Abstract
To date, only few data concerning the biologically active, free form of testosterone (FT) are available in metastatic prostate cancer (mPC) and the impact of FT on disease, therapy and outcome is largely unknown. We retrospectively studied the effect of docetaxel on FT and total testosterone (TT) serum levels in 67 mPC patients monitored between April 2008 and November 2020. FT and TT levels were measured before and weekly during therapy. The primary endpoint was overall survival (OS). Secondary endpoints were prostate-specific antigen response and radiographic response (PSAR, RR), progression-free survival (PFS), FT/TT levels and safety. Median FT and TT serum levels were completely suppressed to below the detection limit during docetaxel treatment (FT: from 0.32 to < 0.18 pg/mL and TT: from 0.12 to < 0.05 ng/mL, respectively). Multivariate Cox regression analyses identified requirement of non-narcotics, PSAR, complete FT suppression and FT nadir values < 0.18 pg/mL as independent parameters for PFS. Prior androgen-receptor targeted therapy (ART), soft tissue metastasis and complete FT suppression were independent prognostic factors for OS. FT was not predictive for treatment outcome in mPC patients with a history of ART.
Similar content being viewed by others
Introduction
Prostate cancer (PC) growth and progression is androgen-dependent and androgen deprivation therapy (ADT) is an effective strategy to manage advanced disease1,2,3. Historically, suppression of total testosterone (TT) to a level lower than 50 ng/dL (< 1.7 nmol/L) has been defined as castration. However, a testosterone suppression target of less than 20 ng/dL improves patient survival and delays tumor progression1,4. Androgen receptor (AR) targeted therapy (ART) in combination with ADT led to testosterone suppression to near zero and further improved patient survival1. Castration-resistance occurs despite castrate testosterone levels through reactivation of AR pathways from multiple mechanisms. Furthermore, neuroendocrine transdifferentiation may also occur in PC and lead to castration resistance, which results in shorter progression-free (PFS) and overall survival (OS)1. Due to its critical role, testosterone levels should be monitored frequently during therapy1.
Ryan et al. found that docetaxel therapy significantly reduced androgen levels, including total testosterone (TT), with increased OS (26.3 vs. 20.9 months), with better outcome in patients with high versus low reduction of androgen levels, respectively5. Total serum androgens (TT, androstenedione, DHEAS) were described as important biomarkers in PC treatment and may be useful in risk stratification in future studies5,6.
Plasma testosterone (total testosterone, TT) circulates specifically bound to sex hormone-binding globulin (SHBG) (50–70%), nonspecifically bound to albumin (20–30%) or other proteins (4%) and unbound (1–3%), or free (FT)7. Bioavailable testosterone (BT) is calculated by adding FT and albumin-bound testosterone levels7,8. Quantification of FT is an efficient method to evaluate BT8. Although FT is a key target in the treatment of advanced prostate cancer, the effects that FT may have on PC treatment and outcome are largely unknown9. The castration level of FT was established to be < 1.7 pg/mL (< 5.9 pmol/L)8. Von Klot et al. identified FT < 0.5 pg/mL as a prognostic marker in second-line therapy, which was associated with improved cancer-related survival (43.6 vs. 17.3 months)10.
Docetaxel seems to be less efficient after treatment with ART, with a reduced PSAR rate after ART (median PSAR group 1: docetaxel → cabazitaxel → ART: 59.8%; group 2: docetaxel → ART → cabazitaxel: 64.3%; group 3: ART → docetaxel → cabazitaxel: 44.0%; p = 0.021) and significantly shorter PFS and OS (median radiographic PFS for group 1: 26.9 (14.8-NR), group 2: 11.0 (9.5–12.9) and group 3: 6.6 (5.0–10.2), p < 0.001; median OS for group 1: 34.8 (32.4–41.5) months; group 2: 35.8 833.9–38.4) months; group 3: 28.9 (23.3–35.9) months; p = 0.007)11. A certain cross-resistance of docetaxel and ART was discussed as a potential explanation for these findings11,12. Studies suggest that docetaxel influences androgen receptor signaling, while testosterone impairs cellular uptake of docetaxel and inhibits stabilization of microtubules. This led to the proposal that testosterone levels should be decreased during chemotherapy with docetaxel13,14.
Even in the castration-resistant setting, PC tumors still rely on androgen signaling15. Hence, the goal of this study was to investigate the effect of docetaxel on FT and TT at different stages of metastatic PC (e.g. castration-naïve (mCNPC, group 1), castration-resistant (mCRPC, group 2) and castration-resistant patients with prior ART (mCRPC-ART, group 3)).
