterial and fungal translocation, which is related to the advancement of liver cirrhosis development.102
In addition, excessive alcohol in ALD damages intestinal barrier components, especially for proteins implicated in innate antibacterial defense such as 3-β and 3-γ, increases adhesion between bacteria and the mucosal surface, causes excessive growth of intestinal bacteria, microbial product translocation103 and ecological imbalances related to intestinal inflammation101 and liver inflammation.104,105
Influencing BAs metabolism in the gut-liver axis
BAs are produced through cytochrome P450 enzymes (CYPs)-mediated oxidation of cholesterol, which includes the classical and alternative pathways in hepatocytes (Fig. 3).106 The classical process contains the enzymatic action of cholesterol 7 alpha-hydroxylase (CYP7A1), sterol 12α-hydroxylase (CYP8B1), and sterol 27-hydroxylase (CYP27A1) to generate the primary BAs such as cholic acid (CA) and chenodeoxycholic acid (CDCA). The alternative process includes CYP27A1 hydroxylating the cholesterol side chain to produce CDCA and then oxysterol 7α-hydroxylase (CYP7B1) 7-α hydroxylating to form the oxysterol intermediates.107
Subsequently, CA and CDCA conjugate taurine (primarily in mice) or glycine (primarily in humans) to form taurocholic acid (TCA), taurochenodeoxycholic acid (TCDCA), glycocholic acid (GCA) and glycochenodeoxycholic acid (GCDCA), respectively, and then are secreted from the liver into the gallbladder via the canalicular bile salt export pump (BSEP).108 In addition, some BAs undergo sulfonation and glucuronidation, and then are transported from the liver into the gallbladder via the multidrug resistance-associated protein 2.109
After BAs are synthesized in hepatocytes, they are secreted from the liver to the gallbladder and then into the intestine. Gut bacteria convert the primary BAs into secondary BAs.110 The main intestinal bacteria taking part in BAs metabolism include Bacteroides, Clostridium, Lactobacillus, Bifidobacterium and Listeria in BAs deconjugation,111 Bacteroides, Eubacterium, Clostridium, Escherichia, Egghertella, Eubacterium, Peptostreptococcus, and Ruminococcus in oxidation and epimerization of hydroxyl groups at C3, C7, and C12 of BAs, Bacteroides, Eubacterium and Lactobacillus in BAs esterification, and Clostridium, Fusobacterium, Peptococcus, and Pesudomonas in BAs desulfatation.112
In this process, intestinal anaerobes including genera Bacteroides, Eubacterium and Clostridium deconjugate taurine-conjugated BAs and glycine-conjugated BAs into unconjugated counterparts via microbiota metabolites like bile salt hydrolase. Subsequently, anaerobes containing the genera Bacteroides, Clostridium, Eubacterium, Lactobacillus and Escherichia convert these unconjugated primary BAs into the secondary BAs such as lithocholic acid (LCA) and deoxycholic acid (DCA) based on 7α-dehydroxylation of CYP7A1.113 Most of CA, CDCA and DCA are then reabsorbed in the gut and transported back to the liver, while the majority of LCA is excreted in feces.114 Besides, BAs as signal molecules and metabolic integrators stimulate nuclear FXR and membrane TGR5, and control cholesterol, lipid, and energy metabolism.115
FXR regulates BAs synthesis and transport in the gut-liver axis
FXR, which is highly expressed in the liver and the intestine tissues, is involved in the BAs metabolism in gut-liver axis (Fig. 5)116,117 and regulates a variety of critical metabolic pathways to maintain BAs homeostasis.118
BAs biosynthesis, transport, and FXR-mediated BAs signaling in the gut-liver axis. BAs are synthesized in hepatocytes via CYPs-mediated oxidation of cholesterol to form CA and CDCA, which conjugate taurine, or glycine to form conjugated BAs and are secreted from the liver to the gallbladder and then into the intestine. Subsequently, gut microbes convert the primary BAs into secondary BAs. During gut dysbiosis, the expression of intestinal FXR is downregulated, leads to the increase of BAs synthesis and BAs influx, and the decrease of BAs efflux, and thus promote the progress of liver diseases. FXR also controls BAs detoxification and inflammation formation. Created with BioRender.com
In the intestine, apical sodium-dependent BA transporter (ASBT) induces BAs influx into the gut and promotes the ileal expression of FXR. In the meantime, FXR negative feedback suppresses the expression of ASBT,119 maintains homeostasis in the intestinal mucosa,120 boosts the expression of intestinal bile acid transport protein like organic solute transporter alpha/beta (OSTα/β), and induces BAs efflux.121 In addition, FXR promotes fibroblast growth factor 19 (FGF19) to enter the liver, and then suppresses CYP7A1 enzyme activity.
In the liver, FXR decreases CYP7A1 enzyme activity via FGF19-fibroblast growth factor receptor 4 (FGFR4) and FXR-small heterodimer partner (SHP) signaling pathways, and hence suppresses BAs production.122 FXR also indirectly down-regulates the expression of organic anion transporter 1/4 and BAs influx,123 and increases BAs efflux based on the up-regulation of OSTα/β, multidrug resistance-associated protein 3/4, BSEP and sodium-dependent taurocholate cotransporting polypeptide transporters.124,125,126,127
In addition, hepatic FXR up-regulates the transporters of choline and cholesterol like ABCG5/8 and MDP3/4128,129 and inhibits the expression of NF-κB and protein kinase C, which regulates the inflammation formation.130 FXR also increases the expression of peroxisome proliferator-activated receptor α and regulates BAs detoxification by encoding CYPs, sulfotransferases (SULTs) and UDP-glucuronosyltransferases (UGTs).131 All in all, FXR inhibits cholestasis and inflammation, and therefore suppresses the development of liver diseases.
TGR5 regulates BAs transport in the gut-liver axis
TGR5 belongs to the transmembrane G protein-coupled receptor that is activated by BAs, increases the intracellular concentration of cyclic AMP (cAMP) and regulates BAs transport. LCA is the most potent natural TGR5 agonist among the BAs pool which contains muricholic acid (MCA), hyocholic acid (HCA), ursodeoxycholic acid (UDCA), CA, CDCA, DCA, LCA, and so on (Fig. 6a, b). TGR5 is found in a variety of cell types and organs such as brown adipocytes, hepatic stellate cells, macrophages, pancreas, Kupffer cells, cholangiocytes, enterocytes, and L cells (Fig. 6c).
BAs as the messengers in the gut-liver axis activate TGR5. a The basic chemical structure of BAs. b BAs pool contains MCA, HCA, UDCA, CA, CDCA, DCA, and LCA. The OH group at R1, R2, or R3 and its spatial orientation determine the type of BA. R2 is the site of dehydrogenation. X is the site of conjugation. – to +++ represents the affinity from low to high based on their affinity to TGR5. c TGR5 is expressed in various cell types and tissues such as brown adipocytes, hepatic stellate cells, macrophages, pancreas, Kupffer cells, cholangiocytes, enterocytes, and L cells. Created with BioRender.com
On L cells, β cells and enterocytes, activation of TGR5 leads to the secretion of GLP-1,132 insulin133 and intestine peristalsis,134 which improves pancreas function and insulin sensitivity. On macrophages and Kupffer cells, activation of TGR5 dampens NF-κB-mediated cytokine expression, modulates immune signals of Treg and TH17, and regulates immunity and inflammation.100,135 TGR5 on brown adipose tissue activates cAMP-dependent iodothyronine deiodinase 2 which converts inactive thyroxine into active thyroid hormone and regulates energy homeostasis.136 TGR5 on hepatic stellate cells promotes the formation of liver fibrosis.137 Besides, TGR5 on cholangiocytes regulates resorptive and secretory mediators, and modulates bile flow and composition138 (Fig. 6c).
Other BAs receptors regulate BAs synthesis and transport in the gut-liver axis
Shingosine-1-phosphate receptor 2 (S1PR2) as another G protein-coupled receptor exists in multiple hepatic cells, bile duct cells, hepatic stellate cells, intestinal endothelial cells and macrophages,139 binds to sphingosine 1-phosphate, and plays a differential role in multiple tissues.140 Importantly, BAs only in their conjugated form such as taurine or glycine conjugated BAs activate S1PR2.
S1PR2 existing around the liver and bile duct promotes hepatic fibrogenesis via influencing the activity of bone marrow-derived macrophages. S1PR2 deficiency dramatically decreases bile duct ligation-induced bile duct cell proliferation and bile stasis damage, as evidenced by a significant decrease in inflammation and hepatic fibrosis in S1PR2 knockout mice. Meanwhile, S1PR2 antagonist JTE-013 drastically lowers blood total BAs and cholestatic liver injury in mice with bile duct ligation.141
Besides, S1PR2 in intestinal endothelial cells is a key protein in maintaining intestinal mucosal barrier function. Inhibition of S1PRs2 restores gut barrier function and M1 macrophage polarization, and decreases ER stress of gut endothelial cells and glycolysis in macrophages.142
The vitamin D receptor (VDR) as the superfamily of nuclear receptors is implicated in immunity, cellular development, insulin production, and secondary bile acid detoxification. VDR has an affinity for dehydro-LCA and LCA,143 which is more sensitive than other BAs receptors. When VDR is activated, it stimulates the expression of cytochrome P450 3 A, which encodes cytochrome P450 enzymes responsible for LCA detoxification in the liver and gut.144 Meanwhile, the levels of VDR is linked to beta-diversity of gut microbiota which corresponds with enhanced Janus kinase (JAK)/ signal transducer of activators of transcription (STAT) signaling, as well as increased secondary BAs and intestinal tumor burden.145 In addition, the amount of Lactobacillus is decreased, while Clostridium and Bacteroidetes species are elevated which link to VDR-induced the change of BAs and fatty acids in VDR-deficient mice.146
Pregnane X receptor (PXR) as a well-known orphan nuclear receptor is enriched in the liver and intestine and responds to xenobiotic and BAs exposure.147,148 PXR is regulated by various endogenous substances, especially for microbial metabolites such as some secondary BAs and 3-indolepropionic acid.149 Among these metabolites, PXR has a better affinity for LCA than for DCA and CA.149,150 When PXR is lack, the relative abundance of Lactobacillus increases, which possesses bile salt hydrolase, and therefore hydrolyzes primary taurine-BAs in feces. Besides, PXR-deficient mice have a characteristic leaky gut physiology which is accompanied by an increase of the TLR signaling pathway.151
Retinoic acid-related orphan receptor (ROR γt) as a nuclear receptor is linked to a number of inflammatory and autoimmune disorders. Th17 cells,152 lymphoid tissue inducer cells,153 type 3 innate lymphoid cells,154 and T cells155 can express ROR γt receptor. Several inverse agonists containing cholesterol intermediates and oxysterols can lower ROR γt. These inverse agonists decrease the transcription binding activity of ROR γt and reduce the production of pro-inflammatory cytokines in inflammation and autoimmune disorders.156
Affecting immunity in the gut-liver axis
The intestinal innate immune system plays an important role in providing the first line of defense against intestinal pathogens. The liver is a central immunological organ with a high exposure to circulating pathogens and endotoxin from the gut microbiota. It’s particularly enriched in multiple immune cells including macrophages, lymphoid cells, mucosal-associated invariant T (MAIT) and γδ T cells.
Intestinal macrophages release inflammatory signals and promote the hepatic recruitment of blood monocytes, which locally develop into monocyte-derived macrophages and increase the size of the macrophage pool in livers.157 Natural killer (NK) cells are a major population of lymphocytes in the liver. They release immunomodulatory cytokines including IFNγ, IL-4, and IL-13 to damage the intestinal barrier. Liver-resident NK cells have many similar characteristics to immune-regulatory lymphocytes (known as innate lymphoid cells), which are frequently present on the intestinal mucosal surfaces of the gut.158 In addition, MAIT cells take part in multiple liver pathogenesis, and inhibit liver inflammation and damage.159,160 γδ T cells as another type of innate-like T cells exist in the steady-state liver, whose development is sustained in a microbiota-dependent way. The increase of γδ T cells in the liver causes hepatic damage.161
Mechanisms linking the gut-brain axis
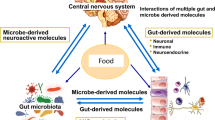
Gut-brain axis is mediated based on the circulatory system, vagus nerve,162 immune system,163 neuroendocrine system,164 and ENS.165 There is a wide range of neuroactive substances, including gut hormones, neuroactive compounds, gut microbiota-derived metabolites, and gut microbiota-derived products in this axis (Fig. 7).166 After metabolites enter the brain, they affect neurological growth and neuronal degeneration under many situations including social and cognitive behavior, fear, stress, and food intake. Furthermore, the brain feeds back to the gut and paracrine through the vagus nerve (Fig. 8).167,168
The mechanisms linking the gut-brain axis. Gut microbiota is capable of synthesizing neurotransmitters like SCFAs and GABA, which have different peripheral and central effects on modifying host metabolism and central regulation of appetite directly via vagal stimulation or indirectly through immune-neuroendocrine mechanisms. Enteroendocrine cells are activated by these microbial-derived metabolites, and lead to the production of gut hormones such as 5-hydroxytryptamine (5-HT), GLP-1, PYY, and cholecystokinin (CCK). These gut hormones are released from the gut to the nucleus tractus solitarius of the brain via the vagus nerve and direct secreted into the circulatory system. Information from the nucleus tractus solitarius is distributed to the arcuate nucleus (ARC) in the hypothalamus, where appetite and energy balance are regulated. The ARC contains neuropeptide Y, agouti-related protein, anorexigenic peptides, cocaine amphetamine-regulated transcript, and pro-opiomelanocortin neurons. Moreover, gut microorganisms also use bile acids and their conjugates to activate FXR and TGR5, and increase GLP-1 secretion by enteroendocrine cells. Additionally, gut microbiota is associated with inflammation via the release of LPS, which activates immune cells, such as B cells and dendritic cells, and promotes the production of cytokines. Created with BioRender.com
Changing BBB in the gut-brain axis
The BBB is a barrier that prevents diffusion between the circulatory system and central nervous system (CNS) cerebrospinal fluid.169 The gut microbiota and its metabolites regulate the expression of TJs proteins, lead to the release of inflammatory cytokines,170 and further induce the structural change of the BBB.171,172 Under normal physiological conditions, cytokines are difficult to pass through the BBB and affect brain regions.173 Once gut disorders occur, the large amounts of IL-1 and IL-6 change BBB permeability, pass through BBB, activate the hypothalamic-pituitary-adrenal (HPA) axis and produce cortisol, which is the most potent stimulator for the pressure system.174 Meanwhile, psychological or physical stress significantly disrupts the HPA axis, which mainly regulates stress response and has a significant impact on the gut-brain axis,175 especially in IBS.
