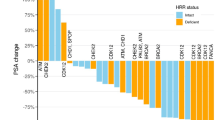
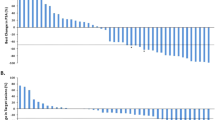
Abstract
Bipolar androgen therapy (BAT) is effective in a subset of metastatic castration-resistant prostate cancer (mCRPC) patients. Treatment selection biomarkers are needed due to other therapies that can be equally efficacious. We performed post-hoc analysis to determine whether baseline serum testosterone (T) is a treatment selection marker in the TRANSFORMER study, a randomized trial of abiraterone-pretreated mCRPC patients assigned to BAT (n = 94) or enzalutamide (n = 101). The findings suggest that patients with poor outcomes to abiraterone and serum T ≥ 20 ng/dL may benefit preferentially from BAT over enzalutamide. Baseline testosterone could be considered in the treatment selection process when BAT is an option.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Denmeade S, Antonarakis ES, Markowski MC. Bipolar androgen therapy (BAT): a patient’s guide. Prostate. 2022;82:753–62.
Denmeade SR, Wang H, Agarwal N, Smith DC, Schweizer MT, Stein MN, et al. TRANSFORMER: a randomized phase II study comparing bipolar androgen therapy versus enzalutamide in asymptomatic men with castration-resistant metastatic prostate cancer. J Clin Oncol. 2021;39:1371–82.
Klotz L, O’Callaghan C, Ding K, Toren P, Dearnaley D, Higano CS, et al. Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: a secondary analysis of the PR-7 trial of intermittent versus continuous ADT. J Clin Oncol. 2015;33:1151–6.
Miura N, Mori K, Mostafaei H, Quhal F, Sari Motlagh R, Abufaraj M, et al. Prognostic value of testosterone for the castration-resistant prostate cancer patients: a systematic review and meta-analysis. Int J Clin Oncol. 2020;25:1881–91.
Vivot A, Boutron I, Beraud-Chaulet G, Zeitoun JD, Ravaud P, Porcher R. Evidence for treatment-by-biomarker interaction for FDA-approved oncology drugs with required pharmacogenomic biomarker testing. Sci Rep. 2017;7:6882.
Tan YG, Quek SZH, Huang HH, Ho HSS, Yuen JSP, Tay KJ, et al. Serum testosterone levels and testosterone ‘bounce’ phenomenon predict response to novel anti-androgen therapies in castration-resistant prostate cancer. Urol Oncol. 2021;39:829.e9–829.e17.
Li Z, Bishop AC, Alyamani M, Garcia JA, Dreicer R, Bunch D, et al. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature. 2015;523:347–51.
Efstathiou E, Titus M, Wen S, Hoang A, Karlou M, Ashe R, et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol. 2015;67:53–60.
Miyazawa Y, Sekine Y, Arai S, Nakamura T, Takezawa Y, Shimizu N, et al. A prospective study of the relationship between clinical outcomes after enzalutamide and serum androgen levels measured via liquid chromatography-tandem mass spectrometry in patients with castration-resistant prostate cancer. Eur Urol Open Sci. 2021;29:59–67.
Vaillancourt J, Turcotte V, Caron P, Villeneuve L, Lacombe L, Pouliot F, et al. Glucuronidation of abiraterone and its pharmacologically active metabolites by UGT1A4, influence of polymorphic variants and their potential as inhibitors of steroid glucuronidation. Drug Metab Dispos. 2020;48:75–84.
Funding
Supported by a Transformative Grant (W81XWH-14-2-0189) (to S.R.D.) from the Department of Defense Prostate Cancer Research Program. ESA is partially supported by NCI Cancer Center Support Grant P30 CA077598 and DOD grant W81XWH-22-2-0025. MK is partially supported by a Prostate Cancer Foundation Young Investigator Award.
Author information
Authors and Affiliations
Contributions
Samuel R Denmeade and Hao Wang had full access to all the data in the study. Jun Luo takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Luo, Antonarakis. Acquisition of data: Samuel R Denmeade and TRANSFORMER investigators. Analysis and interpretation of data: all authors. Drafting of the manuscript: Kanayama, Antonarakis, Luo. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Tsai, Wang. Obtaining funding: Denmeade. Administrative, technical, or material support: Denmeade. Supervision: Luo, Denmeade, Wang, Antonarakis. Other: None.
Corresponding author
Ethics declarations
Competing interests
All authors declare that they have no conflicts of interest in relation to the article. The authors would like to disclose the following financial relationships that are not related to this article. J Luo has served as a paid consultant/advisor for Sun Pharma. J Luo has received research funding for his institution from Sanofi, Constellation, Calibr, and Cardiff Oncology and is a co-inventor of a technology owned by Johns Hopkins University and licensed to Qiagen and A&G. ES Antonarakis has served as a paid consultant/advisor for Sanofi, Dendreon, Janssen Biotech, ESSA, Merck, AstraZeneca, Clovis Oncology, Lilly, Bayer, and received honorarium from Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, Astellas Pharma, Merck, AstraZeneca, Clovis Oncology. E Antonarakis received research funding from Janssen Biotech, Johnson & Johnson, Sanofi, Dendreon, Aragon Pharmaceuticals, Exelixis, Millennium, Genentech, Novartis, Astellas Pharma, Tokai Pharmaceuticals, Merck, AstraZeneca, Clovis Oncology, Constellation Pharmaceuticals, as well as travel accommodations from Sanofi, Dendreon, Medivation. E Antonarakis is a co-inventor of a technology owned by Johns Hopkins University and licensed to Qiagen. S Denmeade has Stock and Other Ownership Interests in Sophiris Bio, served as Consultant/advisor for Sophiris Bio, and received travel accommodations from Sophiris Bio.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kanayama, M., Tsai, HL., Wang, H. et al. Baseline serum testosterone and differential efficacy of bipolar androgen therapy and enzalutamide in the randomized TRANSFORMER trial. Prostate Cancer Prostatic Dis (2024). https://doi.org/10.1038/s41391-024-00844-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-024-00844-w