Methods
Patients
For this translational biomarker study, data from patients with mPC treated with docetaxel at Hannover Medical School from April 2008 to November 2020 were retrospectively analyzed and followed up until death or until December 2020. Eligibility criteria were a histologically confirmed mPC and disease progression according to the PCWG2/3 criteria4,16. Patients who received at least two cycles of docetaxel were included. All data were collected following patient informed consent, in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines, and with the Hannover Medical School institutional review board approval (13th August 2008). Patients were stratified into three subgroups: (1) castration-naïve disease (mCNPC), (2) castration-resistant disease (mCRPC) and (3) mCRPC patients with a history of ART (mCRPC-ART).
Treatment plan
At baseline, medical history and physical examination were performed, including an initial staging. Patients received 75 mg/m2 of docetaxel intravenously every 3 weeks (q3w), 50 mg/m2 every 2 weeks (q2w) or 30–35 mg/m2 weekly on days 1, 8 and 15 (q1w). Concomitant use of dexamethasone and oral prednisone (5 mg) twice a day were part of the regimen. ADT was continued throughout therapy. Morning FT and TT (8–11 am) were obtained before and weekly during treatment (on average 28 samples per patient) using an enzyme immunoassay (ELISA from IBL, International GmbH, Hamburg, Germany) and a direct, competitive, chemiluminescence immunoassay (CLIA) (LIAISON® Testosterone Assay, Diasorin S.p.A., Saluggia, Italy). PSA levels, carcinoembryonic antigen (CEA) levels, neuroendocrine tumor markers (neuron-specific enolase (NSE), chromogranin A (CgA)), lactate dehydrogenase (LDH), alkaline phosphatase (AP), hemoglobin and analgesic requirement were parameters at baseline to possibly predict OS16. Patients were assessed according to PCWG2/3 criteria and RECIST 1.1 by CT and bone scan every three months or if tumor progression was suspected4,17,18. Pain and use of pain medications were monitored by clinician interview. Toxicity was graded according to the Common Terminology Criteria for Adverse Events v3.0 (CTCAE).
Data analysis
The primary study end point was OS, defined by the initiation of docetaxel therapy until death. Secondary endpoints were PFS, defined as the time between the start of docetaxel therapy until progression according to PCWG2/3 criteria4,16, PSA response (PSAR), which is defined by a decline of > 50% from baseline, and FT reduction of 100% from baseline and safety. Radiographic response (RR) was evaluated according to RECIST 1.1.17. Follow-up data were collected throughout December 2020.
Statistical analyses were performed using SPSS statistics v26.0. Categorical variables were summarized, numeric variables were analyzed in median and range. Logistic regression was used to estimate the prognostic significance of FT suppression in predicting ≥ 50% decline in PSA from baseline. Cox proportional hazards regression modeling was used to determine the prognostic significance of baseline characteristics on PFS and OS. Chi-square tests and t-tests were applied to estimate p values of variables at baseline. Uni- and multivariate Cox regression analyses were used to demonstrate the impact of covariates on PFS and OS. Non-proportionality was assessed by plotting the Kaplan–Meier survival distribution as a function of the survival time for each level of the covariate and plotting the function log(-log(survival probability)) as a function of the log survival time19. Additionally, extended Cox modelling with time-by-covariates and conditional landmark analyses were used to remove potential guarantee-time bias, specifically the time-window bias, which is introduced because of differential exposure opportunity time windows between subjects20. Only p values of < 0.05 were considered statistically significant for all comparisons.
Results
Patient characteristics
A total of 67 patients with a histologically confirmed metastatic adenocarcinoma of the prostate were analyzed. Seven patients were castration-naïve (mCNPC, group 1), 26 patients were castration-resistant after ADT (mCRPC, group 2) and 34 patients were castration resistant after ADT plus ART (mCRPC-ART, group 3). The median age at time of diagnosis was 69 years (Table 1).
At baseline, FT levels below the detection limit were more common in group 3 than in groups 1 and 2 (p < 0.001). PSA, FT and TT were higher in group 1 than in groups 2 and 3, whereas hemoglobin was lower. Overall, 95.5% patients had bone metastases and 62.7% had soft tissue metastases. A median of two organs were involved by metastatic disease. All other characteristics were well-balanced (Table 1).