Changing vagus nerve in the gut-brain axis
The vagus nerve serves as a significant two-way highway that connects the brain to the gut.176 It is intrinsically linked to ENS, which alters brain behavior like stress reactivity, anxiety, depressive, and social behaviors as well as cognition.177,178,179 The afferent fibers of the vagus nerve come from the intestinal smooth muscle, and transmit information from the intestine to the CNS.180,181 Furthermore, the vagus nerve may detect microbial messages from CNS and then feed back to the intestine, release bacteria-derived metabolites such as SCFAs, GABA, and 5-HT, or gut hormones like GLP-1, PYY, and CCK, which are affected by enteroendocrine and enterochromaffin cells in the intestinal epithelium.26,182,183
Regulating neurotransmitters in the gut-brain axis
The gut microbiota can synthesize and modulate their hosts to produce neurotransmitters184 including GABA, glutamate, acetylcholine, dopamine, norepinephrine and trace amines.13
GABA in the gut-brain axis
GABA as gut bacteria-derived metabolites is an amino acid derivative of glutamate, which is widely distributed in the mammalian CNS and significantly modulates synaptic suppression and effects on psychological diseases including behavioral disorders, insomnia, and pain.185,186 GABA is produced by several bacteria, including Bacteroides, Bifidobacterium, Lactobacillus and Escherichia spp, which involves ENS homeostasis and disturbance, such as acid secretion, gastric emptying, bowel motion, and sensation of pain.187,188
Importantly, the adhesion from GABA to GABA receptors, and then to postsynaptic neurons inhibits the transfer of Na+, K+, Ca2+, and Cl−.189 Three classes of GABA receptors include GABA-A, GABA-B, and GABA-C, which transfer signals received from hormones, neurotransmitters, and pheromones. Among them, GABA-A190 and GABA-C191 receptors belong to ionic receptors, while GABA-B192 receptors are metabolic receptors. GABA-activating GABA receptors exert a depressant effect and alter behavior in the mammalian CNS.187 GABA-A receptors are the primary inhibitory neurotransmission receptors in the CNS, which means they are involved in the majority of brain physiological processes.193 Besides, gut nerve cells releasing GABA activate GABA-A receptors in the ENS instead of suppressing neurons in the CNS and raise the levels of intracellular chloride via sodium-potassium-chloride transporters.194 Oral administration of gut bacteria like Lactobacillus spp increases blood expression of GABA and the number of GABA-A receptors in the brain, which benefits intestinal health, stress-like behaviors, and growth performance.195,196
Glutamate in the gut-brain axis
Glutamate as the metabolic precursor of GABA is the most abundant excitatory neurotransmitter in the brain. Gut microbiota such as Bacteroides vulgatus Lactobacillus plantarum, Lactobacillus paracasei, Lactococcus lactis and Campylobacter jejuni produce glutamate, and improve cognitive functions and behavior.197 Glutamate regulates the gut-brain axis based on CNS and vagus nerve.198 Glutamate from daily diet or gut microbiota cannot cross the BBB in CNS. Its production in brains is dependent on the collaboration of neurons and astrocytes using intermediate metabolites of glycolysis, and phosphate-activated glutaminase from hydrolytic deamination of glutamine.199 Besides, a subgroup of intestinal enteroendocrine cells can also synthesize glutamate and transmit its downstream signals fast to the brain through the vagus nerve. Neuropod cells belonging to enteroendocrine cells synapse with the vagus nerve, boost expression of vesicular glutamate transporter 1 and produce glutamate to send sensory information from sugars in the gut to the brain in milliseconds, which may improve multiple brain diseases.200,201
Acetylcholine in the gut-brain axis
Acetylcholine as a gut bacteria-derived product is a common cholinergic neurotransmitter, which plays a local mediator in the central and peripheral nervous system by transmitting excitation signals between neurons.202 For example, increased acetylcholine relieves symptoms in AD,203 increases expression of TJs in the colon and hippocampal tissue,204 and prevents cognitive impairment. A variety of gut bacteria, such as Bacillus subtilis, Escherichia coli, Lactobacillus plantarum, and Staphylococcus aureus secrete acetylcholine.205 Among them, Bacillus subtilis releases more acetylcholine than Escherichia coli and Staphylococcus aureus does.206 Generally, acetylcholine cannot cross the BBB. Neurons in the CNS catalyze choline and acetyl-CoA to synthesize acetylcholine based on choline acetyltransferase. Meanwhile, peripherally derived choline crosses the barrier to reach the brain.207
Dopamine in the gut-brain axis
Dopamine as a gut bacteria-derived product is a kind of catecholamine neurotransmitter in the brain.208 It regulates various physiological functions in CNS-related diseases209 such as PD and schizophrenia. Dopamine and its receptors are found throughout the gut, where they influence intestinal functions like mucosal blood flow, gastric secretion and motility.210,211 Dopamine is rich in diet and can be transported to the brain through the BBB.212 Besides, the gut microbiota like Staphylococcus produces more than half of the dopamine in the body. They can absorb and convert the precursor l-3,4-dihydroxy-3phenylalanine into dopamine depending on the staphylococcal aromatic amino acid decarboxylase.
Norepinephrine in the gut-brain axis
Norepinephrine as a gut bacteria-derived product is another kind of catecholamine. Although its content is small, it acts as a neurotransmitter in the central and peripheral nervous system.213 It involves behavior and cognition such as memory, learning, attention, arousal, and alertness. It also triggers acute stress reactions in threatening situations. Norepinephrine is mainly synthesized and secreted by the adrenal medulla. In the brain, norepinephrine is produced by locus coeruleus neurons where the precursor of the neurotransmitter tyrosine is converted into dopamine, and finally forms norepinephrine. Importantly, changes in intestinal microbiota composition at low temperature can regulate the release of norepinephrine from the gut and brown adipose tissue of Lasiopodomys brandtii via the cAMP signaling pathway, thus helping to regulate energetics and thermogenesis.214
Trace amines in the gut-brain axis
Trace amines as gut bacteria-derived products contain β-phenylacetylene amine, p-pyrimidine, tryptamine, p-octopamine, and so forth.215 Although their abundance in the brain is very low, they are regarded as important nerve modulators or neurotransmitters. In common, trace amines are rich in ordinary food and can be produced and degraded by gut microbial.216 For example, Staphylococcus strains in the gut express staphylococcal aromatic amino acid decarboxylase, decarboxylate its corresponding aromatic amino acid substrates and synthesize three types of trace amines including tryptamine, tyramine, and phenethylamine through the decarboxylation of its corresponding aromatic amino acid substrates.216 Besides, Clostridium sporogenes and Ruminococcus gnavus in the gut decarboxylate tryptophan with their own tryptophan decarboxylase and produce tryptamine.217
Changing gut hormones and other microbial metabolites in the gut-brain axis
Gut hormones in the gut-brain axis
Microbiota-mediated enteroendocrine and enterochromaffin cells in the intestinal epithelium can release multiple gut hormones, including 5-HT, PYY, GLP-1, CCK, and ghrelin, which regulate multiple brain disorders, such as anxiety and depression.218,219
5-HT represents one of the most distinctive gut hormones and is generated by enterochromaffin cells. It possesses extensive receptor subtypes and gastrointestinal tract locations,220 including the stomach, small intestine, and large intestine, which regulates intestinal motility and perception of pain in the peripheral nervous system and modulates emotions, sleep, and appetite in the CNS.218 Despite peripheral and central 5-HT are generated differently and divided by the BBB, they are deeply related to CNS.221 First, tryptophan as a 5-HT precursor has multiple benefits for CNS and ENS functioning in the gut-brain axis. The metabolism of tryptophan based on the kynurenine pathway in the peripheral tissues has a significant impact on CNS.222,223 Second, 5-HT plays a crucial role in innate as well as adaptive immunity.224 A recent investigation discovers that endotoxin injection promotes the release of 5-HT from platelets into the plasma and further stimulates lymphocytes and monocytes to secrete cytokines and regulate CNS functioning.224,225 Third, 5-HT produced from enterochromaffin cells also alters vagal afferent action, which in turn changes the gut-brain axis. A recent study displays that chemotherapy causes a quick release of 5-HT release, and thereby induces nausea and emesis, which mainly depends on the stimulation of vagal afferents in the intestines.226,227
Stress-related diseases, neural protection, neurological inflammation, and neurogenesis are all affected by the PYY, which may activate Y4 receptors and then engage in anxiety and depression regulation.228 GLP-1 is well-recognized as a hormone that stimulates glucose-dependent insulin production, and also responds to brain diseases like PD and depression via the GLP-1 receptor. Importantly, endogenous or exogenous glucocorticoids decrease GLP-1 bioavailability.229,230
CCK is widely generated in the CNS and peripheral nervous system and is primarily involved in the regulation of calorie intake219 and anxiety-related actions.231,232 CCK affects neurotransmitters including glutamate, dopamine, acetylcholine, and GABA, which have an impact on the function of the brain.
Ghrelin, recognized for its adipogenic and orexigenic function, is discovered as a stress response, anxiety, and depression regulator.233,234 Numerous stressors such as restraint stress and social defeat raise the levels of ghrelin. Importantly, after hunger, the levels of ghrelin increase and cause stress adaption.235 Ghrelin receptor agonists enhance fear memory generated by stress while its antagonists decrease fear memory, demonstrating that ghrelin increases anxiety and depression-like behaviors.236
SCFAs in the gut-brain axis
SCFAs as gut bacteria-derived metabolites are transported from the gut to the CNS, cross the BBB with the bloodstream,237 and act as signals to affect host metabolism and immunity reaction, which has a substantial impact on human physical and mental health.238,239 For example, SCFAs act on homologous free fatty acid receptors or taste receptors, and regulate intestinal physiological functions including movement, secretion, and inflammation.240,241 Meanwhile, SCFAs pass through the BBB, enter the CNS, reduce LPS-induced neurological inflammation in primary microglia and hippocampus, and decrease circulating pro-inflammatory cytokines.242,243 Therefore, SCFAs are linked to a variety of disorders including anorexia, inflammatory bowel disease, neurological inflammation, and so forth.244,245
Other microbial metabolites in the gut-brain axis
Other microbial metabolites such as BAs and TMAO directly connect with the nervous system to maintain body growth and development.246,247
BAs are synthesized in hepatocytes, secreted into the intestine and metabolized by gut microbiota. They significant impact on the body, particularly on the brain.248,249 For example, gut dysbiosis causes secondary BAs shortage in patients with IBS and intensifies the pro-inflammatory mediators in the CNS, which can be reversed through the increase of secondary BAs activated by TGR5. This pathway is regulated by the increase of secondary BAs activated by TGR5.
Secondary BAs also stimulate FXR transcription in the ileum, and further trigger the synthesis of FGF19, which has the ability to move into the bloodstream, crosse the BBB, and trigger the hypothalamic ARC.250 Subsequently, the hypothalamus regulates glucose homeostasis and inhibits HPA function.251 By boosting GLP-1 secretion from L cells through TGR5 signaling, it has a vital function in managing glucose metabolism, and thereby influences the uptake behavior and food intake.23 In addition, restoring the gut BAs pool in mice with malnutrition increases the amount of gut intraepithelial lymphocytes like RORγt+ Treg cells, reduces the host’s sensitivity to colitis via BAs nuclear receptors, and lowers the risk of neurological inflammation.155 Additionally, gut dysbiosis causes secondary BAs shortage in patients with IBS and intensifies the pro-inflammatory mediators in the CNS, which can be reversed by TGR5, which increases secondary BAs.252
Besides BAs, TMAO is mostly produced by the gut microbiota through the metabolism of choline and betaine, and exerts a direct influence on the CNS.252 For example, TMAO accelerates brain aging and causes age-associated cognitive impairments.253 Moreover, microbiota-related TMAO directly communicates with the mammalian BBB, with implications for cerebrovascular and neurological health.254
Affecting immunity in the gut-brain axis
Innate immunity in the gut-brain axis
The nervous system and innate immune system have capacity to quickly identify and react to potentially harmful signals like TLRs for pathogen detection and other damage-associated molecules.255 Neuron interacts with three gut-resident innate immune cells, including macrophages, lymphoid cells and mast cells.256
Macrophages are present throughout the entirety of the gastrointestinal tract and are crucial for innate immunity. They connect with smooth muscle, capillary cells and glial cells, devour pathogens, absorb microbial products, and maintain ENS homeostasis.257,258 The unique population of macrophages known as muscularis macrophages regulate gut motility,259 release macrophage growth factors like colony-stimulating factor 1 (CSF1),260 and secrete bone morphogenetic protein 2 (BMP2) to alter gut peristalsis activity.261 Gut microbiota regulates the expression of BMP2 and CSF1 in intestinal nerves. Therefore, there is an easily modifiable microbiota-driven interaction between macrophages and intestinal nerves that regulate gastrointestinal motility.260
Although lymphoid cells share the same lymphoid progenitor with lymphocytes, they are regarded as barrier resident lymphocytes and belong to the innate immune system, which initially responds to tissue injury. As the innate counterparts of T cells, lymphoid cells do not have T cell receptors generated by antigen-specific receptor somatic cell recombination.262 However, they effectively regulate the host’s defense and immunological reaction.257,263 For the maintenance of gut homeostasis and inhibiting pathogen infection, neurons positively interact with both type II and type III lymphoid cells.264 Besides, they take an important part in the early stages of the immune reaction by swiftly reacting signals or cytokines generated by other cell types.265
Mast cells exist in the mucosal and submucosal layers of the intestine and have a tight anatomical relationship with sensory and autonomic nerve terminals.266,267 There are numerous receptors binding to typical neurotransmitters such as acetylcholine, corticotropin-releasing hormone, and neuropeptides including substance P, calcitonin gene-related peptides, and hemokinin in mast cells. The function of mast cells is influenced by those nerve-derived substances.268 For example, during stress, corticotropin releases hormone secretion, leads to hypercortisolism, promotes mast cell maturation, and induces neurogenic inflammation.269
Adaptive immunity in the gut-brain axis
Adaptive immunity plays an important roles in modulating interactions between the intestine and brain.270 LPS as an endotoxin exists in the cellular wall of gram-negative microbes that generates endotoxaemia, elicits an extensive immunological response and activates adaptive immune cells,271 such as B and T cells, which can serve as sensors of bacterial, present within the gut, convey signals to the enteric neural system and result in alterations to ENS.272
CD4+ and CD8+ T cells as core regulators of adaptive immunity interact with the peripheral nervous system.273 In the cholinergic anti-inflammatory reflex, the efferent nerve of the vagus nerve delivers the message to the abdominal ganglia, subsequently to the spleen through β2 adrenergic receptor and later conveys them to choline acetyltransferase+ T cells that generate acetylcholine.274 T cell-released acetylcholine acts on nicotinic acetylcholine receptors in macrophages and prevents the release of TNF.275
When B cells are activated, they transform from IgM-producing plasma cells to IgA-producing plasma cells, which increase the reaction of B cells to microorganisms and pathogens.276 It’s still uncertain whether particular neurons connect directly or indirectly with B cells in the gut. In mice with autoimmune encephalomyelitis, colonic motility is decreased, while glial fibrillary acid protein expression is increased and accompanied by increased immunoreactivity towards ENS neurons and glial cells likely due to B cell immunoglobulin synthesis increased.275
Mechanisms linking the liver-brain axis
Inter-organ communication between the liver and brain occurs via the signaling between the nervous and circulatory system.277 The mechanism of liver-brain axis mainly includes BBB permeability, vagus nerve, epigenetic regulation, toxic metabolites, β-amyloid (Aβ) metabolism, and immune response.
Changing BBB in the liver-brain axis
Changing the permeability of BBB by proinflammatory cytokines such as TNF and IL-1β in the liver causes the indiscriminate entry of toxins such as ammonia and xenobiotics, which produce a proinflammatory response.278 For example, BBB is destroyed in mice with acute liver failure, causes TNF and IL-1β to cross the BBB, and further impairs brain function.279
Changing vagus nerve in the liver-brain axis
The hepatic vagal sensory afferent nerves are responsible for indirectly sensing the liver microenvironment and relaying the sensory inputs to the nucleus tractus solitarius of CNS, and then feeding back to the liver vagal parasympathetic nerves.280,281 For example, the signals from the brain regulate VLDL triglyceride secretion and reduce hepatic lipid content via the vagus nerve.282 Besides, an exogenous vagal reflex activity connects hepatic vagal sensory inputs, brainstem, vagal efferents, and intestinal neurons.281
Epigenetic regulation in the liver-brain axis
Methylation is important for development, imprinting, transcriptional control, chromatin structure, and overall genomic stability.283 Hepatic DNA, RNA, and histone methylation are most likely involved in brain development.284 Preeclampsia causes changes in the DNA methylation of numerous critical regulatory genes in the fetal brain and liver, which indicates that liver-brain axis exists.285 Besides, one typical posttranscriptional regulator of mRNA is RNA N6methyladenosine, which is associated with brain activities.286 β-hydroxybutyrate is produced by the liver and goes through the bloodstream to the brain, where it inhibits histone deacetylases.287 Furthermore, injecting β-hydroxybutyrate to the brain results in an increase in brain-derived neurotrophic factor, which is used to treat mental conditions like depression and neurodegenerative disorders.288
MicroRNAs are a kind of tiny, tissue-specific, non-protein-coding RNA that maintains cellular homeostasis by regulation of negative genes in the liver-brain axis. MicroRNAs regulate hepatic lipogenesis and critical brain functions.289 Dysregulation of microRNAs is linked to a variety of liver and cerebral diseases. For example, miR212/132 is expressed in the brain and is sensitive to external cues from the liver. Brain-derived exosomes, known as transport microRNAs, are detected in the bloodstream and liver.290
Toxic metabolites in the liver-brain axis
Ammonia
Ammonia is involved in the pathophysiology of liver-brain axis.291 Astrocytes absorb hepatmogenic ammonia and convert it into glutamine. Glutamine buildup in astrocytes exerts an osmotic impact, causes cerebral edema and neuronal cell death via N-methyl-d-aspartate receptor overactivity, and leads to the formation of Alzheimer’s type 2 astrocytes, which have a bloated appearance and larger nuclei.292 Ex vivo feeding of ammonium salts to healthy animals causes microglial activation as well as elevates expression levels of IL-1β in the brain.293 There is also a strong relationship between circulating ammonia and the levels of TNF in individuals caused by chronic liver failure.292 For example, proinflammatory gene like TNF-α up-regulates in the brains of cirrhotic hepatic encephalopathy (HE) individuals.294 Meanwhile, TNF-α exposing to human cerebrovascular endothelial cells increases ammonia absorption.295
Lactate
The brain concentration of lactate is increased in the acute and chronic liver failure models,296 which correlates with the severity of clinical symptoms, electroencephalogram spectral abnormalities, and degree of microglial activation.297 At the coma phases of encephalopathy in liver failure, brain concentration of lactate reaches 10–12 mM,298 which triggers high concentration of TNF and IL-6 released from microglial cell. Lactate accumulation in the brain is also linked to the ammonia-inhibited ketoglutarate dehydrogenase and phosphofructokinase 1.299
Manganese
Manganese deposition in the basal ganglia region of the brain usually occurs in cirrhotic individuals,300 because of the poor hepatobiliary metal removal and portal-systemic shunting. Manganese deposition is also associated with bilateral T1-weighted signal hyperintensities on magnetic resonance imaging (MRI) as well as dopaminergic cell death in these tissues, providing a compelling explanation for the high occurrence of parkinsonism in cirrhosis. There is substantial evidence that neuroinflammatory processes are involved in the neurotoxic effects of manganese.301 Manganese, for example, is proposed to regulate inflammatory cytokine output from microglia as well as to induce microglia to emit hydrogen peroxide and nitric oxide.302
Aβ metabolism in the liver-brain axis
Imbalanced Aβ generation and clearance are hypothesized to play an important roles in the development of AD.303 The Liver is the main site for peripheral Aβ metabolism whose disorder may lead to AD progression.304 These disturbances are further exacerbated by the pro-inflammatory condition that frequently accompanies liver illnesses, resulting in neuroinflammation.305 Meanwhile, the present eating habits like the Western diet change the bile acid profile in the liver, and also link to both AD and PD. Supplementation with Aβ ameliorates these diseases.306
Besides, Aβ metabolic disorders cause oxidative stress and inflammation, which further lead to chronic hepatic and neural diseases.305 For example, hepatic oxidative inflammation is characterized by the dysregulation of antioxidant enzymes and the HPA axis, as well as the release of pro-inflammatory cytokines such as IL-6 and TNF-α. The oxidative stress factors in the liver are linked to neophobia. These changes might pave the way for a novel route and the identification of prospective integrative system targets for liver-brain axis research.307
Immunity in the liver-brain axis
Some immune cells such as macrophages, dendritic cells and lymphocytes release proinflammatory factors like TNF-α and IL-1β. These inflammatory factors further promote the release of secondary messengers such as prostaglandins and nitric oxide from cerebral endothelial cells, and cause alterations inside the brain.308 For example, the proinflammatory cytokines such as TNF-α and IL-1β induce the release of the inducible nitric oxide synthase (NOS) isoform from macrophages and cerebral endothelial cells.309 In this process, nitric oxide is produced through the NOS-oxidized L-arginine in endothelial and neuronal cells.310 In addition, inhibition of NOS promotes anxiolytic effects in rats.311
Microglial activation usually attracts monocytes into the brain, and causes chronic inflammatory diseases in patients with liver failure.312,313 For example, microglia releases TNF and triggers monocyte recruitment in livers. Microglia also secretes chemokine (CC-motif) ligand 2, facilitates liver monocyte migration into the brain,314 and causes neurological problems in mice with biliary cirrhosis. These discoveries represent a new liver-brain communication route, which leads to increased neuronal excitability and neurological problems in liver disorders.
