Abstract
Background
Three primary strategies for MRI-targeted biopsies (TB) are available: Cognitive TB (COG-TB), MRI-US Fusion TB (FUS-TB), and In Bore TB (IB-TB). Despite nearly a decade of practice, a consensus on the preferred approach is lacking, with previous studies showing comparable PCa detection rates among the three methods.
Methods
We conducted a search of PubMed, EMBASE, PubMed, Web of Science, and Scopus databases from 2014 to 2023, to identify studies comparing at least two of the three methods and reporting clinically significant PCa (csPCa) detection rates. The primary and secondary outcomes were to compare the csPCa and insignificant prostate cancer (iPCa, ISUP GG 1) detection rates between TB techniques. The tertiary outcome was to compare the complication rate between TB techniques. Detection rates were pooled using random-effect models. Planned sensitivity analyses included subgroup analysis according to the definition of csPCa and positive MRI, previous biopsy status, biopsy route, prostate volume, and lesion characteristics.
Results
A total of twenty studies, involving 4928 patients, were included in the quantitative synthesis. The meta-analysis unveiled comparable csPCa detection rates among COG-TB (0.37), FUS-TB (0.39), and IB-TB (0.47). iPCa detection rate was also similar between TB techniques (COG-TB: 0.12, FUS-TB: 0.17, IB-TB: 0.18). All preplanned sensitivity analyses were conducted and did not show any statistically significant difference in the detection of csPCa between TB methods. Complication rates, however, were infrequently reported, and when available, no statistically significant differences were observed among the techniques.
Conclusions
This unique study, exclusively focusing on comparative research, indicates no significant differences in csPCa and iPCa detection rates between COG-TB, FUS-TB, and IB-TB. Decisions between these techniques may extend beyond diagnostic accuracy, considering factors such as resource availability and operator preferences. Well-designed prospective studies are warranted to refine our understanding of the optimal approach for TB in diverse clinical scenarios.
Similar content being viewed by others
Introduction
The advent of magnetic resonance imaging (MRI) and target biopsies (TB) directed at MRI-suspicious lesions has revolutionized the accuracy of Prostate cancer (PCa) diagnosis and biopsy sampling. Indeed, level 1 evidence suggests that this pathway enhances the diagnosis of clinically significant PCa (csPCa) while mitigating the risk of overdiagnosing indolent PCa (iPCa) [1,2,3]. Notably, the incorporation of target sampling alongside standard prostate biopsies achieves higher concordance between biopsy results and final pathology examination on whole gland specimens [4,5,6].
Three distinct strategies for conducting MRI-TB have emerged, each accompanied by its own set of advantages and potential limitations. Cognitive target biopsies (COG-TB) rely on the urologist’s capacity to mentally co-register MRI findings with ultrasound (US) images, demanding a high level of expertise and familiarity with both imaging modalities. In contrast, MRI-US Fusion target biopsies (FUS-TB) employ software capable of overlaying 3D reconstructions from MRI onto real-time US images, guiding the biopsy needle to suspicious areas within the prostate. Lastly, In Bore targeted biopsies (IB-TB) utilize an MRI-compatible device with the patient positioned inside the MRI scanner, enabling direct visualization of the suspicious lesion, needle guide, and biopsy needle throughout the sampling process.
Although these strategies have been practiced for almost a decade, a consensus on the preferred approach to TB is currently lacking [7]. Three randomized controlled trials (RCT) showed similar PCa detection rates between IB-TB, FUS-TB, and COG-TB [8,9,10]. Similarly, the most recent systematic reviews and metanalysis showed no difference between the three techniques [11, 12]. However, the metanalysis was not restricted to comparative studies, and significant differences in baseline characteristics in each cohort might have potentially limited the validity of the findings.
With this in mind, we conducted a systematic review and meta-analysis including only comparative studies between IB-TB, FUS-TB, and COG-TB aiming to evaluate which of the MRI-TB method has the highest diagnostic yield for csPCa and the lowest risk of overdiagnosis of iPCa and complications.
Materials/subjects and methods
Search strategy
A comprehensive systematic review of the literature was conducted by searching the EMBASE, PubMed, Web of Science, Scopus and Cochrane Library (CENTRAL) databases.
Research terms used for the research were the following: “(prostate cancer OR prostate adenocarcinoma) AND (MRI OR magnetic resonance) AND (target* OR biopsy)”.