Effects of docetaxel chemotherapy on TT and FT
In the overall study population, serum levels of TT were reduced from a median of 0.12 ng/mL at baseline to non-detectable levels (< 0.05 ng/mL) at nadir (p = 0.014) and FT levels were reduced from 0.32 pg/mL at baseline to non-detectable levels at nadir (< 0.18 pg/mL) during docetaxel chemotherapy (p = 0.006; Fig. 1). The rate of patients with TT levels under the detection limit (< 0.05 ng/mL) increased from 23/55 (41.8%) at baseline (all 23 receiving abiraterone at that time point) to 49/60 patients (81.7%) at nadir during chemotherapy with docetaxel (p = 0.014) (Fig. 1). Similarly, the rate of patients with FT levels under the detection limit (< 0.18 pg/mL) increased from 17/58 (29.3%) (all 17 receiving abiraterone at that time point) at baseline to 46/61 (75.4%) at nadir during chemotherapy with docetaxel (p = 0.006) (Fig. 1). Complete FT suppression below the detection limit (< 0.18 pg/mL) was observed in 6/7 patients (85.7%) of group 1 (mCNPC), 11/20 (55%) of group 2 (mCRPC) and 8/27 (29.6%) of group 3 (mCRPC-ART) (Fig. 2A). FT suppression below the detection limit (< 0.18 pg/mL) was significantly associated with PSA response (p = 0.008; odds ratio 0.111 95% CI 0.022–0.564) (Fig. 2B). FT suppression was also associated with radiographic response (RECIST) (p = 0.051; odds ratio 0.218 95% CI 0.047–1.005; chi-square test p = 0.006) (Fig. 2C). Partial remission was observed in 5/5 patients of group 1 (mCNPC), 5/13 patients (38.5%) of group 2 (mCRPC) and 8/18 patients (44.4%) of group 3 (mCRPC-ART) (Fig. 2C). The median time to FT nadir was 14 days (95% CI 12.8/28.5, n = 37).
Distribution of baseline, median and nadir values of total testosterone (TT) and free testosterone (FT) levels. Castration level for TT is defined as 0.5 ng/mL4 and for FT as 1.7 pg/mL9. Numbers in brackets demonstrate number of patients with testosterone levels below detection limit. Black dots: mCNPC patients, grey dots: mCRPC patients, white dots: mCRPC-ART patients. The grey bars depict the median values of each group. The discrepancy in sample numbers in the groups is due to missing values in the database.
Response to docetaxel. (A) Free testosterone (FT) reduction, complete reduction (CR) is marked striped in every waterfall plot. White columns show data of patients with no CR and squared columns show missing values. (B) PSA response (PSAR) defined as decrease of 50% from baseline according to PCWG2/3 criteria4,17 during therapy is shown in the second plot. (C) Shrinkage of soft tissue tumor metastases is given as percent from baseline according to RECIST 1.1 (response evaluation criteria in solid tumors)18.
Clinical outcome
Patients underwent a median of eight cycles of docetaxel (range 2–108) and were treated with a median cumulative dosage of 663 mg/m2 (range 100–8100 mg/m2). Group 1 (mCNPC) and group 2 (mCRPC) received a higher cumulative docetaxel dose than group 3 (mCRPC-ART) (1275 mg/m2 vs. 450 mg/m2, p < 0.001, 750 mg/m2 vs. 450 mg/m2, p = 0.004, respectively, Table 2). The overall PSAR rate (≥ 50%) was 43/67 (64.2%): 7/7 (100%) in group 1 (mCNPC), 19/26 (73.1%) in group 2 (mCRPC) and 17/34 (50%) in group 3 (mCRPC-ART) (p = 0.13 and p = 0.014, respectively; Fig. 2B, Table 2). The overall median PSA reduction was − 66.5% (range + 100 to − 99.9%): a median of − 99.7% in group 1; − 70.9% in group 2 and − 50% in group 3, respectively (p = 0.007) (Table 2). The median time to PSAR (≥ 50%) was 44 days (range 7–329).
There was a higher rate of improvement of bone lesions in group 1 than group 2 (42.9% vs. 7.7%, p = 0.034, Table 2). The shrinkage of soft tissue metastases objectified by RECIST 1.1 was significantly higher in group 1 than group 2 (− 75.8% vs. − 21.9, p = 0.002; Fig. 2C and Table 2). The rate of partial remission was also significantly higher in group 1 than in groups 2 and 3 (71.4% vs. 19.2%, p = 0.017 and 71.4% vs. 23.5%, p = 0.02, respectively).