IL-6 as another proinflammatory factor influences brain function via liver-brain axis. The hepatocytes and leukocytes generate IL-6 and express the IL-6 receptor (IL-6R) in the cell surface when they are activated. The IL-6/IL-6R complex subsequently interacts with the transmembrane glycoprotein which exists on the cerebral endothelial cells, and initiates the signaling cascade.315,316 For example, bile duct ligation induces hepatic inflammation and sickness behaviors accompanying with the increased levels of hepatic IL-6 and circulatory IL-6. The sickness behaviors are significantly reduced in IL-6 deficient mice and increased by intravenous injection of recombinant IL-6.317
Therapies targeting the gut-liver-brain axis
Antibiotics application
Antibiotics, especially non-absorbable antibiotics, mainly stay in the intestine, regulate the intestinal microbiota and affect the gut liver brain axis disease progression.318 For example, rifaximin as a non-absorbable, broad-spectrum and gastrointestinal-specific antibiotic display effective and safe in biopsy-proven NAFLD.319 It decreases serum levels of endotoxin, proinflammatory cytokines and cytokeratin (CK)-18, but has no effect on the hepatic lipid content, body mass index (NCT02884037), the serum levels of alanine aminotransferase (ALT), peripheral glucose uptake or hepatic insulin sensitivity (EudraCT 2010-021515-17).320 Meanwhile, rifaximin treatment significantly decreases the relative abundance of gut microbiota including Peptostreptococcaceae, Verrucomicrobiaceae and Enterobacteriaceae, but belongs to a minor, temporary effect on a wide variety of gut bacteria in a 2-week open-label IBS clinical trial (Table 3).321 Other multiple randomized controlled trials with rifaximin also indicate its minor therapeutic improvements in patients with IBS.322 The fact that gut microbial dysbiosis is not a causal factor in IBS symptoms. Several negative trials of fecal microbial transplantation in patients with IBS provide similar data.323 Therefore, further study should reveal if rifaximin impacts IBS.
Solithromycin as a potent next-generation macrolide antibiotic reduces NAFLD activity score (NAS) and the levels of ALT in a phase II 13-week open-label NASH trial (NCT02510599). It also promotes the proliferation of some common gut microbiota like Bifidobacteria and Lactobacilli in short-term treatment,324 but disturbs flora balance in long-term studies (Table 2).325 Additionally, some antibiotics like ampicillin and amoxicillin increase the risk of endocarditis326 and bacteremia.327
Probiotics, prebiotics and synbiotics application
Probiotics are regarded as an adequate number of live microorganisms exerting beneficial effects on the host.328 The most commonly used probiotics in current studies contain Lactobacilli, Streptococci, and Bifidobacteria, which significantly decrease the development of liver and brain-related diseases100 such as NAFLD, NASH, ASD, depression, PD, schizophrenia, epilepsy, migraine, and so on.329 For example, VSL#3 as a probiotic mixture consists of eight distinct microbes such as Bifidobacterium breve, Bifidobacterium infantis, Bifidobacterium longum, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus plantarum, Lactobacillus casei, and Streptococcus thermophilus.31 It’s employed in NAFLD and obese children for four months. In the end, VSL#3 supplementation activates GLP-1, and alleviates fatty liver and body mass index (NCT01650025).330 Multistrain probiotics treatment including Bifidobacterium bifidum, Bifidobacterium longum, Enterococcus faecalis, Enterococcus faecium, Lactobacillus acidophilus, Lactobacillus gasseri, Lactobacillus reuteri, and Lactobacillus rhamnosus displays effective for the treatment of constipation in PD (NCT03377322).32 Besides, leptin as a probiotic substance affects gut microbiota and vagus nerve, which plays an important role in liver and brain function. Leptin action in the liver exerts its anti-steatotic effects and promotes a decreased Firmicutes and an increased Bacteroidetes in the intestine. Leptin action in the CNS also exerts its anti-steatotic effects by increasing hepatic triglyceride secretion and reducing liver de novo lipogenesis (DNL), which requires intact vagal innervation of the liver. In a randomized, placebo-controlled crossover trial, leptin protects from hepatic steatosis independently of food intake by stimulating VLDL secretion and reducing hepatic DNL via a vagal mechanism (EudraCT Nr. 2017-003014-22). Besides, netrin-1 accelerates liver regeneration after partial hepatectomy in mice, and the potential mechanism is related to the promotion of vagus nerve repair and regeneration.331 Therefore, leptin targeting the gut-liver-brain axis is supposed to become a promising drug in the future.
In another clinical study, the treatment of Lactobacillus bulgaricus and Streptococcus thermophilus drastically decreases the levels of ALT, aspartate aminotransferase (AST) and γ-glutamyltransferase in patients with NASH (Table 2).332 In addition, some probiotics also display effectiveness for the treatment of persistent gastrointestinal symptoms and depression in patients with IBS333,334 neurophysiological patterns in patients with ASD,335 and other depressive symptoms.336,337
Prebiotics contain no live microbes and nondigestible dietary components that promote the formation of indigenous microbiota in liver and brain diseases.338,339 Generally, prebiotics boosts bacterial metabolites of SCFAs, promote the growth of indigenous Bifidobacteria and Lactobacilli as well as other beneficial bacterial species, lower luminal pH, increase expression of GLP-2,340 and prevent pathogen growth and endotoxin transfer in liver disease,341 anxiety and depression.342 For example, some soluble fibers alter the neuroendocrine stress response and regulate the processing of information that is significantly associated with anxiety and depression.342 Oligofructose and inulin-type fructans as common prebiotics boost the abundance of Bifidobacterium spp and dramatically reduce liver steatosis and NAS (NCT03184376 and NCT03042494).343,344 Meanwhile, galactooligosaccharides reduce the neuroendocrine response to stress and enhance the processing of positive over negative attentional vigilance in patients with stress-related disorders.33
Synbiotics as a mixture of prebiotics and probiotics improve multiple gut liver brain axis-related diseases. One symbiotic with 28-week treatment includes 200 million bacteria of seven strains such as Bifidobacterium breve, Bifidobacterium longum, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus casei, Lactobacillus rhamnosus, and Streptococcus thermophilus, prebiotics like fructooligosaccharide, and Vitamin A, C and E leads to a substantial decrease of aminotransferases, liver inflammation, and fibrosis formation in patients with NAFLD (NCT01791959)345 (Table 2). Meanwhile, the treatment of Bifidobacterium longum and fructooligosaccharide significantly reduces hepatic fat formation and the NASH activity index compared with lifestyle modification treated alone.346 Besides, some other synbiotics reduce parts of blood lipid markers in patients with obese,347 regulate metabolite synthesis and have complex effects on cognitive, affective, and neurological factors associated with health and illness.348
Fecal microbiota transplantation application
Fecal microbiota transplantation (FMT) as a novel strategy to treat gut liver brain axis-related diseases involves transferring gut microbiota from a healthy donor to a damaged recipient. Firstly, the gut microbiota can be rebuilt in liver disease (Table 2).349 Two studies (NCT03803540 and NCT02469272), not yet recruiting, are registered to explore the potential advantages of FMT on hepatic histological abnormalities (NCT03803540) and MRI-assessed steatosis (NCT02469272). Although promising, it’s required to further assess FMT treatment on liver histological abnormalities in the early stages of NASH and determine whether it delays NASH development. Secondly, FMT rebuilds a healthy microbial composition and displays positive effects on PD through the gut-brain axis. In rotenone-induced gut dysbiosis, FMT therapy repairs gut microbiota dysbiosis and suppresses inflammation induced by the LPS-TLR4 signaling pathway both in the gut and brain.350 Besides, the microbiota from AD mice impairs neurogenesis by increasing colonic inflammation, which contributes to memory loss.351 FMT from senescence-resistant mice to AD mice improves spatial learning and memory.352
Other diets application
Polyphenols as plant-derived components are major metabolized by intestinal microbiota in the colon353 and benefit in many metabolic-related diseases such as type 2 diabetic,354 NASH,355 NAFLD,356 aging, and so on. Therefore, the high percentage of polyphenols is now recommended by the European Association for the Study of Diabetes, European Association for the Study of Obesity, and European Association for the Study of the Liver guidelines for the people with gut-liver-brain axis-related diseases. For example, cranberry extract reverses the high fat & high sucrose-induced gut microbiota alterations (Akkermansia spp.) and improves metabolic syndrome (Table 2).357 Green-Mediterranean diet, amplified with polyphenols and unsaturated fat acids, reduces lipid accumulation and improves NAFLD.358 Besides, a diet rich in polyphenols reduces liver fat accumulation through the inhibition of de novo lipogenesis (NCT03380416).359 Dietary polyphenols, such as isoflavone, lignans, and their metabolites derived from intestinal microorganisms can cross the intestinal barrier and the BBB and prevent neuroinflammatory stimulation.360 Besides, tea polyphenol (-)-epigallocatechin-3-gallate weakens the HPA axis, increases the content of SCFAs, regulates gut-brain communication, and alleviates aging impairment.361
The low-FODMAP diet including fermented oligosaccharides, monosaccharides, disaccharides, and polyols362 is regarded as the first-line therapy for IBS.363 It’s used for short-term therapy of certain IBS symptoms but is not utilized as a long-term treatment.364,365 Short-term therapy of FODMAP reduces dietary consumption which outperforms antispasmodic medication or moderate FODMAP diet (NCT05182593 and NCT02667184)366,367 in alleviating IBS symptoms. Meanwhile, this diet results in less gas and less active microbial metabolite production, which alleviates the sensation of bloating, flatulence, and pain.368,369 On the contrary, the compliance of its long-term therapy is poor,370,371 which causes a drop in gut microbial diversity and richness, notably of butyrate-producing strains, and has detrimental effects on gut health.372
Nanotechnology application
Nanotechnology is constantly developing and improving in the diagnosis and treatment of gut liver brain axis-related diseases. It can manipulate interactions across microscopic and molecular length scales in the microbiome and has the potential to noninvasive and real-time microorganism intervention technique in gut liver brain axis-related diseases. For example, a gut-liver-axis chip contains the gut epithelial cell chamber and a three-dimensional uniform-sized liver spheroid chamber. Its two chambers are separated by a porous membrane to let the hepatocytes in but inhibit microorganisms entering the chamber.373 Nano-poly-boronic acid regulates sugar intake and liver lipogenesis, and finally prevents fructose and glucose absorption in the gut.374 In addition, certain microorganisms’ components are prepared into nanotechnology like light-sensitive Lactococcus lactis which is an oral live biotherapeutic agent that makes communication from the gut to the host more manageable. This engineered microorganism enhances small intestine targeting and exogenous Lactococcus lactis production, allowing for precise regulation of anxiety, vagal afferent and cognitive impairment.375 Besides, the honokiol nanoscale drug delivery system also regulates gut microbiome composition and decreases tau hyperphosphorylation, neurological inflammation and Aβ deposition.376 Therefore, they are a noninvasive and real-time microorganism intervention technique.
Therapies targeting gut-liver axis
Targeting BAs-related pathways
Nowadays, although BAs involved in the pathogenesis of gut-liver and gut-brain axis, targeting BAs-related pathways is only used in liver-related diseases in clinics. BAs metabolism in the gut-liver axis is regulated by two main receptors including FXR and TGR5. FXR activation inhibits BAs production and BAs influx, promotes BAs efflux, and thus alleviates the excessive accumulation of BAs caused by liver disease.377 Currently, the most widely used FXR agonists contain primarily BA derivatives, steroidal compounds, and nonsteroidal compounds (Table 2).
As an FXR agonist, UDCA as a primarily BAs derivative, is used to treat cholestatic liver diseases. It has been proposed as a possible treatment for NASH and NAFLD. However, its clinical effectiveness must be validated further.378
Obeticholic acid as a steroidal FXR agonist reduces fibrosis and essential NASH features in a phase III trial (NCT02548351).379 However, it causes side effects, such as mild to moderate itching, a decrease of high-density lipoprotein-cholesterol (HDL-C), an increase of low-density lipoprotein-cholesterol (LDL-C), and drug-induced hepatotoxicity.380 EDP-305, another powerful steroidal FXR agonist, lowers the levels of hepatic ALT and fat in phase IIa clinical trial (NCT03421431). Its adverse events are the same as that of obeticholic acid such as pruritus, nausea, vomiting, diarrhea, headache, and dizziness.381
Nonsteroidal FXR agonists are constantly emerging in clinical studies. Among these compounds, cilofexor and TERN-101 (NCT04328077) display positive effects in patients with NASH in phase I and II clinical trials.382,383 In a phase I study, although cilofexor has no effect on cholesterol, it dose-dependently reduces the level of FGF19 (NCT02654002). Meanwhile, cilofexor shows well-tolerated and effective in the reduction of hepatic steatosis, liver biochemistry, and serum BAs in a phase II clinical trial (NCT02854605).382 However, some of the nonsteroidal FXR agonists display negative and inconclusive effects. For example, nidufexor fails to improve the level of ALT in a phase II clinical trial (NCT02913105). Tropifexor is also terminated in a phase II clinical trial because of its mild pruritus and minor dose-related increase in LDL (NCT02855164). MET409 being evaluated for the treatment of NASH by Metacrine Investigative Site lowers liver content of fat, but still induces differentiated pruritus and LDL-C profile.384 The safety and efficacy of EYP001a in patients with NASH are also evaluated in a phase IIa trial (NCT03812029). However, its results are not published up to now.