We searched from January 2014 up to November 1, 2023. All the references of included manuscripts and previous reviews were also screened [11, 12]. This systematic review was reported in compliance to the Preferred Reporting Items for Systematic Reviews and Meta-analyses protocol (PRISMA) [13] and was registered within the international prospective registry of systematic reviews (PROSPERO, CRD42024501439).
Initial screening, eligibility/Inclusion criteria
After identifying the initial set of studies, a reviewer (FP) undertook the removal of duplicate entries. Subsequently, two reviewers (FG, AF) assessed independently all the titles and abstracts (and full text, in need of further clarification) for relevance. The eligibility of studies and data extraction were performed with a comprehensive full-text review conducted by two reviewers (FG, AF). In instances of disagreement, a consensus was reached through consultation with a third reviewer (UF).
The population, intervention, comparator, and outcome (PICO) approach [14] was used to define the research question and study eligibility as follows: In patients with a positive MRI (P), what is the best target biopsy (I) technique between cognitive registration, software-assisted image fusion or in-bore sampling fusion (C) to detect clinically significant prostate cancer?(O).
Studies that met the following criteria were eligible for quantitative synthesis and meta-analysis: (A) comparative studies between at least two of the three MRI-TB methods, (B) available detection rate for csPCa by MRI-TB method, (C) mpMRI performed and reported according to ESUR or PIRADS v1 or v2 criteria, (D) available definition of csPCa. Duplicated studies, studies not providing data of the outcomes of interest, review articles, case reports, letters, or conference abstracts were excluded.
Definition of outcomes, data extraction, and quality assessment
The primary outcome of the study was the pooled detection rate of csPCa at MRI-TB using different MRI-TB techniques. Since there is no universally accepted definition of csPCa, definitions used in individual studies were used. The detection rate was defined as the proportion of patients who underwent TB with csPCa at TB. The secondary outcome was the pooled detection rate of iPCa defined as ISUP Grade Group (ISUP GG = 1). Finally, the tertiary outcome was high-grade complications (Clavien-Dindo ≥2) [15].
The number of patients diagnosed with csPCa and iPCa in each study arm was extracted together with the definitions of the outcomes used in each study. When reported, we also extracted the number of patients diagnosed with each ISUP Grade Group.
Study characteristics, study time frame, details for MRI acquisition and reporting, and baseline data of included patients were extracted from each included study. Baseline data included Age, PSA, Prostate volume, PIRADS scores, number of patients with a previous negative prostate biopsy, and number of patients on active surveillance. When available, baseline data were extracted for the overall population and according to each of the study cohorts. The methodological quality of the studies was evaluated using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-C) tool [16]. The assessment was performed by 2 reviewers (AF, FG) and checked by a second (UF). QUADAS-C is a tool recommended for use in systematic reviews to evaluate the risk of bias and the applicability of comparative diagnostic accuracy studies [16].
Statistical analysis
First, a summary table with study characteristics was created.
Then, we employed the accuracy measurements as previously defined and specifically targeted studies that reported one of the MRI-TB techniques, namely IB-TB, FUS-TB, or COG-TB. We synthesized pooled estimates by performing random-effect meta-analyses. All results were reported with 95% confidence intervals (CI). The I2 statistic [17] and the between-study variance (t2) from the random-effect analysis were used to quantify the heterogeneity between the studies. I2 values > 50% indicated large heterogeneity. All models have allowed for different detection rates (random effects) unless otherwise specified. In case of large heterogeneity, random-effect models (using the DerSimonian and Laird approach [18]) were prioritized. A meta-analysis of single means between studies for continuous variables was performed using the inverse variance method for pooling.
Preplanned sensitivity analyses included subgroup analysis according to study design (including only RCTs) definition of csPCa (including studies reporting ISUP GG ≥ 2 detection rates), definition of positive MRI (studies including patients with PI-RADS/Likert score ≥3), previous biopsy status (biopsy naïve vs previous negative prostate biopsy), biopsy route (transrectal vs transperineal), prostate volume (≤50 ml vs >50 ml), target lesion location (Peripheral zone vs Transition Zone) and target lesion size (≤10 mm vs >10 mm). Finally, we repeated our analyses using as outcome the detection of csPCa at biopsies overall. For this analysis, if a patient underwent MRI-TB plus SB the outcome was the detection of csPCa at combined biopsies, whereas if the patient underwent only MRI-TB the outcome was the detection of csPCa at MRI-TB. The extracted data were computed and pre-calculated in Microsoft Excel, while the meta-analyses were executed in R Studio Version 1.2.1335 (Boston, MA, USA).