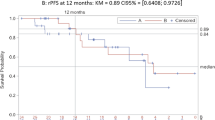
Median OS was 25.7 months (95% CI 18.9–32.5) in all patients. mCNPC patients experienced a median OS of 33.7 months (95% CI 30.9–36.5), whereas patients in group 3 (mCRPC-ART) had a median OS of 16.8 months (95% CI 16.2–17.4; p = 0.002). Median PFS in all patients was 7.8 months (95% CI 3.9–11.7): 15.4 months (95% CI 14.7–16.2) for group 1, 11.3 months (95% CI 6.3–16.4) for group 2 and 5.5 months (95% CI 2.5–8.6) in group 3 (p = 0.033 and p = 0.018, respectively; Table 2, Fig. 3).
Multivariate analyses
Univariate analyses of baseline parameters associated with OS are summarized in Table 3. In multivariate analyses with stepwise regression, only prior ART and the presence of soft tissue metastases (lymphatic, hepatic, pulmonary, brain) remained independent predictors of a shorter OS (Table 3).
In univariate and time-dependent covariate analyses (T_; ln(T_); T_ ≥ 365 days; data not shown) of treatment-dependent parameters (e. g. FT-, PSA-response), only FT median < 0.3 pg/mL and complete FT suppression (reduction of 100%) during docetaxel therapy were associated with a longer OS. In multivariate analysis, complete FT reduction (100%) remained an independent predictor for better OS (Table 3).
In addition to extended time-dependent Cox modelling, log(-log(survival probability)) and conditional landmark analyses were performed and demonstrated that the FT reduction = 100% during docetaxel therapy had no association with time regarding OS (data not shown).
In multivariate analysis of the baseline parameters, only the requirement of non-narcotics remained an independent predictor of a better PFS (Table 3). In univariate analysis of treatment-dependent parameters, PSAR, FT suppression (100%), FT median values < 0.2 pg/mL and FT nadir values < 0.18 pg/mL during docetaxel therapy were associated with a better PFS. In multivariate analysis with stepwise regression, only PSAR, FT reduction = 100% and FT nadir values < 0.18 pg/mL remained independent predictors of a better PFS. Applying extended time-dependent Cox modelling (T_; ln(T_); T_ ≥ 365 days), log(-log(survival probability)) and conditional landmark analyses demonstrated that these parameters had no association with time, with the exception of ln(T_) for PSAR, which revealed a significant time-dependence (p = 0.015, Table 3) (data not shown).
Discussion
In this study, we demonstrate that TT and FT serum levels are reduced during docetaxel chemotherapy and that FT suppression under the detection limit (100%) resulted in better PFS and OS in mCNPC and mCRPC patients, but not in mCRPC patients with a history of ART. Interestingly, in contrast to TT, only FT was a significant predictor for PFS and OS, demonstrating a major biological role of FT for treatment outcome. mCRPC-ART patients had a significantly lower FT reduction rate due to low FT levels at baseline (8/27 vs. 6/7 and 11/20, respectively) and FT reduction was no longer a predictor for better PFS or OS (Table 2). mCRPC-ART patients experienced a lower PSAR and shorter PFS and OS. These results are consistent with several previous studies that showed decreased efficacy of docetaxel in PC patients with a history of ART11,12,21,22. Our data suggest that the worst clinical outcome of mCRPC-ART patients towards docetaxel is due to progressing castration resistance.
Multiple lines of evidence suggest that docetaxel and prednisone might directly interfere with testosterone biosynthesis and metabolism in mPC patients and contribute to this FT suppressing effect. Prednisone was demonstrated to lower serum TT, androstenedione, dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) levels in some metastatic PC patients by suppressing the hypothalamic–pituitary–adrenal axis, but had no antitumor activity in mCRPC23,24. Consistent with this observation, a history of previous prednisone treatment had no effect in multivariate analyses on PFS or OS in our study.
The role of docetaxel in reducing testosterone levels is less clear, although serum androgens (TT, androstenedione and DHEA) decline during docetaxel treatment5. Docetaxel metabolism is largely catalyzed by CYP3A425 and docetaxel was shown to induce CYP3A4, which is responsible for the greatest portion of testosterone 6β- and 16β-hydroxylation26,27,28. CYP3A4 induction may lower testosterone levels by inactivation through 6β- and 16β-hydroxylation. The effect of docetaxel on CYP17A1 is unclear26,27,28.