TGR5 as another BAs receptor exists on the membrane of L cells and influences BAs homeostasis through the gut-liver axis. TGR5 agonists include LCA, DCA, the semi-synthetic BAs, and so on (Table 2).385,386 For example, INT-767 and INT-777 as semi-synthetic BAs activate cAMP, stimulate secretion of GLP-1, and improve hepatic glucose and lipid metabolism in NASH/NAFLD.387
Omega-3 fatty acid as one kind of N-3 PUFA (omega-3 polyunsaturated fatty acids) lowers liver fat and influences BAs metabolism in a variety of ways.388 However, it fails to improve the primary outcome of histological activity in patients with NAFLD (NCT00681408). Now there are another two completed phase II clinical trials without published results involved in Omega-3 fatty acid treatment (NCT01056133 and NCT00845845). Moreover, dietary docosahexaenoic acid as another polyunsaturated fatty acid attenuates blood lipid levels, liver damage and reverses liver metabolism, oxidative stress, and fibrosis formation in NAFLD, which is superior to dietary eicosapentaenoic acid.389
Targeting intestinal mucosa secretions
TGR5 can be stimulated by dietary ingredients and hormonal variables such as insulin and leptin to further trigger the release of gut-derived incretin hormones like GLP-1 (Table 2).390 Although gut hormones also involved in the pathogenesis of the gut-liver and gut-brain axis, targeting intestinal mucosa secretions is only used in clinical liver-related diseases. Meanwhile, GLP-1 receptor antagonists such as liraglutide, semaglutide, ALT-801, and dulaglutide promote pancreatic insulin production and inhibit glucagon secretion in protecting against NAFLD development. Endogenous GLP-1 is degraded by dipeptidyl peptidase-4 in minutes. Long-acting human GLP-1 analogs include liraglutide and semaglutide.391 Liraglutide displays safe, adequately tolerated, and increased either hepatic and global/localized adipose insulin sensitivity, resulting in lowering the blood quantity of lipotoxic metabolites and inflammatory cytokines in a phase II clinical trial (NCT01237119).392,393 Semaglutide possesses a similar mechanism to that of liraglutide and has more dramatic impacts on metabolism and bodyweight reduction in NASH patients than liraglutide does.394 However, it is unable to ameliorate the fibrosis stage (NCT02970942).395,396 Moreover, microbiota analysis illustrates that GLP-1 receptor antagonists alter the variety of gut microbiota by decreasing the relative abundance of Proteobacteria and increasing the relative abundance of Akkermansia muciniphila, which are associated with the treatment of NAFLD.397
FGF19 is released by intestinal cells of the terminal ileum following FXR activation by BAs. FGF19 flows from the intestine into the liver through portal vein circulation and combines with FGFR4 and β-klotho to reduce the production of BAs. FGF19 analogs (NGM282/Aldafermin) modulate BAs production, lipid metabolism, and gluconeogenesis. BAs-activated FXR increases FGF19 gene expression and production.398 NGM282 demonstrates an adequate safety profile in patients with NASH in a 12-week phase IIa trial. It decreases the levels of liver fat, and improves NAS, fibrosis scores, and other liver function indicators (NCT02443116).399 Further stage II research with patients with NASH is active but not recruiting (NCT03912532).
Novel therapeutic applications
Intestinal permeability and microbiota-targeting therapy
Claudins, especially claudin-2 plays a crucial role in the formation of gated paracellular channel and regulation of TJs channels, which may be ideal therapeutic targets for affecting the epithelial barrier and improving intestinal permeability.400 Importantly, occludin S408 dephosphorylation regulates TJs channel gating dynamics and protein molecule interaction, which have therapeutic significance for inflammation-related intestinal barrier disorders.401 However, more clinical studies are needed to determine pharmacological methods for regulating gating activity for therapeutic purposes. In addition, microRNA-155 regulates NF-κB signal, reduces expression of TNF-α, IL-6, ZO-1, and occludin, and then inhibits inflammation and intestinal barrier dysfunction in mice.402
Non-selective beta blockers (NSBBs) play important roles in the management of portal hypertension in liver cirrhosis during the last three decades. NSBBs increase levels of intestinal permeability and bacterial translocation indicators like IL-6/LPS binding proteins.403 They improve intestinal hypomotility in the setting of sympathetic activity, minimize the overgrowth development of small intestine bacteria and are associated with a lower incidence of spontaneous bacterial peritonitis in cirrhosis.404
Bacteriophages as viruses are constantly developed to selectively infect and destroy the defensive system of bacteria.405 Because bacteriophages operate in an entirely orthogonal action mode in comparison with antibiotics, they do not meet their resistance, and even effectively destroy the extremely antibiotic-resistant bacteria.406 Therapeutic bacteriophages delivery under compassionate procedures has antibacterial effect in terminally ill patients with Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species infections.407,408 Therefore, many biotechnological corporations pay attention to this technology and plan to convert it into clinical practice.409
LPS-targeting therapy
High density lipoprotein (HDL) is synthesized in the liver and small intestine where its core structural protein, apolipoprotein A1 (apoA1) is synthesized. HDL removes endotoxins, prevents infection,410 neutralizes LPS, and speeds LPS clearance in mice via SR-BI (scavenger receptor class B, type I)-mediated LPS absorption. LPS attaching to apolipoprotein AI or apolipoprotein E inhibits inflammatory response, while apolipoprotein AII or apolipoprotein CI binding to LPS promotes inflammation. Besides, intestine-derived HDL3 has a unique role in the prevention of liver damage from gut-derived LPS, implying that HDL3 might be a target for treating liver illness linked with gut leakiness.411
Yaq-001 as a newly synthesized non-absorbable carbon has a high adsorption capacity for endotoxin and LPS. Yaq-001 treatment increases the composition and function of the microbiome, as well as the function of circulating innate immune cells in rats with bile duct ligation. Meanwhile, it is regarded as a new strategy to resist the change of gut microbiota and bacterial product translocation in patients with advanced liver disease.412 Therefore, it is mostly given orally in tiny bags and tested in clinical research on decompensated liver cirrhosis (NCT03202498).
Therapies targeting the gut-brain axis
Some therapeutic measures play a special role in gut-brain axis metabolism, such as cognitive behavioral therapy (CBT), antidepressants, and therapies targeting tryptophan metabolism.
Cognitive behavioral therapy
CBT as a viable therapeutic option for IBS symptoms413 reduces anxiety, stimulates health-promoting habits, takes greater responsibility, controls over their treatment and enhances pain tolerance.414 Recent research assesses the effectiveness of clinic-based CBT, home-based CBT, and IBS education for treating IBS symptoms, quality of life changes in feces consistency, emotional distress, and satisfaction with treatment (Table 3).415 As 12-month clinical trial indicates, both clinic-based or home-based CBT reduce IBS symptoms, while IBS education does not display these benefit.416 Patients who received home-based CBT are more likely to persevere owing to their self-monitoring, self-learning and correction of inaccurate threat assessments by imbalanced gut-brain relations.416,417 Furthermore, CBT reduces inappropriate illness cognitions, triggers shift in self-processing, decreases biases in self-referent illness and health processing, and enhances awareness without judgment in patients with IBS (NCT02794376).418
Novel therapeutic applications
Antidepressants application
Antidepressants as neuromodulators at low doses regulate brain-gut communication. Treating inflammatory bowel disease (IBD) involves in activation of vagus nerve-mediated anti-inflammatory capabilities and direct influences on pro-inflammatory cytokines. The primary impact on cytokines depends on the NF-κB and nitric oxide pathway, both of which promote IBD development.419 In a multicenter clinical trial, 5-hydroxytryptophan as one kind of antidepressant significantly regulates depression, anxiety, and stress scores, but not improves IBD-related fatigue (NCT03574948).420
Targeting tryptophan metabolism application
The microbiota has a direct or indirect influence on the three primary tryptophan metabolites including serotonin, kynurenine, and indole derivatives in the gut,421 which is associated with intestinal inflammation and IBD. Reintroducing xanthurenic and kynurenic exerts a defensive impact via reorganizing energy metabolism in aryl hydrocarbon receptors, intestinal epithelial cells, and CD4+ T cells. Therefore, targeting tryptophan metabolism repairs abnormalities in endogenous metabolic pathways in IBD.422
Therapies targeting the liver-brain axis
Targeting epigenetic regulation
Epigenetic regulation in the liver-brain axis, like RNA methylation, should be mentioned in the treatment of liver and brain-related diseases.423 Exercise-mediated restoration of m6A methylation in the mouse medial prefrontal cortex, whose activity is potentiated to produce anxiolytic effects, is demonstrated by a combination of molecular, behavioral, and in vivo recording data. Furthermore, it demonstrates that hepatic manufacture of one methyl donor is required for exercise to enhance brain RNA m6A methylation in order to offset environmental stress. Through the liver-brain axis, exercise training brings fresh insights into the diagnosis and treatment of anxiety disorders.286
Targeting Aβ metabolism
Hepatic soluble epoxide hydrolase targets Aβ metabolism and significantly treats neurological diseases in the liver-brain axis. It bidirectionally regulates the plasma levels of 14,15-epoxyeicosatrienoic acid, which rapidly crosses the BBB and modulates brain Aβ metabolism via multiple pathways. Therefore, hepatic soluble epoxide hydrolase attenuates brain burden, tauopathy, and cognitive deficits, which may be a potential treatment method for liver-brain diseases.424
Future perspective
In the past decades, gut-liver-brain axis communication has become an important research topic.25,425 Researchers make efforts to analyze their communication mechanism among all nodes in the axis.4,426 Now, it is widely accepted that the gut, liver, and brain maintain a stable balance is beneficial for multiple disease progression.427,428,429
The disorder of the gut-liver-brain axis influences disease development and progression, which includes the change of intestinal permeability, SCFAs, FIAF, choline, ethanol, BAs metabolism, BBB, vagus nerve, neurotransmitters, gut hormones, microbial metabolites, immunity, ammonia, lactate and manganese accumulation, epigenetic regulation and Aβ metabolism. Clinical trials using antibiotics, probiotics, prebiotics, synbiotics, FMT, polyphenols, and novel nanotechnology confirm the critical role of the gut-liver-brain axis in different diseases. Besides, some special treatments such as FXR agonists, TGR5 agonists, GLP-1 receptor antagonists, FGF19 analogs have a beneficial effect on the maintenance of gut-liver axis balance. Most importantly, FXR agonists and probiotics enters phase III clinical studies, which increased some bacteria such as Bifidobacterium and Lactobacilli, and displays positive therapeutic effects. The emergence of some promising therapies including intestinal permeability and microbiota and LPS-targeted treatment like claudins, NSBBs, bacteriophages, HDL and Yaq-001 also provides impetus for future multiple liver disease treatments. Moreover, some special therapies regulating the gut-brain axis include CBT, antidepressants and tryptophan metabolism targeted therapy, which also display positive effects in brain diseases. So far, low FODMAP diets and CBT have entered the phase IV and III clinical studies. They exhibit useful for short-term treatment of certain IBS symptoms in multiple clinical trials. Furthermore, emerging manipulations such as antidepressants and tryptophan metabolism targeted therapies may be providing many opportunities for the treatment of gut-brain axis-related diseases. In addition, some promising therapies targeting the liver-brain axis include targeting epigenetic regulation and Aβ metabolism, which opens a new path for future development.
In conclusion, the gut liver brain axis plays an indelible role in influencing multiple diseases. According to the summary of these mechanisms, some metabolites including LPS, SCFAs, BAs, immunity, some inflammatory cytokines and beneficial bacteria such as Bifidobacterium and Lactobacillus significantly influence the pathogenesis of gut liver brain axis-related diseases. So far, high-quality preclinical research and some randomized controlled trials have demonstrated the effectiveness of some therapies based on the theory of gut liver brain axis. It is expected to further develop more clinical candidates to regulate the gut liver brain axis and treat their related diseases.
References
de Vos, W. M., Tilg, H., Van Hul, M. & Cani, P. D. Gut microbiome and health: mechanistic insights. Gut 71, 1020–1032 (2022).
Mayer, E. A., Nance, K. & Chen, S. The gut-brain axis. Annu. Rev. Med. 73, 439–453 (2022).
Tilg, H., Adolph, T. E. & Trauner, M. Gut-liver axis: pathophysiological concepts and clinical implications. Cell Metab 34, 1700–1718 (2022).
Silveira, M. A. D. et al. The gut-liver axis: host microbiota interactions shape hepatocarcinogenesis. Trends Cancer 8, 583–597 (2022).
Liu, L., Huh, J. R. & Shah, K. Microbiota and the gut-brain-axis: Implications for new therapeutic design in the CNS. EBioMedicine 77, 103908 (2022).
Leung, C., Rivera, L., Furness, J. B. & Angus, P. W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 13, 412–425 (2016).
Bajaj, J. S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16, 235–246 (2019).
Marra, F. & Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 68, 280–295 (2018).
Park, J. W. et al. Role of microbiota-derived metabolites in alcoholic and non-alcoholic fatty liver diseases. Int. J. Mol. Sci. 23, 426 (2021).
Kang, Y., Cai, Y. & Yang, Y. The gut microbiome and hepatocellular carcinoma: implications for early diagnostic biomarkers and novel therapies. Liver Cancer 11, 113–125 (2022).
Bence, K. K. & Birnbaum, M. J. Metabolic drivers of non-alcoholic fatty liver disease. Mol. Metab. 50, 101143 (2021).
Tan, A. H., Lim, S. Y. & Lang, A. E. The microbiome-gut-brain axis in Parkinson disease - from basic research to the clinic. Nat. Rev. Neurol. 18, 476–495 (2022).
Doifode, T. et al. The impact of the microbiota-gut-brain axis on Alzheimer’s disease pathophysiology. Pharmacol. Res. 164, 105314 (2021).
Mao, X. Y. et al. Dietary nutrition for neurological disease therapy: Current status and future directions. Pharmacol. Ther. 226, 107861 (2021).
Yu, Y. & Zhao, F. Microbiota-gut-brain axis in autism spectrum disorder. J. Genet. Genomics 48, 755–762 (2021).
Honarpisheh, P., Bryan, R. M. & McCullough, L. D. Aging microbiota-gut-brain axis in stroke risk and outcome. Circ. Res. 130, 1112–1144 (2022).
Simpson, C. A. et al. The gut microbiota in anxiety and depression - A systematic review. Clin. Psychol. Rev. 83, 101943 (2021).
Wang, S. C. et al. Alcohol Addiction, Gut Microbiota, and Alcoholism Treatment: A Review. Int J Mol Sci. 21, (2020).
Dai, X. & Wang, B. Role of gut barrier function in the pathogenesis of nonalcoholic Fatty liver disease. Gastroenterol. Res. Pract. 2015, 287348 (2015).
Wang, P. Y. et al. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature 452, 1012–1016 (2008).
Zmora, N., Suez, J. & Elinav, E. You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16, 35–56 (2019).
Muhammad, F. et al. The molecular gut-brain axis in early brain development. Int J Mol Sci 23, 15389 (2022).
Martin, C. R., Osadchiy, V., Kalani, A. & Mayer, E. A. The brain-gut-microbiome axis. Cell Mol. Gastroenterol. Hepatol. 6, 133–148 (2018).
Bauer, K. C. et al. Nonalcoholic fatty liver disease and the gut-liver axis: exploring an undernutrition perspective. Gastroenterology 162, 1858–1875.e1852 (2022).
Bajaj, J. S., Ng, S. C. & Schnabl, B. Promises of microbiome-based therapies. J. Hepatol. 76, 1379–1391 (2022).
Brescia, P. & Rescigno, M. The gut vascular barrier: a new player in the gut-liver-brain axis. Trends Mol. Med. 27, 844–855 (2021).
Socala, K. et al. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharm. Res. 172, 105840 (2021).
Thompson, R. J. et al. Odevixibat treatment in progressive familial intrahepatic cholestasis: a randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol. Hepatol. 7, 830–842 (2022).
Zhu, J. H. et al. Efficacy and safety of vibrating capsule for functional constipation (VICONS): a randomised, double-blind, placebo-controlled, multicenter trial. EClinicalMedicine 47, 101407 (2022).
Wang, X. et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res 29, 787–803 (2019).
Jena, P. K., Sheng, L., Li, Y. & Wan, Y. Y. Probiotics VSL#3 are effective in reversing non-alcoholic steatohepatitis in a mouse model. Hepatobiliary Surg. Nutr. 9, 170–182 (2020).
Tan, A. H. et al. Probiotics for constipation in parkinson disease: a randomized placebo-controlled study. Neurology 96, e772–e782 (2021).
Schmidt, K. et al. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 232, 1793–1801 (2015).
Huang, H. et al. Integrated analysis of microbiome and host transcriptome reveals correlations between gut microbiota and clinical outcomes in HBV-related hepatocellular carcinoma. Genome Med 12, 102 (2020).
Marascio, N. et al. The role of the microbiota gut-liver axis during HCV chronic infection: a schematic overview. J Clin Med 11, 5936 (2022).
Han, H. et al. Intestinal dysbiosis in nonalcoholic fatty liver disease (NAFLD): focusing on the gut-liver axis. Crit. Rev. Food Sci. Nutr. 63, 1689–1706 (2023).
Kong, L. et al. Alcoholic fatty liver disease inhibited the co-expression of Fmo5 and PPARalpha to activate the NF-kappaB signaling pathway, thereby reducing liver injury via inducing gut microbiota disturbance. J. Exp. Clin. Cancer Res. 40, 18 (2021).
Gong, S. et al. Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice. J. Hepatol. 69, 51–59 (2018).
Luo, W. et al. Hepatocellular carcinoma: how the gut microbiota contributes to pathogenesis, diagnosis, and therapy. Front. Microbiol. 13, 873160 (2022).
Weiss, G. A. & Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol. Life Sci 74, 2959–2977 (2017).
Hu, H. et al. Intestinal microbiome and NAFLD: molecular insights and therapeutic perspectives. J. Gastroenterol. 55, 142–158 (2020).
Safari, Z. & Gerard, P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol. Life Sci 76, 1541–1558 (2019).
Pujo, J. et al. Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis. Gut 70, 1088–1097 (2021).
Sherriff, J. L. et al. Choline, its potential role in nonalcoholic fatty liver disease, and the case for human and bacterial genes. Adv. Nutr. 7, 5–13 (2016).
Alvares, D., Hoffman, S., Stankovic, B. & Adeli, K. Gut peptide and neuroendocrine regulation of hepatic lipid and lipoprotein metabolism in health and disease. Biochim Biophys. Acta Mol. Cell Biol. Lipids 1864, 326–334 (2019).
Zhu, L. et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 57, 601–609 (2013).
Arab, J. P. et al. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology 65, 350–362 (2017).