Results
Study characteristics
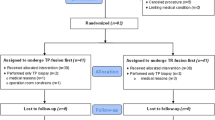
Twenty studies were deemed eligible for the quantitative analyses including a total of 4928 patients (1931 COG-TB, 2432 FUS-TB and 1050 IB-TB). PRISMA flow chart is presented in Fig. 1.
Study design, details for MRI acquisition and reporting, and baseline data of included patients are presented in Table 1. Eight of the included studies were prospective including three RCTs. Four studies had a within patient’s comparison design with each patient undergoing each of the MRI-TB technique [9, 19, 20]. In one of these the order for preforming TB was randomized [9]. Three studies compared all three MRI-TB modalities [10, 21, 22] while most studies (n = 17) compared any combination of two techniques. The mean age ranged from 59 to 72 years and the mean PSA value ranged from 4.2 ng/ml to 14.1 ng/ml. The populations varied with respect to the inclusion of patients with previous biopsy, 6 and 5 studies respectively included only biopsy naïve and previous negative biopsy patients. The remaining (n = 9) included mixed cohorts with three also including patients with a previous positive biopsy with iPCa on active surveillance [9, 19, 23]. PIRADS score was the preferred reporting system in 18 studies (PIRADSv2 in 12) and 3 Tesla (T) MRI scanners were used in 14 studies. Results of MRI-TB only were reported by most studies except 4 that only reported the final Gleason score from the combination of Target and Random cores.
Quality assessment
Quality was evaluated for studies included in the meta-analysis (n = 20) (Supplementary Table 1). All studies were estimated to have low risk regarding applicability to the current review. Thirteen studies were deemed to have a high risk of selection bias.
Diagnosis of csPCa, and iPCa at MRI-TB according to MRI-TB technique
For the primary outcome (csPCa at MRI-TB), data were extracted from 13 studies for COG-TB (1578 patients), 12 studies for FUS-TB (1729 patients), and 8 studies for IB-TB (1050 patients). Pooled meta-analyses with random-effect models demonstrated a csPCa detection rate of 0.37 (CI: 0.25; 0.50) for COG-TB, 0.39 (CI: 0.29; 0.49) for FUS-TB, and 0.47 (CI: 0.32, 0.63) for IB-TB (Fig. 2).
Ten studies for COG-TB (1009 patients), 9 studies for FUS-TB (857 patients), and 8 studies for IB-TB (1050 patients) reported data to evaluate the secondary outcome (iPCa at MRI-TB). Pooled meta-analyses with random-effect models demonstrated iPCa detection rate of 0.12 (CI: 0.09; 0.16) for COG-TB, 0.17 (CI: 0.12; 0.23) for FUS-TB, and 0.18 (CI: 0.13; 0.24) for IB-TB (Fig. 3). All analyses were characterized by significant heterogeneity (I2 > 77%, p < 0.01). The differences between MRI-TB techniques were not statistically different at univariable metaregression (p > 0.05). Since Funnel plots showed evidence for a potential publication bias (Supplementary Fig. 2), we performed a sensitivity analysis including only studies with >100 patients and >50 anyPCa cases showing a statistically significant difference in only the detection rate of anyPCa (p: 0.05) (Supplementary Fig. 3). Indeed, this was 0.79 (CI: 0.52; 0.97) for COG-TB, 0.62 (CI: 0.53; 0.70) for FUS-TB, and 0.79 (CI: 0.66; 0.89) for IB-TB.
The sensitivity analysis including only RCTs did not show any differences in the diagnosis of csPCa at MRI-TB between MRI-TB techniques (Supplementary Fig. 4).
Complications
Out of the 20 studies included, 4 had a within patients design and were excluded for the evaluation of complications according to MRI-TB method [9, 19, 20, 24]. Eleven studies did not comment on complications [23, 25,26,27,28,29,30,31,32,33,34]. The remaining 5 studies [8, 10, 21, 35, 36], including a total of 821 patients, reported 19 (2.3%) Grade 2 adverse events. Out of these, 11 were infective complications (with 7 requiring hospitalization), 4 urinary tract symptoms progression for which treatment was initiated. Two studies reported no significant complications [21, 35]. None of the studies reported any difference between groups in terms of complication except for the FUTURE trial in the setting of repeat biopsy. In the latter, a lower rate of minor adverse events was noted in patients who underwent IB-TB that was likely caused by the omission of standard biopsies [37].