Franke et al. reported castration-dependent pharmacokinetics of docetaxel in PC patients. Docetaxel clearance was increased by approximately 100% in castrated men and was associated with a two-fold reduction in area under the curve, although hepatic activity of CYP3A4 was unchanged29. Conversely, castration-naïve patients were exposed to higher amounts of the drug, which was accompanied by more severe hematotoxicity29. This study also demonstrated that lower intracellular docetaxel levels caused by lower baseline levels of testosterone resulted in a lower response rate to treatment29. Consistent with these results, group 1 patients (mCNPC) had a significantly higher rate of grade 3 and 4 neutropenia compared to group 3 patients (mCRPC-ART) in our study (57.1% vs. 20.6%, p = 0.047; Table 2) and a significantly better clinical outcome (Figs. 2, 3, Table 2).
Ryan et al. showed that conversion from higher to lower androgen levels (e.g. above/below median) during docetaxel therapy contributed to superior survival as the reduction is the driving mechanism behind the clinical responses5. Consistent with these findings, 6/7 (85.7%) mCNPC patients in our study underwent a complete (= 100%) and one patient a nearly complete (99.3%) FT reduction and had a PSA response rate of 100%.
In recent years, several large phase 3 trials in patients with mCNPC (e.g. CHAARTED, STAMPEDE, GETUG-3, LATTITUDE, TITAN, PREVAIL) demonstrated that the addition of docetaxel and ART (abiraterone, apalutamide and enzalutamide) to ADT is associated with significantly improved PFS and/or OS compared to ADT alone30. Our data demonstrate that docetaxel therapy is associated with similarly low testosterone levels (FT + TT) as achieved by Abiraterone + ADT (Fig. 1, Tables 1, 2).
As with many retrospective analyses, the retrospective design of our study also has some limitations. For example, the patient population size is small, and although high numbers of FT and TT measurements were accomplished, these were not always assessed on a regular basis (e.g. weekly). Furthermore, progression was mainly due to PSA progression (e.g. PSA progression or radiographic progression, whichever presented first). Despite all of our efforts to address possible lead-time bias (e.g. use of extended time-dependent Cox modelling (T_; ln(T_); T_ ≥ 365 days), log(-log(survival probability)) and conditional landmark analyses), there is still the risk that our analyses are subject to lead-time bias, as group 3 patients (mCRPC-ART) had more advanced disease at baseline compared to groups 1 (mCNPC) and 2 (mCRPC). Group 1 was small due to a recent trend towards abiraterone treatment in this setting and patients had a very high-volume disease that required intensive treatment. In addition, scanning intervals were not always uniformly assessed and confirmatory scans were not conducted in general.
Conclusion
This study represents the strongest evidence to date that FT plays a fundamental role during docetaxel chemotherapy. In mCNPC and mCRPC patients, complete FT suppression (= 100%) during chemotherapy was an independent predictor of PSAR, RR, PFS and OS. However, in mCRPC patients with a history of ART, FT was not linked to the clinical outcome. Our data suggest that castration-dependent pharmacokinetics of docetaxel seem to reduce its clinical effectiveness in mCRPC-ART patients.
References
Crawford, E. D. et al. Androgen-targeted therapy in men with prostate cancer: Evolving practice and future considerations. Prostate Cancer Prostatic Dis. 22, 24–38 (2019).
Teo, M. Y., Rathkopf, D. E. & Kantoff, P. Treatment of advanced prostate cancer. Annu Rev Med. 70, 479–499 (2019).
Sartor, O. & de Bono, J. S. Metastatic prostate cancer. N. Engl. J. Med. 378, 645–657 (2018).
Scher, H. I. et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 26, 1148–1159 (2008).
Ryan, C. J. et al. Androgen decline and survival during docetaxel therapy in metastatic castration resistant prostate cancer (mCRPC). Prostate Cancer Prostatic Dis. 23, 66–73 (2020).
Ryan, C. J. et al. Serum androgens as prognostic biomarkers in castration-resistant prostate cancer: Results from an analysis of a randomized phase III trial. J. Clin. Oncol. 31, 2791–2798 (2013).
Diver, M. Laboratory measurement of testosterone. Front. Horm. Res. 37, 21–31 (2009).
Morote, J. et al. Behavior of free testosterone in patients with prostate cancer on androgen deprivation therapy. Int. J. Biol. Mark. 20, 119–222 (2005).
Rove, K. O. et al. Maximal testosterone suppression in prostate cancer—Free vs total testosterone. Urology 83, 1217–1222 (2014).
von Klot, C. A. et al. Role of free testosterone levels in patients with metastatic castration-resistant prostate cancer receiving second-line therapy. Oncol. Lett. 13, 22–28 (2017).