Lackner, C. & Tiniakos, D. Fibrosis and alcohol-related liver disease. J. Hepatol. 70, 294–304 (2019).
Gerard, P. Gut microbiota and obesity. Cell Mol. Life Sci 73, 147–162 (2016).
Allam-Ndoul, B., Castonguay-Paradis, S. & Veilleux, A. Gut microbiota and intestinal trans-epithelial permeability. Int. J. Mol. Sci. 21, 6402 (2020).
Lang, S. & Schnabl, B. Microbiota and fatty liver disease-the known, the unknown, and the future. Cell Host Microbe 28, 233–244 (2020).
Chopyk, D. M. & Grakoui, A. Contribution of the intestinal microbiome and gut barrier to hepatic disorders. Gastroenterology 159, 849–863 (2020).
Delgado, T. C., de Las Heras, J. & Martinez-Chantar, M. L. Understanding gut-liver axis nitrogen metabolism in Fatty Liver Disease. Front. Endocrinol. (Lausanne) 13, 1058101 (2022).
Meijnikman, A. S. et al. Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat. Med 28, 2100–2106 (2022).
Fang, J. et al. Gut dysbiosis in nonalcoholic fatty liver disease: pathogenesis, diagnosis, and therapeutic implications. Front. Cell. Infect. Microbiol. 12, 997018 (2022).
Tsukita, S., Tanaka, H. & Tamura, A. The claudins: from tight junctions to biological systems. Trends Biochem. Sci. 44, 141–152 (2019).
Gunzel, D. & Fromm, M. Claudins and other tight junction proteins. Compr. Physiol. 2, 1819–1852 (2012).
Kuo, W. T. et al. The tight junction protein ZO-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterology 161, 1924–1939 (2021).
Gao, B., Ahmad, M. F., Nagy, L. E. & Tsukamoto, H. Inflammatory pathways in alcoholic steatohepatitis. J. Hepatol. 70, 249–259 (2019).
De Schepper, S. et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 176, 676 (2019).
Delfini, M., Stakenborg, N., Viola, M. F. & Boeckxstaens, G. Macrophages in the gut: masters in multitasking. Immunity 55, 1530–1548 (2022).
Ciesielska, A., Matyjek, M. & Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 78, 1233–1261 (2021).
Li, X. et al. The interplay between the gut microbiota and NLRP3 activation affects the severity of acute pancreatitis in mice. Gut Microbes 11, 1774–1789 (2020).
Huang, Y., Xu, W. & Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 18, 2114–2127 (2021).
He, Y., Hara, H. & Nunez, G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 41, 1012–1021 (2016).
Boursier, J. et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 63, 764–775 (2016).
He, J. et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 21, 6356 (2020).
Fu, X. et al. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 59, S130–S152 (2019).
May, H. D., Evans, P. J. & LaBelle, E. V. The bioelectrosynthesis of acetate. Curr. Opin. Biotechnol. 42, 225–233 (2016).
Nogal, A., Valdes, A. M. & Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 13, 1–24 (2021).
Hu, J., Lin, S., Zheng, B. & Cheung, P. C. K. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 58, 1243–1249 (2018).
McNabney, S. M. & Henagan, T. M. Short chain fatty acids in the colon and peripheral tissues: a focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients 9, 1348 (2017).
Alhabeeb, H. et al. Gut hormones in health and obesity: the upcoming role of short chain fatty acids. Nutrients 13, 481 (2021).
Salvi, P. S. & Cowles, R. A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 10, 1775 (2021).
Stoeva, M. K. et al. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 13, 1–28 (2021).
Coppola, S., Avagliano, C. & Calignano, A. & Berni Canani, R. The protective role of butyrate against obesity and obesity-related diseases. Molecules 26, 682 (2021).
Kawasoe, J. et al. Propionic acid, induced in gut by an inulin diet, suppresses inflammation and ameliorates liver ischemia and reperfusion injury in mice. Front. Immunol. 13, 862503 (2022).
Steinert, R. E. et al. Ghrelin, CCK, GLP-1, and PYY(3-36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 97, 411–463 (2017).
Holst, J. J. The physiology of glucagon-like peptide 1. Physiol. Rev. 87, 1409–1439 (2007).
Svegliati-Baroni, G. et al. Gut-Pancreas-Liver Axis as a Target for Treatment of NAFLD/NASH. Int. J. Mol. Sci. 21, 5820 (2020).
Jiang, H., Chen, C. & Gao, J. Extensive summary of the important roles of indole propionic acid, a gut microbial metabolite in host health and disease. Nutrients 15, 151 (2022).
van der Hee, B. & Wells, J. M. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol 29, 700–712 (2021).
Singh, V. et al. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell 175, 679–694.e622 (2018).
Machate, D. J. et al. Fatty acid diets: regulation of gut microbiota composition and obesity and its related metabolic dysbiosis. Int. J. Mol. Sci. 21, 4093 (2020).
Shi, X. et al. Hepatic and fecal metabolomic analysis of the effects of Lactobacillus rhamnosus GG on alcoholic fatty liver disease in mice. J. Proteome Res. 14, 1174–1182 (2015).
Atshaves, B. P. et al. Liver fatty acid-binding protein and obesity. J. Nutr. Biochem. 21, 1015–1032 (2010).
Alex, S. et al. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor gamma. Mol. Cell. Biol. 33, 1303–1316 (2013).
Backhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl Acad. Sci. USA 101, 15718–15723 (2004).
van Eenige, R. et al. Angiopoietin-like 4 governs diurnal lipoprotein lipase activity in brown adipose tissue. Mol. Metab. 60, 101497 (2022).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Ter Horst, K. W. & Serlie, M. J. Fructose consumption, lipogenesis, and non-alcoholic fatty liver disease. Nutrients 9, 981 (2017).
Zeisel, S. H. & Blusztajn, J. K. Choline and human nutrition. Annu. Rev. Nutr. 14, 269–296 (1994).
Bernhard, W. Choline in cystic fibrosis: relations to pancreas insufficiency, enterohepatic cycle, PEMT and intestinal microbiota. Eur. J. Nutr. 60, 1737–1759 (2021).
Spencer, M. D. et al. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 140, 976–986 (2011).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Hosseinkhani, F. et al. The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes 13, 1–22 (2021).
Yoo, W. et al. High-fat diet-induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science 373, 813–818 (2021).
Arias, N. et al. The relationship between choline bioavailability from diet, intestinal microbiota composition, and its modulation of human diseases. Nutrients 12, 2340 (2020).
Huang, W. & Kong, D. The intestinal microbiota as a therapeutic target in the treatment of NAFLD and ALD. Biomed. Pharmacother. 135, 111235 (2021).
Chen, J. & Vitetta, L. Gut microbiota metabolites in NAFLD pathogenesis and therapeutic implications. Int. J. Mol. Sci. 21, 5214 (2020).
Meroni, M., Longo, M. & Dongiovanni, P. Alcohol or gut microbiota: who is the guilty? Int. J. Mol. Sci. 20, 4568 (2019).
Parlesak, A. et al. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J. Hepatol. 32, 742–747 (2000).
Park, J. H. et al. Alcohol stimulates the proliferation of mouse small intestinal epithelial cells via Wnt signaling. Biochem. Biophys. Res. Commun. 534, 639–645 (2021).
Mendes, B. G. & Schnabl, B. From intestinal dysbiosis to alcohol-associated liver disease. Clin. Mol. Hepatol. 26, 595–605 (2020).
Yang, Y. et al. Non-alcoholic components in huangjiu as potential factors regulating the intestinal barrier and gut microbiota in mouse model of alcoholic liver injury. Foods 11, 1537 (2022).
Thibaut, M. M. & Bindels, L. B. Crosstalk between bile acid-activated receptors and microbiome in entero-hepatic inflammation. Trends Mol. Med. 28, 223–236 (2022).
Fiorucci, S. et al. Bile acid modulators for the treatment of nonalcoholic steatohepatitis (NASH). Expert Opin. Investig. Drugs 29, 623–632 (2020).
Guzior, D. V. & Quinn, R. A. Review: microbial transformations of human bile acids. Microbiome 9, 140 (2021).
Fiorucci, S. et al. Bile acids and their receptors in metabolic disorders. Prog. Lipid Res. 82, 101094 (2021).
Simbrunner, B., Trauner, M. & Reiberger, T. Review article: therapeutic aspects of bile acid signalling in the gut-liver axis. Aliment. Pharmacol. Ther. 54, 1243–1262 (2021).
Chavez-Talavera, O., Tailleux, A., Lefebvre, P. & Staels, B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 152, 1679–1694.e1673 (2017).
Wahlstrom, A., Sayin, S. I., Marschall, H. U. & Backhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 24, 41–50 (2016).
Funabashi, M. et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 582, 566–570 (2020).
Kakiyama, G. et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J. Hepatol. 58, 949–955 (2013).
Schoeler, M. & Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 20, 461–472 (2019).
Radun, R. & Trauner, M. Role of FXR in bile acid and metabolic homeostasis in NASH: pathogenetic concepts and therapeutic opportunities. Semin Liver Dis 41, 461–475 (2021).
Winston, J. A. & Theriot, C. M. Diversification of host bile acids by members of the gut microbiota. Gut Microbes 11, 158–171 (2020).
Tu, H., Okamoto, A. Y. & Shan, B. FXR, a bile acid receptor and biological sensor. Trends Cardiovasc. Med. 10, 30–35 (2000).
Li, T. & Chiang, J. Y. L. Bile acid-based therapies for non-alcoholic steatohepatitis and alcoholic liver disease. Hepatobiliary Surg. Nutr. 9, 152–169 (2020).
Kunst, R. F., Verkade, H. J., Oude Elferink, R. P. J. & van de Graaf, S. F. J. Targeting the four pillars of enterohepatic bile salt cycling; lessons from genetics and pharmacology. Hepatology 73, 2577–2585 (2021).
Deng, F. & Bae, Y. H. Bile acid transporter-mediated oral drug delivery. J. Control Release 327, 100–116 (2020).
Liu, W. Y. et al. Targeting fibroblast growth factor 19 in liver disease: a potential biomarker and therapeutic target. Expert Opin. Ther. Targets 19, 675–685 (2015).
Oswald, S. Organic Anion Transporting Polypeptide (OATP) transporter expression, localization and function in the human intestine. Pharmacol. Ther. 195, 39–53 (2019).
Beaudoin, J. J., Brouwer, K. L. R. & Malinen, M. M. Novel insights into the organic solute transporter alpha/beta, OSTalpha/beta: from the bench to the bedside. Pharmacol. Ther. 211, 107542 (2020).
Deeley, R. G. & Cole, S. P. Function, evolution and structure of multidrug resistance protein (MRP). Semin. Cancer Biol. 8, 193–204 (1997).
Telbisz, A. & Homolya, L. Recent advances in the exploration of the bile salt export pump (BSEP/ABCB11) function. Expert Opin. Ther. Targets 20, 501–514 (2016).
Appelman, M. D. et al. Molecular regulation of the hepatic bile acid uptake transporter and HBV entry receptor NTCP. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1866, 158960 (2021).
Wittenburg, H. et al. FXR and ABCG5/ABCG8 as determinants of cholesterol gallstone formation from quantitative trait locus mapping in mice. Gastroenterology 125, 868–881 (2003).
Ding, L., Yang, L., Wang, Z. & Huang, W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharmacol. Sin. B 5, 135–144 (2015).
Fiorucci, S. et al. Targeting FXR in cholestasis: hype or hope. Expert Opin. Ther. Targets 18, 1449–1459 (2014).
Preidis, G. A., Kim, K. H. & Moore, D. D. Nutrient-sensing nuclear receptors PPARalpha and FXR control liver energy balance. J. Clin. Invest. 127, 1193–1201 (2017).
Holter, M. M., Chirikjian, M. K., Govani, V. N. & Cummings, B. P. TGR5 signaling in hepatic metabolic health. Nutrients 12, 2598 (2020).
Maczewsky, J. et al. TGR5 activation promotes stimulus-secretion coupling of pancreatic beta-cells via a PKA-dependent pathway. Diabetes 68, 324–336 (2019).
Deutschmann, K. et al. Bile acid receptors in the biliary tree: TGR5 in physiology and disease. Biochim Biophys. Acta Mol. Basis Dis 1864, 1319–1325 (2018).
Hogenauer, K. et al. G-protein-coupled bile acid receptor 1 (GPBAR1, TGR5) agonists reduce the production of proinflammatory cytokines and stabilize the alternative macrophage phenotype. J. Med. Chem. 57, 10343–10354 (2014).
Hodge, R. J. & Nunez, D. J. Therapeutic potential of Takeda-G-protein-receptor-5 (TGR5) agonists. Hope or hype? Diabetes Obes. Metab. 18, 439–443 (2016).
Xie, G. et al. Conjugated secondary 12alpha-hydroxylated bile acids promote liver fibrogenesis. EBioMedicine 66, 103290 (2021).
Keitel, V. & Haussinger, D. Role of TGR5 (GPBAR1) in liver disease. Semin. Liver Dis. 38, 333–339 (2018).
Burg, N., Salmon, J. E. & Hla, T. Sphingosine 1-phosphate receptor-targeted therapeutics in rheumatic diseases. Nat. Rev. Rheumatol. 18, 335–351 (2022).
Cartier, A. & Hla, T. Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science 366, eaar5551 (2019).
Wang, Y. et al. The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology 65, 2005–2018 (2017).
Wang, X. et al. S1PR2/RhoA/ROCK1 pathway promotes inflammatory bowel disease by inducing intestinal vascular endothelial barrier damage and M1 macrophage polarization. Biochem. Pharmacol. 201, 115077 (2022).
Poland, J. C. & Flynn, C. R. Bile acids, their receptors, and the gut microbiota. Physiology (Bethesda) 36, 235–245 (2021).
Qin, X. & Wang, X. Role of vitamin D receptor in the regulation of CYP3A gene expression. Acta Pharmacol. Sin. B 9, 1087–1098 (2019).
Wang, J. et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 48, 1396–1406 (2016).
Zhang, J., Zhang, Y., Xia, Y. & Sun, J. Imbalance of the intestinal virome and altered viral-bacterial interactions caused by a conditional deletion of the vitamin D receptor. Gut Microbes 13, 1957408 (2021).
Carazo, A., Mladenka, P. & Pavek, P. Marine ligands of the pregnane X receptor (PXR): an overview. Mar. Drugs 17, 554 (2019).
Kim, S. et al. Pregnane X receptor exacerbates nonalcoholic fatty liver disease accompanied by obesity- and inflammation-prone gut microbiome signature. Biochem. Pharmacol. 193, 114698 (2021).
Dutta, M., Lim, J. J. & Cui, J. Y. Pregnane X receptor and the gut-liver axis: a recent update. Drug Metab. Dispos. 50, 478–491 (2022).
Sayaf, K. et al. The nuclear receptor PXR in chronic liver disease. Cells 11, 61 (2021).
Venkatesh, M. et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41, 296–310 (2014).
Hall, J. A. et al. Transcription factor RORalpha enforces stability of the Th17 cell effector program by binding to a Rorc cis-regulatory element. Immunity 55, 2027–2043.e2029 (2022).
Lyu, M. et al. ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature 610, 744–751 (2022).
Kumar, R., Theiss, A. L. & Venuprasad, K. RORgammat protein modifications and IL-17-mediated inflammation. Trends Immunol 42, 1037–1050 (2021).
Song, X. et al. Microbial bile acid metabolites modulate gut RORgamma(+) regulatory T cell homeostasis. Nature 577, 410–415 (2020).
Jetten, A. M. & Cook, D. N. (Inverse) agonists of retinoic acid-related orphan receptor gamma: regulation of immune responses, inflammation, and autoimmune disease. Annu. Rev. Pharmacol. Toxicol. 60, 371–390 (2020).
Guilliams, M. & Scott, C. L. Liver macrophages in health and disease. Immunity 55, 1515–1529 (2022).
Gu, X. et al. New insights into iNKT cells and their roles in liver diseases. Front. Immunol. 13, 1035950 (2022).
Naimimohasses, S. et al. Differential effects of dietary versus exercise intervention on intrahepatic MAIT cells and histological features of NAFLD. Nutrients 14, 2198 (2022).
Mabire, M. et al. MAIT cell inhibition promotes liver fibrosis regression via macrophage phenotype reprogramming. Nat. Commun. 14, 1830 (2023).
Li, F. et al. Erratum: the microbiota maintain homeostasis of liver-resident gammadeltaT-17 cells in a lipid antigen/CD1d-dependent manner. Nat. Commun. 8, 15265 (2017).
Berding, K. et al. Diet and the microbiota-gut-brain axis: sowing the seeds of good mental health. Adv. Nutr. 12, 1239–1285 (2021).
Long-Smith, C. et al. Microbiota-gut-brain axis: new therapeutic opportunities. Annu Rev. Pharmacol. Toxicol. 60, 477–502 (2020).
Farzi, A., Frohlich, E. E. & Holzer, P. Gut microbiota and the neuroendocrine system. Neurotherapeutics 15, 5–22 (2018).
