Abstracts
Background
Due to the discomfort and incidence of complications increases with the increasing number of biopsy cores, the protocol of prostate biopsy has been promoted from systematic biopsy (SB) to targeted biopsy (TB) in many studies. However, the optimal prostate sampling scheme to balance the incidence of biopsy complications and accuracy of biopsy remains controversial. Our objective is to explore an optimal prostate cancer (PCa) sampling scheme with fewer SB cores.
Methods
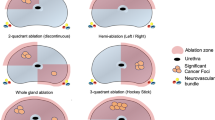
Patients with at least one lesion of Prostate Imaging Reporting and Data System ≥3 were prospectively enrolled. TB and SB were performed for each patient as reference. The hypothetical biopsy sampling schemes were TB only, SB only, and TB followed by SB of the nontargeted sector (TB+nSB). The PCa and clinically significant PCa (csPCa) detection rates and cores of the three hypothetical biopsy schemes were compared with TB+SB.
Results
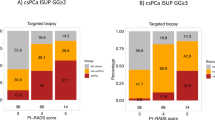
Among 165 patients, 107 (64.8%) were diagnosed with PCa and 91 (55.2%) with csPCa via TB+SB. There were 54 (50.5%) and 42 (46.2%) magnetic resonance imaging (MRI) true negative cases and 53 (49.5%) and 49 (53.8%) false negative cases of nontargeted sectors among PCa and csPCa patients, respectively. The maximal cancer proportion in positive biopsy cases differed significantly between the true and false groups of these cohorts. There was no difference between TB+nSB and TB+SB for PCa or csPCa detection.
Conclusions
The optimal sampling scheme TB+nSB with fewer SB cores showed same PCa and csPCa detection rates as that of standard TB+SB with MRI/ultrasound fusion biopsy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Surveillance, E., and End Results Program, Cancer Stat Facts: Prostate Cancer. Nation Cancer Institute, http://seer.cancer.gov, 2020.
Mohler JL, S. S, and A. ES, NCCN clinical practice guidelines in oncology: prostate cancer (Version 4.2019). J Natl Compr Cancer Netw., 2019-08-19.
Mottet N, v.d. BR, Biers E et al. EAU Guideline Prostate Cancer, 2019. http://uroweb.org/guideline/prostate-cancer/. Accessed March 2019.
Schoots Ivo G, Roobol Monique J, Nieboer Daan, Bangma Chris H, Steyerberg Ewout W, Hunink M G Myriam. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol., 2015, 68:438–50.
Marco Borghesi, Hashim Ahmed, Robert Nam, Edward Schaeffer, Riccardo Schiavina, Samir Taneja, et al. Complications after systematic, random, and image-guided prostate biopsy. Eur. Urol. 2017;71:353–65.
Stabile Armando, Giganti Francesco, Rosenkrantz AndrewB, Taneja SamirS, Villeirs Geert, Gill InderbirS, et al. Multiparametric MRI for prostate cancer diagnosis: current status and future directions. Nat Rev Urol. 2020;17:41–61.
Yuval Freifeld, Yin Xi, Niccolo Passoni, Solomon Woldu, Brad Hornberger, Kenneth Goldberg, et al. Optimal sampling scheme in men with abnormal multiparametric MRI undergoing MRI-TRUS fusion prostate biopsy. Urol Oncol. 2019;37:57–62.
Epstein JIWP, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (Stage T1 c) prostate cancer. JAMA. 1994;271:368–74.
Gawlitza JR-ZM, Thörmer G, Schaudinn A, Linder N, Garnov N, Horn LC, et al. Impact of the use of an endorectal coil for 3 T prostate MRI on image quality and cancer detection rate. Sci Rep. 2017;7:40640.
Weinreb Jeffrey C, Barentsz Jelle O, Choyke Peter L, Cornud Francois, Haider MasoomA, Macura KatarzynaJ, et al. PI-RADS prostate imaging – reporting and data system: 2015, Version 2. Eur Urol. 2016;69:16–40.
Ahdoot Michael, Wilbur AndrewR, Reese SarahE, Lebastchi AmirH, Mehralivand Sherif, Gomella PatrickT, et al. MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N Engl J Med. 2020;382:917–28.
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. PROMIS study group. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22.
Kasivisvanathan Veeru, Rannikko AnttiS, Borghi Marcelo, Panebianco Valeria, Mynderse LanceA, Vaarala MarkkuH, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767–77.
Pokorny MR, Thompson LC. Is magnetic resonance imaging-targeted biopsy now the standard of care? Eur Urol. 2019;76:304–5.