Delanoy, N. et al. Sequencing of taxanes and new androgen-targeted therapies in metastatic castration-resistant prostate cancer: Results of the international multicentre retrospective CATS database. Eur. Urol. Oncol. 1, 467–475 (2018).
Mezynski, J. et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: Clinical evidence for cross-resistance?. Ann. Oncol. 23, 2943–2947 (2012).
Lolli, C. et al. Testosterone levels and androgen receptor copy number variations in castration-resistant prostate cancer treated with abiraterone or enzalutamide. Prostate 79, 1211–1220 (2019).
Gan, L. et al. Inhibition of the androgen receptor as a novel mechanism of taxol chemotherapy in prostate cancer. Cancer Res. 69, 8386–8394 (2009).
Montgomery, R. B. et al. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 68, 4447–4454 (2008).
Halabi, S. et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 32, 671–677 (2014).
Scher, H. I. et al. Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 34, 1402–1418 (2016).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (1990).
Bellera, C. A. et al. Variables with time-varying effects and the Cox model: Some statistical concepts illustrated with a prognostic factor study in breast cancer. BMC Med. Res. Methodol. 10, 20 (2010).
Giobbie-Hurder, A., Gelber, R. D. & Regan, M. M. Challenges of guarantee-time bias. J. Clin. Oncol. 31, 2963–2969 (2013).
de Bono, J. S. et al. Subsequent chemotherapy and treatment patterns after abiraterone acetate in patients with metastatic castration-resistant prostate cancer: Post hoc analysis of COU-AA-302. Eur. Urol. 71, 656–664 (2017).
Schweizer, M. T. et al. The influence of prior abiraterone treatment on the clinical activity of docetaxel in men with metastatic castration-resistant prostate cancer. Eur. Urol. 66, 646–652 (2014).
Tannock, I. et al. Treatment of metastatic prostatic cancer with low-dose prednisone: Evaluation of pain and quality of life as pragmatic indices of response. J. Clin. Oncol. 7, 590–597 (1989).
Amato, R. J., Ellerhorst, J., Finn, L. & Logothetis, C. J. Absence of antitumor activity with prednisone in patients with progressive androgen-independent prostate carcinoma. Urol. Oncol. 2, 70–73 (1996).
Marre, F. et al. Hepatic biotransformation of docetaxel (Taxotere) in vitro: Involvement of the CYP3A subfamily in humans. Cancer Res. 56, 1296–1302 (1996).
Kawano, S., Kamataki, T., Yasumori, T., Yamazoe, Y. & Kato, R. Purification of human liver cytochrome P-450 catalyzing testosterone 6 beta-hydroxylation. J. Biochem. (Tokyo) 102, 493–501 (1987).
Yamazaki, H. & Shimada, T. Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes. Arch. Biochem. Biophys. 346, 161–169 (1997).
Nallani, S. C., Goodwin, B., Buckley, A. R., Buckley, D. J. & Desai, P. B. Differences in the induction of cytochrome P450 3A4 by taxane anticancer drugs, docetaxel and paclitaxel, assessed employing primary human hepatocytes. Cancer Chemother. Pharmacol. 54, 219–229 (2004).
Franke, R. M., Carducci, M. A., Rudek, M. A., Baker, S. D. & Sparreboom, A. Castration-dependent pharmacokinetics of docetaxel in patients with prostate cancer. J. Clin. Oncol. 28, 4562–4567 (2010).
Gillessen, S. et al. Management of patients with advanced prostate cancer: Report of the advanced prostate cancer consensus conference 2019. Eur. Urol. 77, 508–547 (2020).
Acknowledgements
We thank the patients and their families who participated in the study.
Funding
Open Access funding enabled and organized by Projekt DEAL. This is an academic study. This work was sponsored and supported by Hannover Medical School (MHH). The sponsor (MHH) had no role in study design, in the collection, analysis and interpretation of data. Chemotherapy was provided as standard of care.
Author information
Authors and Affiliations
Contributions
P.K., M.A.M., P.I., S.J.B. and C.W.M.R. helped write the manuscript. P.K. and C.W.M.R. designed the study. A.G. supervised this research project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kappler, P., Morgan, M.A., Ivanyi, P. et al. Prognostic role of docetaxel-induced suppression of free testosterone serum levels in metastatic prostate cancer patients. Sci Rep 11, 16457 (2021). https://doi.org/10.1038/s41598-021-95874-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-95874-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.