Agirman, G. & Hsiao, E. Y. SnapShot: the microbiota-gut-brain axis. Cell 184, 2524–2524.e2521 (2021).
Chakrabarti, A. et al. The microbiota-gut-brain axis: pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell. Mol. Life Sci. 79, 80 (2022).
Toledo, A. R. L. et al. Gut-brain axis as a pathological and therapeutic target for neurodegenerative disorders. Int. J. Mol. Sci. 23, 1184 (2022).
Shobeiri, P., Kalantari, A., Teixeira, A. L. & Rezaei, N. Shedding light on biological sex differences and microbiota-gut-brain axis: a comprehensive review of its roles in neuropsychiatric disorders. Biol. Sex. Differ. 13, 12 (2022).
Terstappen, G. C., Meyer, A. H., Bell, R. D. & Zhang, W. Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug Discov. 20, 362–383 (2021).
Andersson, E. A., Mallard, C. & Ek, C. J. Circulating tight-junction proteins are potential biomarkers for blood-brain barrier function in a model of neonatal hypoxic/ischemic brain injury. Fluids Barriers CNS 18, 7 (2021).
Braniste, V. et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 6, 263ra158 (2014).
Takata, F., Nakagawa, S., Matsumoto, J. & Dohgu, S. Blood-brain barrier dysfunction amplifies the development of neuroinflammation: understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Front. Cell. Neurosci. 15, 661838 (2021).
Sweeney, M. D. et al. Blood-brain barrier: from physiology to disease and back. Physiol. Rev. 99, 21–78 (2019).
Anand, N., Gorantla, V. R. & Chidambaram, S. B. The role of gut dysbiosis in the pathophysiology of neuropsychiatric disorders. Cells 12, 54 (2022).
Herman, J. P. et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 6, 603–621 (2016).
Prescott, S. L. & Liberles, S. D. Internal senses of the vagus nerve. Neuron 110, 579–599 (2022).
Berthoud, H. R., Albaugh, V. L. & Neuhuber, W. L. Gut-brain communication and obesity: understanding functions of the vagus nerve. J. Clin. Investig. 131, e143770 (2021).
Mendez-Hernandez, R., Escobar, C. & Buijs, R. M. Suprachiasmatic nucleus-arcuate nucleus axis: interaction between time and metabolism essential for health. Obesity (Silver Spring) 28, S10–S17 (2020).
Jais, A. & Bruning, J. C. Arcuate nucleus-dependent regulation of metabolism-pathways to obesity and diabetes mellitus. Endocr. Rev. 43, 314–328 (2022).
Bonaz, B., Sinniger, V. & Pellissier, S. Vagus nerve stimulation at the interface of brain-gut interactions. Cold Spring Harb. Perspect. Med. 9, a034199 (2019).
Xie, Z. et al. The gut-to-brain axis for toxin-induced defensive responses. Cell 185, 4298–4316.e4221 (2022).
Bonaz, B., Sinniger, V. & Pellissier, S. Vagus nerve stimulation: a new promising therapeutic tool in inflammatory bowel disease. J. Intern. Med. 282, 46–63 (2017).
Bravo, J. A. et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA 108, 16050–16055 (2011).
Goralczyk-Binkowska, A., Szmajda-Krygier, D. & Kozlowska, E. The microbiota-gut-brain axis in psychiatric disorders. Int. J. Mol. Sci. 23, 11245 (2022).
Valles-Colomer, M. et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632 (2019).
Dicks, L. M. T. Gut bacteria and neurotransmitters. Microorganisms 10, 1838 (2022).
Strandwitz, P. et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 4, 396–403 (2019).
Duranti, S. et al. Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Sci. Rep. 10, 14112 (2020).
Auteri, M., Zizzo, M. G. & Serio, R. GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol. Res. 93, 11–21 (2015).
Engin, E., Benham, R. S. & Rudolph, U. An emerging circuit pharmacology of GABA(A) receptors. Trends Pharm. Sci. 39, 710–732 (2018).
Evenseth, L. S. M., Gabrielsen, M. & Sylte, I. The GABA(B) receptor-structure, ligand binding and drug development. Molecules 25, 3093 (2020).
Enz, R. GABA(C) receptors: a molecular view. Biol. Chem. 382, 1111–1122 (2001).
Sakimoto, Y. et al. Significance of GABA(A) receptor for cognitive function and hippocampal pathology. Int. J. Mol. Sci. 22, 12456 (2021).
Zhao, H. et al. GABAergic system dysfunction in autism spectrum disorders. Front. Cell Dev. Biol. 9, 781327 (2021).
Zheng, P. et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 5, eaau8317 (2019).
Sun, T. et al. Decoding the contributions of gut microbiota and cerebral metabolism in acute liver injury mice with and without cognitive dysfunction. CNS Neurosci. Ther. Suppl 1, 31–42 (2022).
Chang, C. H., Lin, C. H. & Lane, H. Y. d-glutamate and gut microbiota in Alzheimer’s disease. Int. J. Mol. Sci. 21, 2676 (2020).
Baj, A. et al. Glutamatergic signaling along the microbiota-gut-brain axis. Int. J. Mol. Sci. 20, 1482 (2019).
Andersen, J. V., Schousboe, A. & Verkhratsky, A. Astrocyte energy and neurotransmitter metabolism in Alzheimer’s disease: Integration of the glutamate/GABA-glutamine cycle. Prog. Neurobiol. 217, 102331 (2022).
Kaelberer, M. M. et al. A gut-brain neural circuit for nutrient sensory transduction. Science 361, eaat5236 (2018).
Liu, W. W. & Bohorquez, D. V. The neural basis of sugar preference. Nat. Rev. Neurosci. 23, 584–595 (2022).
Tsetlin, V. I. Acetylcholine and acetylcholine receptors: textbook knowledge and new data. Biomolecules 10, 852 (2020).
Sun, Q. et al. Acetylcholine deficiency disrupts extratelencephalic projection neurons in the prefrontal cortex in a mouse model of Alzheimer’s disease. Nat. Commun. 13, 998 (2022).
Wang, W. et al. Neurotransmitter disturbances caused by methylmercury exposure: Microbiota-gut-brain interaction. Sci. Total Environ. 873, 162358 (2023).
Horiuchi, Y. et al. Evolutional study on acetylcholine expression. Life Sci 72, 1745–1756 (2003).
Koussoulas, K. et al. Neurally released GABA acts via GABA(C) receptors to modulate Ca(2+) transients evoked by trains of synaptic inputs, but not responses evoked by single stimuli, in myenteric neurons of mouse ileum. Front. Physiol. 9, 97 (2018).
Inazu, M. Functional expression of choline transporters in the blood-brain barrier. Nutrients 11, 2265 (2019).
Costa, K. M. & Schoenbaum, G. Dopamine. Curr. Biol. 32, R817–R824 (2022).
Wiseman, S. Dopamine surprises. Nat. Neurosci. 25, 531 (2022).
Wang, Y. et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal. Transduct. Target. Ther. 6, 77 (2021).
Gianessi, C. A. & Kash, T. L. A gut feeling about dopamine. Neuron 106, 703–704 (2020).
Monzani, E. et al. Dopamine, oxidative stress and protein-quinone modifications in Parkinson’s and other neurodegenerative diseases. Angew. Chem. Int Ed. Engl. 58, 6512–6527 (2019).
Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res 128–133, 2018 (1693).
Bo, T. B. et al. The microbiota-gut-brain interaction in regulating host metabolic adaptation to cold in male Brandt’s voles (Lasiopodomys brandtii). ISME J 13, 3037–3053 (2019).
Gainetdinov, R. R., Hoener, M. C. & Berry, M. D. Trace amines and their receptors. Pharmacol. Rev. 70, 549–620 (2018).
Luqman, A. et al. SadA-expressing staphylococci in the human gut show increased cell adherence and internalization. Cell Rep 22, 535–545 (2018).
Williams, B. B. et al. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 16, 495–503 (2014).
Sun, L. J., Li, J. N. & Nie, Y. Z. Gut hormones in microbiota-gut-brain cross-talk. Chin. Med. J. (Engl.) 133, 826–833 (2020).
Lach, G., Schellekens, H., Dinan, T. G. & Cryan, J. F. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics 15, 36–59 (2018).
Liu, N. et al. The mechanism of secretion and metabolism of gut-derived 5-hydroxytryptamine. Int. J. Mol. Sci. 22, (2021).
Huang, F. & Wu, X. Brain neurotransmitter modulation by gut microbiota in anxiety and depression. Front. Cell Dev. Biol. 9, 649103 (2021).
Roth, W. et al. Tryptophan metabolism and gut-brain homeostasis. Int. J. Mol. Sci. 22, 2973 (2021).
Jenkins, T. A., Nguyen, J. C., Polglaze, K. E. & Bertrand, P. P. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 8, 56 (2016).
Wu, H., Denna, T. H., Storkersen, J. N. & Gerriets, V. A. Beyond a neurotransmitter: the role of serotonin in inflammation and immunity. Pharm. Res. 140, 100–114 (2019).
McLean, P. G., Borman, R. A. & Lee, K. 5-HT in the enteric nervous system: gut function and neuropharmacology. Trends Neurosci 30, 9–13 (2007).
Lund, M. L. et al. Enterochromaffin 5-HT cells - a major target for GLP-1 and gut microbial metabolites. Mol. Metab. 11, 70–83 (2018).
Wan, S. & Browning, K. N. Glucose increases synaptic transmission from vagal afferent central nerve terminals via modulation of 5-HT3 receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G1050–G1057 (2008).
Wu, Y. et al. The Role of neuropeptide Y and peptide YY in the development of obesity via gut-brain axis. Curr. Protein Pept. Sci. 20, 750–758 (2019).
Manfready, R. A. et al. Gut-Brain Communication in Parkinson’s Disease: Enteroendocrine Regulation by GLP-1. Curr. Neurol. Neurosci. Rep. 22, 335–342 (2022).
Sun, J. et al. Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson’s disease via gut microbiota-GLP-1 pathway. Brain Behav. Immun. 91, 703–715 (2021).
Rubio, C. et al. Aging in male wistar rats associates with changes in intestinal microbiota, gut structure, and cholecystokinin-mediated gut-brain axis function. J. Gerontol. A Biol. Sci. Med. Sci. 76, 1915–1921 (2021).
Del Boca, C. et al. Cholecystokinin knock-down in the basolateral amygdala has anxiolytic and antidepressant-like effects in mice. Neuroscience 218, 185–195 (2012).
Stasi, C. & Milani, S. Functions of ghrelin in brain, gut and liver. CNS Neurol. Disord. Drug Targets 15, 956–963 (2016).
Decarie-Spain, L. & Kanoski, S. E. Ghrelin and glucagon-like peptide-1: a gut-brain axis battle for food reward. Nutrients 13, 977 (2021).
Hirano, Y. et al. Fasting launches CRTC to facilitate long-term memory formation in Drosophila. Science 339, 443–446 (2013).
Meyer, R. M. et al. A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol. Psychiatry 19, 1284–1294 (2014).
Doroszkiewicz, J., Groblewska, M. & Mroczko, B. The role of gut microbiota and gut-brain interplay in selected diseases of the central nervous system. Int J Mol Sci. 22, 10028 (2021).
O’Riordan, K. J. et al. Short chain fatty acids: microbial metabolites for gut-brain axis signalling. Mol. Cell Endocrinol. 546, 111572 (2022).
Tran, S. M. & Mohajeri, M. H. The role of gut bacterial metabolites in brain development, aging and disease. Nutrients 13, 732 (2021).
Cerreto, M. et al. Bariatric surgery and liver disease: general considerations and role of the gut-liver axis. Nutrients 13, 2649 (2021).
Dalile, B., Van Oudenhove, L., Vervliet, B. & Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478 (2019).
Su, Y. L. et al. Phlorizin alleviates cholinergic memory impairment and regulates gut microbiota in d-galactose induced mice. Exp. Gerontol. 165, 111863 (2022).
Xiao, Q. et al. Crocin-I alleviates the depression-like behaviors probably via modulating “microbiota-gut-brain” axis in mice exposed to chronic restraint stress. J. Affect Disord. 276, 476–486 (2020).
Xiao, W. et al. The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids. Microbiome 10, 62 (2022).
Xiao, L., Liu, Q., Luo, M. & Xiong, L. Gut microbiota-derived metabolites in irritable bowel syndrome. Front. Cell Infect. Microbiol. 11, 729346 (2021).
Joyce, S. A. & O’Malley, D. Bile acids, bioactive signalling molecules in interoceptive gut-to-brain communication. J. Physiol. 600, 2565–2578 (2022).
Wang, S. Z., Yu, Y. J. & Adeli, K. Role of gut microbiota in neuroendocrine regulation of carbohydrate and lipid metabolism via the microbiota-gut-brain-liver axis. Microorganisms 8, 527 (2020).
Perino, A., Demagny, H., Velazquez-Villegas, L. & Schoonjans, K. Molecular physiology of bile acid signaling in health, disease, and aging. Physiol. Rev. 101, 683–731 (2021).
Cai, J., Sun, L. & Gonzalez, F. J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 30, 289–300 (2022).
Hsuchou, H., Pan, W. & Kastin, A. J. Fibroblast growth factor 19 entry into brain. Fluids Barriers CNS 10, 32 (2013).
Perry, R. J. et al. FGF1 and FGF19 reverse diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat. Commun. 6, 6980 (2015).
Sinha, S. R. et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe 27, 659–670.e655 (2020).
Li, D. et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 17, e12768 (2018).
Hoyles, L. et al. Regulation of blood-brain barrier integrity by microbiome-associated methylamines and cognition by trimethylamine N-oxide. Microbiome 9, 235 (2021).
Chavan, S. S., Pavlov, V. A. & Tracey, K. J. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 46, 927–942 (2017).
Harms, A. S., Ferreira, S. A. & Romero-Ramos, M. Periphery and brain, innate and adaptive immunity in Parkinson’s disease. Acta Neuropathol 141, 527–545 (2021).
Kazankov, K. et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 16, 145–159 (2019).
Grubisic, V. et al. Enteric Glia Modulate Macrophage Phenotype and Visceral Sensitivity following Inflammation. Cell Rep 32, 108100 (2020).
Muller, P. A. et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 300–313 (2014).
Nerurkar, N. L., Mahadevan, L. & Tabin, C. J. BMP signaling controls buckling forces to modulate looping morphogenesis of the gut. Proc. Natl Acad. Sci. USA 114, 2277–2282 (2017).
Chen, W. et al. A Special network comprised of macrophages, epithelial cells, and gut microbiota for gut homeostasis. Cells 11, 307 (2022).
Xiong, L., Nutt, S. L. & Seillet, C. Innate lymphoid cells: More than just immune cells. Front Immunol 13, 1033904 (2022).
Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature 517, 293–301 (2015).
Karagiannis, F. & Wilhelm, C. Innate lymphoid cells-key immune integrators of overall body homeostasis. Semin. Immunopathol. 40, 319–330 (2018).
Spits, H. et al. Innate lymphoid cells-a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013).
Aguilera-Lizarraga, J., Hussein, H. & Boeckxstaens, G. E. Immune activation in irritable bowel syndrome: what is the evidence? Nat. Rev. Immunol. 22, 674–686 (2022).
Schemann, M. & Camilleri, M. Functions and imaging of mast cell and neural axis of the gut. Gastroenterology 144, 698–704.e694 (2013).
Toyoshima, S. & Okayama, Y. Neuro-allergology: mast cell-nerve cross-talk. Allergol. Int 71, 288–293 (2022).
Fukuoka, H., Shichi, H., Yamamoto, M. & Takahashi, Y. The mechanisms underlying autonomous adrenocorticotropic hormone secretion in cushing’s disease. Int. J. Mol. Sci. 21, 9132 (2020).
Agirman, G., Yu, K. B. & Hsiao, E. Y. Signaling inflammation across the gut-brain axis. Science 374, 1087–1092 (2021).
Cryan, J. F. et al. The microbiota-gut-brain axis. Physiol. Rev. 99, 1877–2013 (2019).
Morais, L. H., Schreiber, H. L. T. & Mazmanian, S. K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol 19, 241–255 (2021).
Tang, X. et al. Regenerative role of t cells in nerve repair and functional recovery. Front. Immunol. 13, 923152 (2022).
Rosas-Ballina, M. et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011).
Zhang, X. et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature 581, 204–208 (2020).
Otten, U., Ehrhard, P. & Peck, R. Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc. Natl Acad. Sci. USA 86, 10059–10063 (1989).
Smith, M. L., Wade, J. B., Wolstenholme, J. & Bajaj, J. S. Gut microbiome-brain-cirrhosis axis. Hepatology (2023).
Cheon, S. Y. & Song, J. The association between hepatic encephalopathy and diabetic encephalopathy: the brain-liver axis. Int. J. Mol Sci. 22, 463 (2021).
Liu, L. et al. Altered function and expression of abc transporters at the blood-brain barrier and increased brain distribution of phenobarbital in acute liver failure mice. Front Pharmacol 9, 190 (2018).
Coulter, A. A., Rebello, C. J. & Greenway, F. L. Centrally acting agents for obesity: past, present, and future. Drugs 78, 1113–1132 (2018).