Wong MCGW, Wang HH, Fung FD, Leung C, Wong SY, et al. Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur Urol. 2016;70:862–74.
Chang AJAK, Roach M III, Scher HI. High-risk prostate cancer-classification and therapy. Nat Rev Clin Oncol. 2014;11:308–23.
Serrano NA, Anscher MS. Favorable vs unfavorable intermediate-risk prostate cancer: a review of the new classification system and its impact on treatment recommendations. Oncology (Williston Park). 2016;30:229–36.
Bryk DarrenJ, Llukani Elton, Taneja SamirS, Rosenkrantz AndrewB, Huang WilliamC, Lepor Herbert. The role of ipsilateral and contralateral transrectal ultrasound-guided systematic prostate biopsy in men with unilateral magnetic resonance imaging lesion undergoing magnetic resonance imaging-ultrasound fusion-targeted prostate biopsy. Urology. 2017;102:178–82.
Wei-Wei Shen, Li-Gang Cui, Wei-Qiang Ran, Yan Sun, Jie Jiang, Xin-Long Pei, et al. Targeted biopsy with reduced number of cores: optimal sampling scheme in patients undergoing magnetic resonance imaging/transrectal ultrasound fusion prostate biopsy. Ultrasound Med Biol. 2020;46:1197–207.
Aminsharifi Alireza, Gupta RajanT, Tsivian Efrat, Sekar Sitharthan, Sze Christina, Polascik ThomasJ. Reduced Core Targeted (RCT) biopsy: combining multiparametric magnetic resonance imaging – transrectal ultrasound fusion targeted biopsy with laterally-directed sextant biopsies – An alternative template for prostate fusion biopsy. Eur J Radiol. 2019;110:7–13.
Glaser Alexander P, Novakovic Kristian, Helfand Brian T. The impact of prostate biopsy on urinary symptoms, erectile function, and anxiety. Curr Urol Rep. 2012;13:447–54.
Baojun Wang, Jie Gao, Qing Zhang, Chengwei Zhang, Guangxiang Liu, Wang Wei, et al. Investigating the equivalent performance of biparametric compared to multiparametric MRI in detection of clinically significant prostate cancer. Abdom Radiol. 2020;45:547–55.
Francesco Porpiglia, Matteo Manfredi, Fabrizio Mele, Marco Cossu, Enrico Bollito, Andrea Veltri, et al. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy-naïve patients with suspected prostate cancer. Eur Urol. 2017;72:282–88.
Heijmink SWFJ, Hambrock T, Takahashi S, Scheenen TW, Huisman HJ, et al. Prostate cancer: body-array versus endorectal coil MR imaging at 3 T-comparison of image quality, localization, and staging performance. Radiology. 2007;244:184–95.
Hamoen EstherHJ, de Rooij Maarten, Witjes JAlfred, Barentsz Jelle O, Rovers MaroeskaM. Use of the prostate imaging reporting and data system (PI-RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: a diagnostic meta-analysis. Eur Urol. 2015;67:1112–21.
Hambrock Thomas, Somford DiederikM, Huisman HenkjanJ, van Oort Inge M, Witjes JAlfred, Hulsbergen-van de Kaa ChristinaA, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011;259:453–61.
Acknowledgements
HL and MJR contributed equally to this work. We thank the entire staff of the Department of Urology, Peking University First Hospital.
Author contributions
HL and MJR contributed equally to this work. GS conceived and designed the study. HL, MJR, Hao Wang, and GS collected the data. He Wang analyzed mpMRI results. HL, MJR, and XYL analyzed and interpreted the data. HL drafted the manuscript. GS revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by Joint Fund of Peking University (BMU2020MI003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, H., Ruan, M., Wang, H. et al. Can fewer transperineal systematic biopsy cores have the same prostate cancer detection rate as of magnetic resonance imaging/ultrasound fusion biopsy?. Prostate Cancer Prostatic Dis 23, 589–595 (2020). https://doi.org/10.1038/s41391-020-0260-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-020-0260-0
This article is cited by
-
Re: Can fewer transperineal systematic biopsy cores have the same prostate cancer detection rate as of magnetic resonance imaging/ultrasound fusion biopsy?
Prostate Cancer and Prostatic Diseases (2023)
-
Novel sampling scheme with reduced cores in men with multiparametric MRI-visible lesions undergoing prostate biopsy
Abdominal Radiology (2023)
-
A clinical available decision support scheme for optimizing prostate biopsy based on mpMRI
Prostate Cancer and Prostatic Diseases (2022)