Abstract
Background
Bipolar androgen therapy (BAT) results in rapid fluctuation of testosterone (T) between near-castrate and supraphysiological levels and has shown promise in metastatic castration-resistant prostate cancer (mCRPC). Its clinical effects may be mediated through induction of DNA damage, and preclinical studies suggest synergy with PARP inhibitors.
Patients and methods
This was a single-center, Phase II trial testing olaparib plus BAT (T cypionate/enanthate 400 mg every 28 days) with ongoing androgen deprivation. Planned recruitment was 30 subjects (equal proportions with/without homologous recombination repair [HRR] gene mutations) with mCRPC post abiraterone and/or enzalutamide. The primary objective was to determine PSA50 response (PSA decline ≥50% from baseline) rate at 12-weeks. The primary analysis utilized the entire (intent-to-treat [ITT]) cohort, with those dropping out early counted as non-responders. Secondary/exploratory analyses were in those treated beyond 12-weeks (response-evaluable cohort).
Results
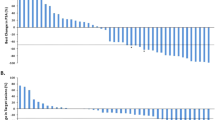
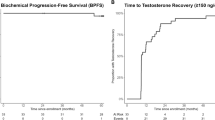
Thirty-six patients enrolled and 6 discontinued prior to response assessment. In the ITT cohort, PSA50 response rate at 12-weeks was 11/36 (31%; 95% CI 17–48%), and 16/36 (44%, 95% CI 28–62%) had a PSA50 response at any time on-study. After a median follow-up of 19 months, the median clinical/radiographic progression-free survival in the ITT cohort was 13.0 months (95% CI 7–17). Clinical outcomes were similar regardless of HRR gene mutational status.
Conclusions
BAT plus olaparib is associated with high response rates and long PFS. Clinical benefit was observed regardless of HRR gene mutational status.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the paper and its Supplementary Materials.
References
Haffner MC, Aryee MJ, Toubaji A, Esopi DM, Albadine R, Gurel B, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet. 2010;42:668–75.
Schweizer MT, Antonarakis ES, Wang H, Ajiboye AS, Spitz A, Cao H, et al. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: results from a pilot clinical study. Sci Transl Med. 2015;7:269ra2.
Chatterjee P, Schweizer MT, Lucas JM, Coleman I, Nyquist MD, Frank SB, et al. Supraphysiological androgens suppress prostate cancer growth through androgen receptor-mediated DNA damage. The. J Clin Investig. 2019;129:4245–60.
Denmeade SR, Wang H, Agarwal N, Smith DC, Schweizer MT, Stein MN, et al. TRANSFORMER: A Randomized Phase II Study Comparing Bipolar Androgen Therapy Versus Enzalutamide in Asymptomatic Men With Castration-Resistant Metastatic Prostate Cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2021:Jco2002759.
Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N. Engl J Med. 2015;373:1697–708.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J cancer (Oxf, Engl: 1990). 2009;45:228–47.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016.
Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy–Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health. 2009;12:124–9.
Rosen RC, Allen KR, Ni X, Araujo AB. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. Eur Urol. 2011;60:1010–6.
Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16:110–20.
Teply BA, Wang H, Luber B, Sullivan R, Rifkind I, Bruns A, et al. Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: an open-label, phase 2, multicohort study. Lancet Oncol. 2018;19:76–86.
De Sarkar N, Dasgupta S, Chatterjee P, Coleman I, Ha G, Ang LS, et al. Genomic attributes of homology-directed DNA repair deficiency in metastatic prostate cancer. JCI Insight. 2021;6.
Sztupinszki Z, Diossy M, Krzystanek M, Reiniger L, Csabai I, Favero F, et al. Migrating the SNP array-based homologous recombination deficiency measures to next generation sequencing data of breast cancer. NPJ Breast Cancer. 2018;4:16.
Takaya H, Nakai H, Takamatsu S, Mandai M, Matsumura N. Homologous recombination deficiency status-based classification of high-grade serous ovarian carcinoma. Sci Rep. 2020;10:2757.
Z S. scarHRD R package Manual 2020. https://github.com/sztup/scarHRD.
Markowski MC, Wang H, Sullivan R, Rifkind I, Sinibaldi V, Schweizer MT, et al. A Multicohort Open-label Phase II Trial of Bipolar Androgen Therapy in Men with Metastatic Castration-resistant Prostate Cancer (RESTORE): A Comparison of Post-abiraterone Versus Post-enzalutamide Cohorts. Eur Urol. 2021;79:692–9.
Schweizer MT, Cheng HH, Nelson PS, Montgomery RB. Two Steps Forward and One Step Back for Precision in Prostate Cancer Treatment. J Clin Oncol: Off J Am Soc Clin Oncol. 2020;38:3740–2.
Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res: Off J Am Assoc Cancer Res. 2007;13:2728–37.
Delaney CA, Wang LZ, Kyle S, White AW, Calvert AH, Curtin NJ, et al. Potentiation of temozolomide and topotecan growth inhibition and cytotoxicity by novel poly(adenosine diphosphoribose) polymerase inhibitors in a panel of human tumor cell lines. Clin Cancer Res: Off J Am Assoc Cancer Res. 2000;6:2860–7.