Patterson, T. T. et al. Complex feed-forward and feedback mechanisms underlie the relationship between traumatic brain injury and the gut-microbiota-brain axis. Shock 52, 318–325 (2019).
Metz, M. et al. Leptin increases hepatic triglyceride export via a vagal mechanism in humans. Cell Metab 34, 1719–1731.e1715 (2022).
Conole, E. L. S. et al. DNA methylation and protein markers of chronic inflammation and their associations with brain and cognitive aging. Neurology 97, e2340–e2352 (2021).
Li, Y., Chen, X. & Lu, C. The interplay between DNA and histone methylation: molecular mechanisms and disease implications. EMBO Rep 22, e51803 (2021).
Jeong, H. et al. Evolution of DNA methylation in the human brain. Nat. Commun. 12, 2021 (2021).
Yan, L. et al. Physical exercise prevented stress-induced anxiety via improving brain RNA methylation. Adv. Sci. (Weinh.) 9, e2105731 (2022).
Sleiman, S. F. et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife 5, e15092 (2016).
Ieraci, A., Mallei, A., Musazzi, L. & Popoli, M. Physical exercise and acute restraint stress differentially modulate hippocampal brain-derived neurotrophic factor transcripts and epigenetic mechanisms in mice. Hippocampus 25, 1380–1392 (2015).
Dos Santos, J. A. C. et al. Physical exercise and the functions of microRNAs. Life Sci. 304, 120723 (2022).
Benito, E. et al. RNA-dependent intergenerational inheritance of enhanced synaptic plasticity after environmental enrichment. Cell Rep 23, 546–554 (2018).
Fallahzadeh, M. A. & Rahimi, R. S. Hepatic encephalopathy: current and emerging treatment modalities. Clin. Gastroenterol. Hepatol. 20, S9–S19 (2022).
Claeys, W. et al. The neurogliovascular unit in hepatic encephalopathy. JHEP Rep 3, 100352 (2021).
Rodrigo, R. et al. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology 139, 675–684 (2010).
Hsu, S. J. et al. Enhanced meningeal lymphatic drainage ameliorates neuroinflammation and hepatic encephalopathy in cirrhotic rats. Gastroenterology 160, 1315–1329.e1313 (2021).
Duchini, A. et al. Effects of tumor necrosis factor-alpha and interleukin-6 on fluid-phase permeability and ammonia diffusion in CNS-derived endothelial cells. J. Investig. Med. 44, 474–482 (1996).
Proia, P. et al. Lactate as a metabolite and a regulator in the central nervous system. Int. J. Mol. Sci. 17, 1450 (2016).
Bosoi, C. R. et al. Increased brain lactate is central to the development of brain edema in rats with chronic liver disease. J. Hepatol. 60, 554–560 (2014).
Oria, M. & Jalan, R. Brain lactate in hepatic encephalopathy: friend or foe? J. Hepatol. 60, 476–477 (2014).
Rose, C. et al. Association of reduced extracellular brain ammonia, lactate, and intracranial pressure in pigs with acute liver failure. Hepatology 46, 1883–1892 (2007).
Tarnacka, B., Jopowicz, A. & Maslinska, M. Copper, iron, and manganese toxicity in neuropsychiatric conditions. Int J Mol Sci. 22, 7820 (2021).
Martins, A. C. et al. Manganese in the diet: bioaccessibility, adequate intake, and neurotoxicological effects. J. Agric. Food Chem. 68, 12893–12903 (2020).
Kirkley, K. S. et al. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J. Neuroinflammation 14, 99 (2017).
Ray, W. J. & Buggia-Prevot, V. Novel targets for alzheimer’s disease: a view beyond amyloid. Annu. Rev. Med. 72, 15–28 (2021).
Cheng, Y. et al. Physiological beta-amyloid clearance by the liver and its therapeutic potential for Alzheimer’s disease. Acta Neuropathol 145, 717–731 (2023).
Huang, Z., Lin, H. W. K., Zhang, Q. & Zong, X. Targeting Alzheimer’s disease: the critical crosstalk between the liver and brain. Nutrients 14, 4298 (2022).
Wang, J., Gu, B. J., Masters, C. L. & Wang, Y. J. A systemic view of Alzheimer disease - insights from amyloid-beta metabolism beyond the brain. Nat. Rev. Neurol. 13, 612–623 (2017).
Fraile-Ramos, J., Garrit, A., Reig-Vilallonga, J. & Gimenez-Llort, L. Hepatic oxi-inflammation and neophobia as potential liver-brain axis targets for alzheimer’s disease and aging, with strong sensitivity to sex, isolation, and obesity. Cells 12, 1517 (2023).
Chen, A. Q. et al. Microglia-derived TNF-alpha mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis 10, 487 (2019).
Dhapola, R. et al. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 29, 1669–1681 (2021).
Gantner, B. N., LaFond, K. M. & Bonini, M. G. Nitric oxide in cellular adaptation and disease. Redox Biol 34, 101550 (2020).
Papageorgoulis, A. et al. Repeated but not acute exposure with a low dose range of the nitric oxide (NO) donor sodium nitroprusside (SNP) induces anxiolytic-like behaviour in a dose-independent manner in two different rat models of anxiety. Nitric Oxide 99, 1–6 (2020).
D’Mello, C., Le, T. & Swain, M. G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci. 29, 2089–2102 (2009).
Kerfoot, S. M. et al. TNF-alpha-secreting monocytes are recruited into the brain of cholestatic mice. Hepatology 43, 154–162 (2006).
Jordan, S. et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell 178, 1102–1114.e1117 (2019).
Wang, J. et al. Classical signaling and trans-signaling pathways stimulated by megalobrama amblycephala IL-6 and IL-6R. Int. J. Mol. Sci. 23, 2019 (2022).
Rose-John, S. Local and systemic effects of interleukin-6 (IL-6) in inflammation and cancer. FEBS Lett 596, 557–566 (2022).
Nguyen, K. et al. Regulatory T cells suppress sickness behaviour development without altering liver injury in cholestatic mice. J. Hepatol. 56, 626–631 (2012).
Baumler, A. J. & Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535, 85–93 (2016).
Cobbold, J. F. L. et al. Rifaximin in non-alcoholic steatohepatitis: An open-label pilot study. Hepatol. Res. 48, 69–77 (2018).
Hvas, C. L. et al. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent clostridium difficile infection. Gastroenterology 156, 1324–1332.e1323 (2019).
Fodor, A. A. et al. Rifaximin is associated with modest, transient decreases in multiple taxa in the gut microbiota of patients with diarrhoea-predominant irritable bowel syndrome. Gut Microbes 10, 22–33 (2019).
Nee, J. & Lembo, A. Review article: current and future treatment approaches for IBS with diarrhoea (IBS-D) and IBS mixed pattern (IBS-M). Aliment. Pharmacol. Ther. 54, S63–S74 (2021).
El-Salhy, M. et al. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 69, 859–867 (2020).
Modi, S. R., Collins, J. J. & Relman, D. A. Antibiotics and the gut microbiota. J. Clin. Invest. 124, 4212–4218 (2014).
Ianiro, G., Tilg, H. & Gasbarrini, A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut 65, 1906–1915 (2016).
Gavalda, J. et al. Brief communication: treatment of Enterococcus faecalis endocarditis with ampicillin plus ceftriaxone. Ann. Intern. Med. 146, 574–579 (2007).
Hao, W. Z., Li, X. J., Zhang, P. W. & Chen, J. X. A review of antibiotics, depression, and the gut microbiome. Psychiatry Res 284, 112691 (2020).
Xie, C. & Halegoua-DeMarzio, D. Role of probiotics in non-alcoholic fatty liver disease: does gut microbiota matter? Nutrients 11, 2837 (2019).
Snigdha, S. et al. Probiotics: Potential novel therapeutics for microbiota-gut-brain axis dysfunction across gender and lifespan. Pharm. Ther. 231, 107978 (2022).
Alisi, A. et al. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 39, 1276–1285 (2014).
Wang, Z. et al. Netrin-1 promotes liver regeneration possibly by facilitating vagal nerve repair after partial hepatectomy in mice. Cell. Signal. 91, 110227 (2022).
Aller, R. et al. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur. Rev. Med. Pharm. Sci. 15, 1090–1095 (2011).
Aaldijk, E. & Vermeiren, Y. The role of serotonin within the microbiota-gut-brain axis in the development of Alzheimer’s disease: a narrative review. Ageing Res. Rev. 75, 101556 (2022).
Sonali, S. et al. Mechanistic insights into the link between gut dysbiosis and major depression: an extensive review. Cells 11, 1362 (2022).
Santocchi, E. et al. Gut to brain interaction in Autism Spectrum Disorders: a randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry 16, 183 (2016).
Schaub, A. C. et al. Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: a randomized controlled trial. Transl. Psychiatry 12, 227 (2022).
Tian, P. et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 100, 233–241 (2022).
Beyaz Coskun, A. & Sagdicoglu Celep, A. G. Therapeutic modulation methods of gut microbiota and gut-liver axis. Crit. Rev. Food Sci. Nutr. 62, 6505–6515 (2022).
Alfonsetti, M., Castelli, V. & d’Angelo, M. Are we what we Eat? Impact of diet on the gut-brain axis in Parkinson’s Disease. Nutrients 14, 380 (2022).
Cani, P. D. et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–1103 (2009).
Holscher, H. D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8, 172–184 (2017).
Cerdo, T., Ruiz, A., Suarez, A. & Campoy, C. Probiotic, prebiotic, and brain development. Nutrients 9, 1247 (2017).
Bomhof, M. R. et al. Histological improvement of non-alcoholic steatohepatitis with a prebiotic: a pilot clinical trial. Eur. J. Nutr. 58, 1735–1745 (2019).
Reimer, R. A. et al. Effect of chicory inulin-type fructan-containing snack bars on the human gut microbiota in low dietary fiber consumers in a randomized crossover trial. Am. J. Clin. Nutr. 111, 1286–1296 (2020).
Eslamparast, T. et al. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am. J. Clin. Nutr. 99, 535–542 (2014).
Malaguarnera, M. et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig. Dis. Sci. 57, 545–553 (2012).
Safavi, M. et al. The effects of synbiotic supplementation on some cardio-metabolic risk factors in overweight and obese children: a randomized triple-masked controlled trial. Int J. Food Sci. Nutr. 64, 687–693 (2013).
Sarkar, A. et al. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci 39, 763–781 (2016).
Ooijevaar, R. E. et al. Clinical application and potential of fecal microbiota transplantation. Annu. Rev. Med. 70, 335–351 (2019).
Zhao, Z. et al. Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome 9, 226 (2021).
Kim, N. et al. Transplantation of gut microbiota derived from Alzheimer’s disease mouse model impairs memory function and neurogenesis in C57BL/6 mice. Brain Behav. Immun. 98, 357–365 (2021).
Zhan, G. et al. Abnormal gut microbiota composition contributes to cognitive dysfunction in SAMP8 mice. Aging (Albany NY) 10, 1257–1267 (2018).
Cao, H. et al. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 59, 3371–3379 (2019).
Cardona, F. et al. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 24, 1415–1422 (2013).
Abenavoli, L. et al. Dietary polyphenols and non-alcoholic fatty liver disease. Nutrients 13, 494 (2021).
Rodriguez-Ramiro, I., Vauzour, D. & Minihane, A. M. Polyphenols and non-alcoholic fatty liver disease: impact and mechanisms. Proc. Nutr. Soc. 75, 47–60 (2016).
Anhe, F. F. et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 64, 872–883 (2015).
Yaskolka Meir, A. et al. Effect of green-Mediterranean diet on intrahepatic fat: the DIRECT PLUS randomised controlled trial. Gut 70, 2085–2095 (2021).
Costabile, G. et al. Reduction of de novo lipogenesis mediates beneficial effects of isoenergetic diets on fatty liver: mechanistic insights from the MEDEA Randomized Clinical Trial. Nutrients 14, 2178 (2022).
Johnson, S. L. et al. Polyphenol microbial metabolites exhibit gut and blood(-)brain barrier permeability and protect murine microglia against LPS-induced inflammation. Metabolites 9, 78 (2019).
Luo, Y. P. et al. Epigallocatechin-3-gallate alleviates galactose-induced aging impairment via gut-brain communication. Food Funct 13, 11200–11209 (2022).
Staudacher, H. M. & Whelan, K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut 66, 1517–1527 (2017).
Algera, J. P. et al. Low FODMAP diet reduces gastrointestinal symptoms in irritable bowel syndrome and clinical response could be predicted by symptom severity: a randomized crossover trial. Clin. Nutr. 41, 2792–2800 (2022).
Black, C. J., Staudacher, H. M. & Ford, A. C. Efficacy of a low FODMAP diet in irritable bowel syndrome: systematic review and network meta-analysis. Gut 71, 1117–1126 (2022).
Xie, C. R. et al. Low FODMAP diet and probiotics in irritable bowel syndrome: a systematic review with network meta-analysis. Front. Pharmacol. 13, 853011 (2022).
Colombel, J. F., Shin, A. & Gibson, P. R. AGA clinical practice update on functional gastrointestinal symptoms in patients with inflammatory bowel disease: expert review. Clin. Gastroenterol. Hepatol. 17, 380–390.e381 (2019).
Simoes, C. D., Maganinho, M. & Sousa, A. S. FODMAPs, inflammatory bowel disease and gut microbiota: updated overview on the current evidence. Eur. J. Nutr. 61, 1187–1198 (2022).
Lis, D. M. Exit gluten-free and enter low FODMAPs: a novel dietary strategy to reduce gastrointestinal symptoms in athletes. Sports Med 49, 87–97 (2019).
Erickson, J., Korczak, R., Wang, Q. & Slavin, J. Gastrointestinal tolerance of low FODMAP oral nutrition supplements in healthy human subjects: a randomized controlled trial. Nutr. J. 16, 35 (2017).
Bellini, M. et al. A low-FODMAP diet for irritable bowel syndrome: some answers to the doubts from a long-term follow-Up. Nutrients 12, 2360 (2020).
Altobelli, E., Del Negro, V., Angeletti, P. M. & Latella, G. Low-FODMAP diet improves irritable bowel syndrome symptoms: a meta-analysis. Nutrients 9, 940 (2017).
Reznikov, E. A. & Suskind, D. L. Current nutritional therapies in inflammatory bowel disease: improving clinical remission rates and sustainability of long-term dietary therapies. Nutrients 15, 668 (2023).
Kang, S. G. et al. Effect of gut microbiome-derived metabolites and extracellular vesicles on hepatocyte functions in a gut-liver axis chip. Nano Converg 10, 5 (2023).
Zhao, X. et al. Orally administered saccharide-sequestering nanocomplex to manage carbohydrate metabolism disorders. Sci Adv 7, eabf7311 (2021).
Pan, H. et al. Light-sensitive lactococcus lactis for microbe-gut-brain axis regulating via upconversion optogenetic micro-nano system. ACS Nano 16, 6049–6063 (2022).
Qu, C. et al. Nano-Honokiol ameliorates the cognitive deficits in TgCRND8 mice of Alzheimer’s disease via inhibiting neuropathology and modulating gut microbiota. J. Adv. Res. 35, 231–243 (2022).
Tully, D. C. et al. Discovery of tropifexor (LJN452), a highly potent non-bile acid FXR agonist for the treatment of cholestatic liver diseases and nonalcoholic steatohepatitis (NASH). J. Med. Chem. 60, 9960–9973 (2017).
Ratziu, V. et al. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J. Hepatol. 54, 1011–1019 (2011).
Younossi, Z. M. et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 394, 2184–2196 (2019).
Neuschwander-Tetri, B. A. et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 (2015).
Ratziu, V. et al. EDP-305 in patients with NASH: A phase II double-blind placebo-controlled dose-ranging study. J. Hepatol. 76, 506–517 (2021).
Patel, K. et al. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology 72, 58–71 (2020).
Wang, Y. et al. Safety, pharmacokinetics, pharmacodynamics, and formulation of liver-distributed farnesoid X-receptor agonist TERN-101 in healthy volunteers. Clin. Pharmacol. Drug Dev. 10, 1198–1208 (2021).
Harrison, S. A. et al. A structurally optimized FXR agonist, MET409, reduced liver fat content over 12 weeks in patients with non-alcoholic steatohepatitis. J. Hepatol. 75, 25–33 (2021).
Kawamata, Y. et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 278, 9435–9440 (2003).
Sorrentino, G. et al. Bile acids signal via TGR5 to activate intestinal stem cells and epithelial regeneration. Gastroenterology 159, 956–968.e958 (2020).
Pathak, P. et al. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J. Biol. Chem. 292, 11055–11069 (2017).
Argo, C. K. et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J. Hepatol. 62, 190–197 (2015).
Jump, D. B., Depner, C. M., Tripathy, S. & Lytle, K. A. Potential for dietary omega-3 fatty acids to prevent nonalcoholic fatty liver disease and reduce the risk of primary liver cancer. Adv. Nutr. 6, 694–702 (2015).
Yang, T., Richards, E. M., Pepine, C. J. & Raizada, M. K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 14, 442–456 (2018).
Knudsen, L. B. et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J. Med. Chem. 43, 1664–1669 (2000).