Preplanned sub-analysis
Definition of csPCa (ISUP GG ≥ 2)
Seven studies did not define csPCa as ISUP GG ≥ 2 [9, 21, 24, 27, 30, 34, 36]. Among these, 4 studies provided data to evaluate ISUP GG ≥ 2 at MRI-TB as outcome [9, 21, 24, 27]. Therefore, data were extracted from 10 studies for COG-TB (1009 patients), 9 studies for FUS-TB (857 patients), and 8 studies for IB-TB (1050 patients). Pooled meta-analyses with random-effect models demonstrated csPCa detection rate of 0.36 (CI: 0.23; 0.50) for COG-TB, 0.35 (CI: 0.27; 0.43) for FUS-TB, and 0.45 (CI: 0.34; 0.55) for IB-TB (Supplementary Fig. 5), with large heterogeneity (I2 > 93%, p < 0.01). This difference was not statistically significant at univariable metaregression (p > 0.05).
Definition of positive MRI (PI-RADS/Likert score ≥ 3)
Only 8 studies defined positive MRI as PI-RADS ≥ 3 [9, 10, 20, 23, 24, 28, 31, 33]. Therefore, we considered only these studies for the next steps. For these analyses, data were extracted from 4 studies for COG-TB (478 patients), 4 studies for FUS-TB (479 patients), and 2 studies for IB-TB (180 patients). The pooled detection rate of csPCa were 0.38 (CI: 0.23; 0.55) for COG-TB, 0.39 (CI: 0.24; 0.55) for FUS-TB, and 0.47 (CI: 0.20; 0.74) for IB-TB at meta-analyses with random-effect models (Supplementary Fig. 6), with significant heterogeneity (I2 51%, p: 0.03) and no statistically significant difference at univariable metaregression (p > 0.05).
Previous biopsy status
We repeated our analysis in studies including only biopsy-naive patients and previous negative biopsy patients or studies reporting their results separately for patients with different biopsy histories. Five studies for COG-TB (721 patients), 4 studies for FUS-TB (939 patients), and 1 study for IB-TB (88 patients) reported results on biopsy naïve patients and had complete data to evaluate the detection of csPCa. Pooled meta-analyses with random-effect models demonstrated a csPCa detection rate of 0.33 (CI: 0.18; 0.50) for COG-TB, 0.48 (CI: 0.29; 0.68) for FUS-TB, and 0.30 (CI: 0.20, 0.40) for IB-TB (Supplementary Fig. 7A), with large heterogeneity (I2 95%, p < 0.01).
Four study for COG-TB (319 patients), 6 studies for FUS-TB (510 patients), and 4 studies for IB-TB (455 patients) reported results on previous negative biopsy patients and had complete data to evaluate the detection of csPCa. Pooled meta-analyses with random-effect models demonstrated a csPCa detection rate of 0.26 (CI: 0.20; 0.34) for COG-TB, 0.33 (CI: 0.26; 0.39) for FUS-TB, and 0.40 (CI: 0.23, 0.59) for IB-TB (Supplementary Fig. 7B), with significant heterogeneity (I2 86%, p < 0.01). In both cases, we did not find a statistically significant difference in csPCa detection rate between MRI-TB techniques at univariable metaregression (p > 0.05).
Biopsy route (transrectal vs transperineal)
Five studies for COG-TB (392 patients), 6 studies for FUS-TB (483 patients), and 5 study for IB-TB (668 patients) reported results on transrectal MRI-TB and had complete data to evaluate the detection of csPCa. Pooled meta-analyses with random-effect models demonstrated a csPCa detection rate of 0.25 (CI: 0.18; 0.32) for COG-TB, 0.29 (CI: 0.22; 0.37) for FUS-TB, and 0.45 (CI: 0.31, 0.59) for IB-TB (Supplementary Fig. 8A), with large heterogeneity (I2 91%, p < 0.01). This difference was statistically significant (p: 0.04).
None of the studies reported results of transperineal IB-TB and we included in the subanalysis 5 studies for transperineal COG-TB (869 patients) and 5 studies for transperineal FUS-TB (1173 patients) (Supplementary Fig. 8B). csPCa detection rates were 0.44 (CI: 0.32; 0.57) and 0.51 (CI: 0.36; 0.66) respectively (I2 94%, p < 0.01). Univariable metaregression did not show any statistically significant difference in csPCa detection rate according to biopsy route (p > 0.05).
Prostate volume, target lesion location, and target lesion size
Four studies reported subgroups csPCa detection rates according to prostate volume, target lesion location, and target lesion size [10, 23, 32, 33]. Since there was no univocal cutoff for prostate volume subanalysis, preplanned meta-analyses were performed only according to target lesion location (Peripheral zone lesions vs transitional zone lesions Supplementary Fig. 9A, B) and target lesion size (≤10 mm vs >10 mm, Supplementary Fig. 10A, B). No difference was found in csPCa detection rates at univariable metaregression (p > 0.05).