Evers B, Drost R, Schut E, de Bruin M, van der Burg E, Derksen PW, et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res: Off J Am Assoc Cancer Res. 2008;14:3916–25.
Miknyoczki SJ, Jones-Bolin S, Pritchard S, Hunter K, Zhao H, Wan W, et al. Chemopotentiation of temozolomide, irinotecan, and cisplatin activity by CEP-6800, a poly(ADP-ribose) polymerase inhibitor. Mol cancer therapeutics. 2003;2:371–82.
Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA. 2008;105:17079–84.
Luo L, Keyomarsi K. PARP inhibitors as single agents and in combination therapy: the most promising treatment strategies in clinical trials for BRCA-mutant ovarian and triple-negative breast cancers. Expert Opin Investig Drugs. 2022;31:607–31.
Matulonis UA, Monk BJ. PARP inhibitor and chemotherapy combination trials for the treatment of advanced malignancies: does a development pathway forward exist? Ann Oncol: Off J Eur Soc Med Oncol/ESMO. 2017;28:443–7.
Denmeade SR, Isaacs JT. Bipolar androgen therapy: the rationale for rapid cycling of supraphysiologic androgen/ablation in men with castration resistant prostate cancer. Prostate 2010;70:1600–7.
Lam HM, Nguyen HM, Labrecque MP, Brown LG, Coleman IM, Gulati R, et al. Durable Response of Enzalutamide-resistant Prostate Cancer to Supraphysiological Testosterone Is Associated with a Multifaceted Growth Suppression and Impaired DNA Damage Response Transcriptomic Program in Patient-derived Xenografts. Eur Urol. 2020;77:144–55.
Sena LA, Kumar R, Sanin DE, Thompson EA, Rosen DM, Dalrymple SL, et al. Prostate cancer androgen receptor activity dictates efficacy of bipolar androgen therapy through MYC. The Journal of clinical investigation. 2022.
Kari V, Mansour WY, Raul SK, Baumgart SJ, Mund A, Grade M, et al. Loss of CHD1 causes DNA repair defects and enhances prostate cancer therapeutic responsiveness. EMBO Rep. 2016;17:1609–23.
Shenoy TR, Boysen G, Wang MY, Xu QZ, Guo W, Koh FM, et al. CHD1 loss sensitizes prostate cancer to DNA damaging therapy by promoting error-prone double-strand break repair. Ann Oncol: Off J Eur Soc Med Oncol/ESMO. 2017;28:1495–507.
Zhu Y, Wen J, Huang G, Mittlesteadt J, Wen X, Lu X. CHD1 and SPOP synergistically protect prostate epithelial cells from DNA damage. Prostate. 2021;81:81–8.
Acknowledgements
This study was supported by a research grant from AstraZeneca. MTS was supported by US DOD Award W81XWH-16-1-0484 and a Prostate Cancer Foundation Young Investigator Award. RG was supported by NIH/NCI grants P50 CA097186 and R50 CA221836. HHC and PSN were supported by NIH/NCI Cancer Center Support Grant P30 CA015704. MCH was supported by the US DOD Awards W81XWH-20-1-0111 and W81XWH-21-1-0229, Grant 2021184 from the Doris Duke Charitable, and the V Foundation. NDS was supported by a Prostate Cancer Foundation Young Investigator Award. PSN was supported by NIH/NCI grants P50 CA097186, 5P01 CA163227, R01CA266452-01 and PC200262.
Author information
Authors and Affiliations
Contributions
Conceptualization: MTS, RG, PSN, EYY. Collection and assembly of data: MTS, TY, HHC, EM, RD, BW, AL, PP, NM, M, KN, JH, PG, AH, BM, PSN, EYY. Data analysis and interpretation: MTS, RG, EM, MCH, RP, NDS, GH, PSN, EYY. Paper writing-original draft: MTS, RG. Paper review and editing: All authors.
Corresponding author
Ethics declarations
Competing interests
MTS: Paid consultant and/or received Honoria from Sanofi, AstraZeneca, PharmaIn and Resverlogix. He has received research funding to his institution from Zenith Epigenetics, Bristol Myers Squibb, Merck, Immunomedics, Janssen, AstraZeneca, Pfizer, Madison Vaccines, Hoffman-La Roche, Tmunity, SignalOne Bio and Ambrx, Inc. HHC: Paid consultant for AstraZeneca. She received royalties from UpToDate. Research funds to her institution were from Clovis Oncology, Color Genomics, Janssen, Medivation, Promontory Therapeutics (formerly Phosplatin) and Sanofi. PSN: Paid consultant for Janssen and Pfizer. He received royalties from UpToDate, Research funds to his institution were from Janssen.
Ethics approval and consent
This clinical study was approved by the Fred Hutchinson Cancer Center institutional review board. All research subjects provided informed consent prior to participating in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schweizer, M.T., Gulati, R., Yezefski, T. et al. Bipolar androgen therapy plus olaparib in men with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 26, 194–200 (2023). https://doi.org/10.1038/s41391-022-00636-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-022-00636-0