Armstrong, M. J. et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J. Hepatol. 64, 399–408 (2016).
Armstrong, M. J. et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 387, 679–690 (2016).
O’Neil, P. M. et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 392, 637–649 (2018).
Newsome, P. N. et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med. 384, 1113–1124 (2021).
Harrison, S. A. et al. Semaglutide for the treatment of non-alcoholic steatohepatitis: Trial design and comparison of non-invasive biomarkers. Contemp. Clin. Trials 97, 106174 (2020).
Flynn, C. R., Albaugh, V. L. & Abumrad, N. N. Metabolic effects of bile acids: potential role in bariatric surgery. Cell Mol. Gastroenterol. Hepatol. 8, 235–246 (2019).
Talukdar, S. & Kharitonenkov, A. FGF19 and FGF21: In NASH we trust. Mol. Metab. 46, 101152 (2021).
Harrison, S. A. et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 391, 1174–1185 (2018).
Meoli, L. & Gunzel, D. The role of claudins in homeostasis. Nat. Rev. Nephrol. 19, 587–603 (2023).
Srivastava, A. K. et al. Serine 408 phosphorylation is a molecular switch that regulates structure and function of the occludin alpha-helical bundle. Proc. Natl Acad. Sci. USA 119, e2204618119 (2022).
Cao, Y. Y. et al. Inhibition of miR-155 alleviates sepsis-induced inflammation and intestinal barrier dysfunction by inactivating NF-kappaB signaling. Int. Immunopharmacol. 90, 107218 (2021).
Yoon, K. T., Liu, H. & Lee, S. S. Beta-blockers in advanced cirrhosis: More friend than enemy. Clin. Mol. Hepatol. 27, 425–436 (2021).
Bernardi, M. & Caraceni, P. Novel perspectives in the management of decompensated cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 15, 753–764 (2018).
Strathdee, S. A., Hatfull, G. F., Mutalik, V. K. & Schooley, R. T. Phage therapy: from biological mechanisms to future directions. Cell 186, 17–31 (2023).
Hatfull, G. F., Dedrick, R. M. & Schooley, R. T. Phage therapy for antibiotic-resistant bacterial infections. Annu. Rev. Med. 73, 197–211 (2022).
Chang, R. Y. K., Nang, S. C., Chan, H. K. & Li, J. Novel antimicrobial agents for combating antibiotic-resistant bacteria. Adv. Drug Deliv. Rev. 187, 114378 (2022).
Duan, Y. et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 575, 505–511 (2019).
Nakamoto, N. et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat. Microbiol. 4, 492–503 (2019).
Chen, S. & Wang, D. Intestinal-derived HDL: the portal guardian of the liver. Immunity 54, 1903–1905 (2021).
Kim, J. & Seki, E. An intestine-derived HDL as a novel regulator of the activity of gut-derived LPS: ushering in a new era of research on the gut-liver axis. Gastroenterology 162, 651–652 (2022).
Trebicka, J. et al. The microbiota in cirrhosis and its role in hepatic decompensation. J. Hepatol. 75, S67–S81 (2021).
Chey, W. D., Keefer, L., Whelan, K. & Gibson, P. R. Behavioral and diet therapies in integrated care for patients with irritable bowel syndrome. Gastroenterology 160, 47–62 (2021).
Hertenstein, E. et al. Cognitive behavioral therapy for insomnia in patients with mental disorders and comorbid insomnia: a systematic review and meta-analysis. Sleep. Med. Rev. 62, 101597 (2022).
Wechsler, E. V. & Shah, E. D. Diarrhea-predominant and constipation-predominant irritable bowel syndrome: current prescription drug treatment options. Drugs 81, 1953–1968 (2021).
Lackner, J. M. et al. Durability and decay of treatment benefit of cognitive behavioral therapy for irritable bowel syndrome: 12-month follow-up. Am. J. Gastroenterol. 114, 330–338 (2019).
Didari, T., Mozaffari, S., Nikfar, S. & Abdollahi, M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J. Gastroenterol. 21, 3072–3084 (2015).
Henrich, J. F. et al. A randomized clinical trial of mindfulness-based cognitive therapy for women with irritable bowel syndrome-effects and mechanisms. J. Consult Clin. Psychol. 88, 295–310 (2020).
Macer, B. J., Prady, S. L. & Mikocka-Walus, A. Antidepressants in inflammatory bowel disease: a systematic review. Inflamm. Bowel Dis. 23, 534–550 (2017).
Truyens, M. et al. Effect of 5-hydroxytryptophan on fatigue in quiescent inflammatory bowel disease: a randomized controlled trial. Gastroenterology 163, 1294–1305.e1293 (2022).
Agus, A., Planchais, J. & Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724 (2018).
Michaudel, C. et al. Rewiring the altered tryptophan metabolism as a novel therapeutic strategy in inflammatory bowel diseases. Gut 72, 1296–1307 (2023).
Fernandes, J., Arida, R. M. & Gomez-Pinilla, F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev. 80, 443–456 (2017).
Wu, Y. et al. Hepatic soluble epoxide hydrolase activity regulates cerebral Abeta metabolism and the pathogenesis of Alzheimer’s disease in mice. Neuron 111, 2847–2862.e10 (2023).
Hofer, U. Gut-brain axis in ageing. Nat. Rev. Microbiol. 20, 446 (2022).
Borgmann, D. et al. Gut-brain communication by distinct sensory neurons differently controls feeding and glucose metabolism. Cell Metab 33, 1466–1482.e1467 (2021).
Brandl, K., Kumar, V. & Eckmann, L. Gut-liver axis at the frontier of host-microbial interactions. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G413–G419 (2017).
Fianchi, F. et al. NonalcohoLic Fatty Liver Disease (NAFLD) as model of gut-liver axis interaction: from pathophysiology to potential target of treatment for personalized therapy. Int. J. Mol. Sci. 22, 6485 (2021).
Yu, Z. et al. Crosstalk between adipose tissue and the microbiota-gut-brain axis in metabolic diseases. Int. J. Biol. Sci. 18, 1706–1723 (2022).
Chang, L. et al. Efficacy of linaclotide in reducing abdominal symptoms of bloating, discomfort, and pain: a phase 3B trial using a novel abdominal scoring system. Am. J. Gastroenterol. 116, 1929–1937 (2021).
Rao, S. S. C. et al. Randomised clinical trial: linaclotide vs placebo-a study of bi-directional gut and brain axis. Aliment. Pharmacol. Ther. 51, 1332–1341 (2020).
Murillo Perez, C. F. et al. Greater transplant-free survival in patients receiving obeticholic acid for primary biliary cholangitis in a clinical trial setting compared to real-world external controls. Gastroenterology 163, 1630–1642.e1633 (2022).
Kjaergaard, K. et al. Obeticholic acid improves hepatic bile acid excretion in patients with primary biliary cholangitis. J. Hepatol. 74, 58–65 (2021).
Ray, K. Positive phase III results for odevixibat for progressive familial intrahepatic cholestasis. Nat. Rev. Gastroenterol. Hepatol. 19, 556 (2022).
Rao, S. S. C. et al. Randomized placebo-controlled phase 3 trial of vibrating capsule for chronic constipation. Gastroenterology 164, 1202–1210.e1206 (2023).
Syed, Y. Y. Sodium oligomannate: first approval. Drugs 80, 441–444 (2020).
Xiao, S. et al. A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia. Alzheimers Res. Ther. 13, 62 (2021).
Bureau, C. et al. The use of rifaximin in the prevention of overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt : a randomized controlled trial. Ann. Intern. Med. 174, 633–640 (2021).
Wong, V. W. et al. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann. Hepatol. 12, 256–262 (2013).
Silva-Sperb, A. S. et al. Effect of probiotic supplementation in nonalcoholic steatohepatitis patients: PROBILIVER TRIAL protocol. Trials 20, 580 (2019).
Kirpich, I. A. et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol 42, 675–682 (2008).
Stadlbauer, V. et al. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J. Hepatol. 48, 945–951 (2008).
Han, S. H. et al. Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study. Eur. J. Gastroenterol. Hepatol. 27, 1300–1306 (2015).
Nicolucci, A. C. et al. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology 153, 711–722 (2017).
Lambert, J. E. et al. Gut microbiota manipulation with prebiotics in patients with non-alcoholic fatty liver disease: a randomized controlled trial protocol. BMC Gastroenterol 15, 169 (2015).
Scorletti, E. et al. Synbiotics alter fecal microbiomes, but not liver fat or fibrosis, in a randomized trial of patients with nonalcoholic fatty liver disease. Gastroenterology 158, 1597–1610.e1597 (2020).
Harrison, S. A. et al. Efficacy and safety of aldafermin, an engineered FGF19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic steatohepatitis. Gastroenterology 160, 219–231.e211 (2021).
Armstrong, M. J. et al. Liraglutide efficacy and action in non-alcoholic steatohepatitis (LEAN): study protocol for a phase II multicentre, double-blinded, randomised, controlled trial. BMJ Open 3, e003995 (2013).
Craven, L. et al. Allogenic fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: a randomized control trial. Am. J. Gastroenterol. 115, 1055–1065 (2020).
Bajaj, J. S. et al. Fecal microbial transplant capsules are safe in hepatic encephalopathy: a phase 1, randomized, placebo-controlled trial. Hepatology 70, 1690–1703 (2019).
Bajaj, J. S. et al. Microbial functional change is linked with clinical outcomes after capsular fecal transplant in cirrhosis. JCI Insight 4, e133410 (2019).
Yu, E. W. et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med 17, e1003051 (2020).
Allegretti, J. R. et al. Effects of fecal microbiota transplantation with oral capsules in obese patients. Clin. Gastroenterol. Hepatol. 18, 855–863.e852 (2020).
Philips, C. A. et al. Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic hepatitis: a pilot study. Clin. Gastroenterol. Hepatol. 15, 600–602 (2017).
Fazelzadeh, P. et al. Global testing of shifts in metabolic phenotype. Metabolomics 14, 139 (2018).
Strozyk, A., Horvath, A., Muir, J. & Szajewska, H. Effect of a low-FODMAP diet for the management of functional abdominal pain disorders in children: a study protocol for a randomized controlled trial. Nutr. J. 20, 1 (2021).
van Megen, F. et al. A low FODMAP diet reduces symptoms in treated celiac patients with ongoing symptoms-a randomized controlled trial. Clin. Gastroenterol. Hepatol. 20, 2258–2266.e2253 (2022).
Wu, J. et al. Gut-brain axis dysfunction underlies FODMAP-induced symptom generation in irritable bowel syndrome. Aliment. Pharmacol. Ther. 55, 670–682 (2022).
Masuy, I., Van Oudenhove, L., Tack, J. & Biesiekierski, J. R. Effect of intragastric FODMAP infusion on upper gastrointestinal motility, gastrointestinal, and psychological symptoms in irritable bowel syndrome vs healthy controls. Neurogastroenterol. Motil 30 (2018).
Bennet, S. M. P. et al. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut 67, 872–881 (2018).
Zhang, Y. et al. Low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet compared with traditional dietary advice for diarrhea-predominant irritable bowel syndrome: a parallel-group, randomized controlled trial with analysis of clinical and microbiological factors associated with patient outcomes. Am. J. Clin. Nutr. 113, 1531–1545 (2021).
Laatikainen, R. et al. Randomised clinical trial: effect of low-FODMAP rye bread versus regular rye bread on the intestinal microbiota of irritable bowel syndrome patients: association with individual symptom variation. BMC Nutr 5, 12 (2019).
Huaman, J. W. et al. Effects of prebiotics vs a diet low in FODMAPs in patients with functional gut disorders. Gastroenterology 155, 1004–1007 (2018).
Ankersen, D. V. et al. Long-term effects of a web-based low-FODMAP diet versus probiotic treatment for irritable bowel syndrome, including shotgun analyses of microbiota: randomized, double-crossover clinical trial. J. Med. Internet Res. 23, e30291 (2021).
Rafferty, A. J., Hall, R. & Johnston, C. S. A novel mobile app (Heali) for disease treatment in participants with irritable bowel syndrome: randomized controlled pilot trial. J. Med. Internet Res. 23, e24134 (2021).
Nordin, E., Hellstrom, P. M., Brunius, C. & Landberg, R. Modest conformity between self-reporting of bristol stool form and fecal consistency measured by stool water content in irritable bowel syndrome and a FODMAP and gluten trial. Am. J. Gastroenterol. 117, 1668–1674 (2022).
Dieterich, W. et al. Influence of low FODMAP and gluten-free diets on disease activity and intestinal microbiota in patients with non-celiac gluten sensitivity. Clin. Nutr. 38, 697–707 (2019).
Rej, A. et al. Efficacy and acceptability of dietary therapies in non-constipated irritable bowel syndrome: a randomized trial of traditional dietary advice, the low FODMAP diet, and the gluten-free diet. Clin. Gastroenterol. Hepatol. 20, 2876–2887.e2815 (2022).
Berentsen, B. et al. Study protocol of the Bergen brain-gut-microbiota-axis study: A prospective case-report characterization and dietary intervention study to evaluate the effects of microbiota alterations on cognition and anatomical and functional brain connectivity in patients with irritable bowel syndrome. Medicine 99, e21950 (2020).
Carbone, F. et al. Diet or medication in primary care patients with IBS: the DOMINO study - a randomised trial supported by the Belgian Health Care Knowledge Centre (KCE Trials Programme) and the Rome Foundation Research Institute. Gut 71, 2226–2232 (2022).
Menees, S. B. et al. A randomized pilot study to compare the effectiveness of a low FODMAP diet vs psyllium in patients with fecal incontinence and loose stools. Clin. Transl. Gastroenterol. 13, e00454 (2022).
Silva, A. R. et al. An anti-inflammatory and low fermentable oligo, di, and monosaccharides and polyols diet improved patient reported outcomes in fibromyalgia: A randomized controlled trial. Front. Nutr. 9, 856216 (2022).
Wang, Y. et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol. Res. 157, 104784 (2020).
Pinto-Sanchez, M. I. et al. Probiotic bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 153, 448–459.e448 (2017).
Mack, I. et al. A nonviable probiotic in irritable bowel syndrome: a randomized, double-blind, placebo-controlled, multicenter study. Clin. Gastroenterol. Hepatol. 20, 1039–1047.e1039 (2022).
Andresen, V., Gschossmann, J. & Layer, P. Heat-inactivated Bifidobacterium bifidum MIMBb75 (SYN-HI-001) in the treatment of irritable bowel syndrome: a multicentre, randomised, double-blind, placebo-controlled clinical trial. Lancet Gastroenterol. Hepatol. 5, 658–666 (2020).
Ghaderi, A. et al. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry 19, 77 (2019).
Okubo, R. et al. Effect of bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: a proof-of-concept study. J. Affect Disord. 245, 377–385 (2019).
Lu, C. S. et al. The add-on effect of lactobacillus plantarum ps128 in patients with parkinson’s disease: a pilot study. Front. Nutr. 8, 650053 (2021).
Ibrahim, A. et al. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: a randomised controlled trial. PLoS ONE 15, e0244680 (2020).
Barichella, M. et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology 87, 1274–1280 (2016).
Tamtaji, O. R. et al. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Clin. Nutr. 38, 1031–1035 (2019).
Gomez-Eguilaz, M., Ramon-Trapero, J. L., Perez-Martinez, L. & Blanco, J. R. The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: a pilot study. Benef. Microbes 9, 875–881 (2018).
Ghavami, A. et al. Effect of synbiotic supplementation on migraine characteristics and inflammatory biomarkers in women with migraine: results of a randomized controlled trial. Pharmacol. Res. 169, 105668 (2021).
Martami, F. et al. The effects of a multispecies probiotic supplement on inflammatory markers and episodic and chronic migraine characteristics: A randomized double-blind controlled trial. Cephalalgia 39, 841–853 (2019).
de Roos, N. M. et al. The effects of a multispecies probiotic on migraine and markers of intestinal permeability-results of a randomized placebo-controlled study. Eur. J. Clin. Nutr. 71, 1455–1462 (2017).
Jacobs, J. P. et al. Cognitive behavioral therapy for irritable bowel syndrome induces bidirectional alterations in the brain-gut-microbiome axis associated with gastrointestinal symptom improvement. Microbiome 9, 236 (2021).
Lackner, J. M. et al. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel syndrome. Gastroenterology 155, 47–57 (2018).
Acknowledgements
The National Natural Science Foundation of China (82074069) and Tianjin Municipal Science and Technology Committee (20YFZCSY00560) funded this work.
Author information
Authors and Affiliations
Contributions
M.Y.Y. conceived, designed, and wrote the manuscript, and designed the figure. S.L.M. critically revised the manuscript and contributed to writing the final manuscript. L.M. critically revised the manuscript. B.Y.S., L.P.G. and L.Q.H. wrote-review and edited. W.Y.G. supervised the completion of the manuscript. All authors read and approved the manuscript for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, M., Man, S., Sun, B. et al. Gut liver brain axis in diseases: the implications for therapeutic interventions. Sig Transduct Target Ther 8, 443 (2023). https://doi.org/10.1038/s41392-023-01673-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-023-01673-4