Combined biopsy
Finally, we tested the difference in the detection of csPCa at a combined approach (MRI-TB plus SB). Data for this analysis were available from 9 studies for COG-TB (724 patients), 9 studies for FUS-TB (1005 patients), and 8 studies for IB-TB (1050 patients). All patients underwent MRI-TB and SB, but those undergoing IB-TB received only MRI-TB. Pooled meta-analyses with random-effect models demonstrated a csPCa detection rate of 0.38 (CI: 0.22; 0.56) for COG-TB, 0.45 (CI: 0.28; 0.62) for FUS-TB, and 0.47 (CI: 0.32, 0.63) for IB-TB (Supplementary Fig. 11), with significant heterogeneity (I2 96%, p < 0.01). This difference was not significant at univariable metaregression (p > 0.05)
Discussion
In this systematic review and meta-analysis comparing COG-TB, FUS-TB, and IB-TB we found no significant differences in the detection rates of csPCa among the three biopsy techniques. Study results were consistent in all preplanned subgroup analysis.
Current knowledge comparing MRI-TB techniques is limited. The FUTURE trial is the only three-arm RCT so far available in men undergoing repeat biopsy and showed no difference in PCa and csPCa detection among the three techniques [10].
The presence of several confounding factors makes any comparison extremely challenging and may limit the validity of the latest meta-analysis including non-comparative reports [11, 12].
Our study aimed to contribute insights into the ongoing debate on the optimal approach for MRI-TB by including only comparative studies and thus reducing the potential risk of inclusion bias.
COG-TB results are intuitively affected by a longer learning curve. Similarly, in a patient with a small MRI lesion within a big prostate, the probabilities of hitting the target via COG-TB or FUS-TB are lower [38]. In our preplanned subgroup analysis, we did not find any difference in csPCa detection rates based on lesion characteristics. However, we could only compare 4 studies reporting subgroup’s CDRs and we only stratified the analysis according to lesion size (<10 mm) and lesion location (PZ vs TZ). It may be relevant to stratify for other lesion-specific factors such as the presence of anterior vs posterior lesions. Indeed, Wysock et al. demonstrated some benefits associated with a FUS-TB for anterior tumors, but that study was conducted using a transrectal approach, which might have made the sampling of these tumors that were furthest away from the needle deployment subject to some systematic error [19]. Only one study compared csPCa CDR in anterior or posterior lesions showing no statistically significant differences. However, these subanalyses should be interpreted with caution due to the small sample size [10].
The choice between these strategies may be influenced by factors other than diagnostic accuracy, such as resource availability, cost-effectiveness, and operator preferences.
An important consideration may be the difference in complication rates between the three MRI-TB. In the present study, we attempted to evaluate if any difference in complication rates could justify the choice of one MRI-TB technique over the others. None of the studies was specifically designed with complications as primary outcome and 5 out of 20 studies [8, 10, 21, 35, 36] reported adverse events according to biopsy techniques. A post-hoc analysis of the FUTURE trial showed a higher low-grade complication rate in patients undergoing FUS-TB or COG-TB compared to IB-TB [37]. A possible explanation is the lowest number of cores needed with the IB biopsy technique. Indeed, standard sampling is not usually performed in patients undergoing IB-TB while it is considered standard of care in patients undergoing COG-TB and FUS-TB. Additionally, IB-TB in all the included studies was performed by a transrectal approach that is inherently associated with a higher risk of infective complications [39].
It is crucial to acknowledge the limitations of our study, which may restrict the overall generalizability of our findings.
First, the high heterogeneity among the included studies reflects the absence of strict guidelines on performing prostate biopsies in patients with suspicious MRI lesions.
Second, there is a potential for publication bias, as most studies are published by high-volume centers with significant expertise.
Third, despite our efforts to reduce inclusion bias by excluding non-comparative studies, the evidence quality remains low. Most studies fail to adjust for all possible confounders that can impact the clinically significant cancer detection rate at the target biopsy. Additionally, we were unable to perform some preplanned sub-analyses stratifying by PIRADS score and the number of target cores.
It is noteworthy that the included studies not only compare MRI-targeted biopsy techniques but also assess the accuracy of MRI and the indications for prostate biopsy. These three aspects are interconnected with the accuracy of the diagnosis and should be standardized in future studies addressing this issue. MRI and MRI-TB indications should be explicitly stated in the study protocol, enrolling consecutive series of patients selected for MRI [11]. MRI acquisition and reporting should adhere to the latest guidelines and be performed by the same radiologists [40]. A standardized biopsy protocol should be adopted, ensuring an equal number of cores taken at MRI-TB, consistent biopsy routes, and uniform pathology evaluation.
Some of the variability in CDRs between techniques can be attributed to the skill of the urologist or radiologist performing the biopsy. These may impact each of the TB techniques differently and future studies should aim to compare the learning curves for each biopsy method [41].
Finally, target biopsy platforms and imaging may completely change over the next years. Indeed, artificial intelligence is showing promising results for image analysis, real time delineation of suspicious lesions on ultrasound images, and elastic registration between ultrasound and MRI [42].
Similarly, novel imaging techniques such as PSMA PET/CT have been proposed to further improve patient selection for prostate biopsy [43] and new methods to target PSMA PET/CT findings are under evaluation [44].
Conclusion
Our study adds to the ongoing debate on the optimal approach for MRI-TB by providing a comprehensive comparison of COG-TB, FUS-TB, and IB-TB. With the caveat of heterogeneity and suboptimal quality of the included studies, the lack of significant differences in csPCa detection rates among the techniques may suggest that the choice between them may be influenced by factors beyond diagnostic accuracy, such as resource availability, cost-effectiveness, and operator preferences. Further research, including well-designed prospective studies, is warranted to address the limitations of current evidence and refine our understanding of the optimal approach for MRI target biopsies in diverse clinical scenarios.
References
Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl J Med. 2018;378:1767–77.
Rouviere O, Puech P, Renard-Penna R, Claudon M, Roy C, Mege-Lechevallier F, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019;20:100–9.
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22.
Lantz A, Falagario UG, Ratnani P, Jambor I, Dovey Z, Martini A, et al. Expanding Active Surveillance Inclusion Criteria: A Novel Nomogram Including Preoperative Clinical Parameters and Magnetic Resonance Imaging Findings. Eur Urol Oncol. 2022;5:187–94.
Ahdoot M, Wilbur AR, Reese SE, Lebastchi AH, Mehralivand S, Gomella PT, et al. MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N Engl J Med. 2020;382:917–28.
Tan N, Margolis DJ, Lu DY, King KG, Huang J, Reiter RE, et al. Characteristics of Detected and Missed Prostate Cancer Foci on 3-T Multiparametric MRI Using an Endorectal Coil Correlated With Whole-Mount Thin-Section Histopathology. Am J Roentgenol. 2015;205:W87–92.
Mottet N, van den Bergh RCN, Briers E, Cornford P, De Santis M, Fanti S, et al. EAU - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer 2022. European Association of Urology Guidelines 2022 Edition. presented at the EAU Annual Congress Amsterdam 2022. Arnhem, The Netherlands: European Association of Urology Guidelines Office; 2022.
Arsov C, Rabenalt R, Blondin D, Quentin M, Hiester A, Godehardt E, et al. Prospective randomized trial comparing magnetic resonance imaging (MRI)-guided in-bore biopsy to MRI-ultrasound fusion and transrectal ultrasound-guided prostate biopsy in patients with prior negative biopsies. Eur Urol. 2015;68:713–20.
Hamid S, Donaldson IA, Hu Y, Rodell R, Villarini B, Bonmati E, et al. The SmartTarget Biopsy Trial: A Prospective, Within-person Randomised, Blinded Trial Comparing the Accuracy of Visual-registration and Magnetic Resonance Imaging/Ultrasound Image-fusion Targeted Biopsies for Prostate Cancer Risk Stratification. Eur Urol. 2019;75:733–40.
Wegelin O, Exterkate L, van der Leest M, Kummer JA, Vreuls W, de Bruin PC, et al. The FUTURE Trial: A Multicenter Randomised Controlled Trial on Target Biopsy Techniques Based on Magnetic Resonance Imaging in the Diagnosis of Prostate Cancer in Patients with Prior Negative Biopsies. Eur Urol. 2019;75:582–90.
Bass EJ, Pantovic A, Connor MJ, Loeb S, Rastinehad AR, Winkler M, et al. Diagnostic accuracy of magnetic resonance imaging targeted biopsy techniques compared to transrectal ultrasound guided biopsy of the prostate: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2022;25:174–9.
Wegelin O, van Melick HHE, Hooft L, Bosch J, Reitsma HB, Barentsz JO, et al. Comparing Three Different Techniques for Magnetic Resonance Imaging-targeted Prostate Biopsies: A Systematic Review of In-bore versus Magnetic Resonance Imaging-transrectal Ultrasound fusion versus Cognitive Registration. Is There a Preferred Technique? Eur Urol. 2017;71:517–31.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Yang B, Mallett S, Takwoingi Y, Davenport CF, Hyde CJ, Whiting PF, et al. QUADAS-C: A Tool for Assessing Risk of Bias in Comparative Diagnostic Accuracy Studies. Ann Intern Med. 2021;174:1592–9.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Wysock JS, Rosenkrantz AB, Huang WC, Stifelman MD, Lepor H, Deng FM, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol. 2014;66:343–51.
Petov V, Bazarkin A, Morozov A, Taratkin M, Ganzha T, Danilov S, et al. A Prospective Comparison of Transrectal Standard, Cognitive, Transperineal Fusion, and Mapping Prostate Biopsy for Cancer Detection. J Endourol. 2023;37:940–7.
Kaufmann S, Russo GI, Bamberg F, Lowe L, Morgia G, Nikolaou K, et al. Prostate cancer detection in patients with prior negative biopsy undergoing cognitive-, robotic- or in-bore MRI target biopsy. World J Urol. 2018;36:761–8.
Kilic M, Acar O, Vural M, Colakoglu B, Cil BE, Koseoglu E, et al. Pathological Accuracy in Prostate Cancer: Single-Center Outcomes of 3 Different Magnetic Resonance Imaging-Targeted Biopsy Techniques and Random Systematic Biopsy. Turk J Urol. 2022;48:346–53.
Costa DN, Goldberg K, Leon AD, Lotan Y, Xi Y, Aziz M, et al. Magnetic Resonance Imaging-guided In-bore and Magnetic Resonance Imaging-transrectal Ultrasound Fusion Targeted Prostate Biopsies: An Adjusted Comparison of Clinically Significant Prostate Cancer Detection Rate. Eur Urol Oncol. 2019;2:397–404.
Simmons LAM, Kanthabalan A, Arya M, Briggs T, Barratt D, Charman SC, et al. Accuracy of Transperineal Targeted Prostate Biopsies, Visual Estimation and Image Fusion in Men Needing Repeat Biopsy in the PICTURE Trial. J Urol. 2018;200:1227–34.
Osses DF, van Asten JJ, Tijsterman JD. Cognitive-Targeted versus Magnetic Resonance Imaging-Guided Prostate Biopsy in Prostate Cancer Detection. Curr Urol. 2018;11:182–8.
Venderink W, van der Leest M, van Luijtelaar A, van de Ven WJM, Futterer JJ, Sedelaar JPM, et al. Retrospective comparison of direct in-bore magnetic resonance imaging (MRI)-guided biopsy and fusion-guided biopsy in patients with MRI lesions which are likely or highly likely to be clinically significant prostate cancer. World J Urol. 2017;35:1849–55.
Yaxley AJ, Yaxley JW, Thangasamy IA, Ballard E, Pokorny MR. Comparison between target magnetic resonance imaging (MRI) in-gantry and cognitively directed transperineal or transrectal-guided prostate biopsies for Prostate Imaging-Reporting and Data System (PI-RADS) 3-5 MRI lesions. BJU Int. 2017;120:43–50.
Oberlin DT, Casalino DD, Miller FH, Matulewicz RS, Perry KT, Nadler RB, et al. Diagnostic Value of Guided Biopsies: Fusion and Cognitive-registration Magnetic Resonance Imaging Versus Conventional Ultrasound Biopsy of the Prostate. Urology. 2016;92:75–9.
Guerra-Lacambra M, Yanez-Castillo Y, Folgueral-Corral M, Melgarejo-Segura MT, Del Carmen Cano-Garcia M, Sanchez-Tamayo FJ, et al. Results of fusion prostate biopsy comparing with cognitive and systematic biopsy. J Cancer Res Clin Oncol. 2023;149:15085–90.
Izadpanahi MH, Elahian A, Gholipour F, Khorrami MH, Zargham M, Mohammadi Sichani M, et al. Diagnostic yield of fusion magnetic resonance-guided prostate biopsy versus cognitive-guided biopsy in biopsy-naive patients: a head-to-head randomized controlled trial. Prostate Cancer Prostatic Dis. 2021;24:1103–9.
Turkay R, Inci E, Yildiz O, Ozgur E, Tasci AI. Cognitive Versus Magnetic Resonance-Ultrasound Fusion Prostate Biopsy: Which One Is Worthier to Perform? Ultrasound Q. 2020;36:345–9.
Khoo CC, Eldred-Evans D, Peters M, van Son M, van Rossum PSN, Connor MJ, et al. A Comparison of Prostate Cancer Detection between Visual Estimation (Cognitive Registration) and Image Fusion (Software Registration) Targeted Transperineal Prostate Biopsy. J Urol. 2021;205:1075–81.
Yamada Y, Shiraishi T, Ueno A, Ueda T, Fujihara A, Naitoh Y, et al. Magnetic resonance imaging-guided targeted prostate biopsy: Comparison between computer-software-based fusion versus cognitive fusion technique in biopsy-naive patients. Int J Urol. 2020;27:67–71.
Zhang K, Zhang Z, Liu M, Zhu G, Roobol MJ. Comparison of clinically significant prostate cancer detection by MRI cognitive biopsy and in-bore MRI-targeted biopsy for naive biopsy patients. Transl Androl Urol. 2020;9:243–9.
Oderda M, Faletti R, Battisti G, Dalmasso E, Falcone M, Marra G, et al. Prostate Cancer Detection Rate with Koelis Fusion Biopsies versus Cognitive Biopsies: A Comparative Study. Urol Int. 2016;97:230–7.
Ito M, Yonese I, Toide M, Ikuta S, Kobayashi S, Koga F. Superior detection of significant prostate cancer by transperineal prostate biopsy using MRI-transrectal ultrasound fusion image guidance over cognitive registration. Int J Clin Oncol. 2023;28:1545–53.
Wegelin O, Exterkate L, van der Leest M, Kelder JC, Bosch J, Barentsz JO, et al. Complications and Adverse Events of Three Magnetic Resonance Imaging-based Target Biopsy Techniques in the Diagnosis of Prostate Cancer Among Men with Prior Negative Biopsies: Results from the FUTURE Trial, a Multicentre Randomised Controlled Trial. Eur Urol Oncol. 2019;2:617–24.
Falagario U, Jambor I, Taimen P, Syvanen KT, Kahkonen E, Merisaari H, et al. Added value of systematic biopsy in men with a clinical suspicion of prostate cancer undergoing biparametric MRI-targeted biopsy: multi-institutional external validation study. World J Urol. 2021;39:1879–87.
Pradere B, Veeratterapillay R, Dimitropoulos K, Yuan Y, Omar MI, MacLennan S, et al. Nonantibiotic Strategies for the Prevention of Infectious Complications following Prostate Biopsy: A Systematic Review and Meta-Analysis. J Urol. 2021;205:653–63.
Jambor I, Falagario U. Does prostate magnetic resonance imaging (MRI) reporting system affect performance of MRI in men with a clinical suspicion of prostate cancer? BJU Int. 2020;125:4–5.
Halstuch D, Baniel J, Lifshitz D, Sela S, Ber Y, Margel D. Characterizing the learning curve of MRI-US fusion prostate biopsies. Prostate Cancer Prostatic Dis. 2019;22:546–51.
Baydoun A, Jia AY, Zaorsky NG, Kashani R, Rao S, Shoag JE, et al. Artificial intelligence applications in prostate cancer. Prostate Cancer Prostatic Dis. 2024;27:37–45.
Guo S, Zhang J, Wang Y, Jiao J, Li Z, Cui C, et al. Avoiding unnecessary biopsy: the combination of PRIMARY score with prostate-specific antigen density for prostate biopsy decision. Prostate Cancer Prostatic Dis. 2023. Online ahead of print.
Zhang LL, Li WC, Xu Z, Jiang N, Zang SM, Xu LW, et al. (68)Ga-PSMA PET/CT targeted biopsy for the diagnosis of clinically significant prostate cancer compared with transrectal ultrasound guided biopsy: a prospective randomized single-centre study. Eur J Nucl Med Mol Imaging. 2021;48:483–92.
Funding
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
Conception and design: UGF, GG, FP; acquisition of data: UGF, FG, AF; analysis and interpretation of data: UGF, GG, FP; drafting of the manuscript: UGF, GG, FP; critical revision of the manuscript for important intellectual content: All authors; supervision: GC, RB, HC, GG.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Falagario, U.G., Pellegrino, F., Fanelli, A. et al. Prostate cancer detection and complications of MRI-targeted prostate biopsy using cognitive registration, software-assisted image fusion or in-bore guidance: a systematic review and meta-analysis of comparative studies. Prostate Cancer Prostatic Dis (2024). https://doi.org/10.1038/s41391-024-00827-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-024-00827-x