Abstract
Background
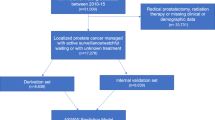
Expectant management (EM) has been widely recommended for men with low-risk prostate cancers (PCa). We evaluated trends in EM and the sociodemographic and clinical factors associated with EM, initiating a National Comprehensive Cancer Network guideline-concordant active surveillance (AS) monitoring protocol, and switching from EM to active treatment (AT).
Methods
We used the SEER-Medicare database to identify men ages 66+ diagnosed with a low-risk PCa (PSA < 10 ng/mL, Gleason ≤ 6, stage ≤ T2a) in 2010–2013 with ≥1 year of follow-up. We used claims data to capture (1) PCa treatments, including surgical procedures, radiotherapy, and hormone therapy, and (2) AS monitoring procedures, including PSA tests and prostate biopsy. We defined EM as receiving no AT within 1 year of diagnosis. We used multivariable regression techniques to identify factors associated with EM, initiating AS monitoring, and switching to AT.
Results
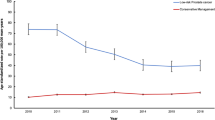
During the study period, EM increased from 29.4% to 49.0%, p < 0.01. Age < 77, being married/partnered, non-Hispanic ethnicity, higher median ZIP code income, lower PSA levels, stage T1c, and more recent year of diagnosis were associated with EM. Nearly 39% of the EM cohort initiated AS monitoring; age <77, White race, being married/partnered, higher median ZIP code income, and lower PSA levels were associated with initiating AS. By three years after diagnosis, 21.3% of the EM cohort had switched to AT, usually after undergoing AS monitoring procedures.
Discussion
We found increasing uptake of EM over time, though over 50% still received AT. About 60% of EM patients did not initiate AS monitoring, even among those with life expectancy >10 years, implying that a substantial proportion was being managed by watchful waiting. AS monitoring was associated with switching to AT, suggesting that treatment decisions likely were based on cancer progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the National Cancer Institute, but restrictions apply to the availability of these data, which are under license for the study, and so are not publicly available.
References
Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374–83.
National Comprehensive Cancer Network. Prostate cancer. NCCN Guidelines Version 2.2021. 2021. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. part i: risk stratification, shared decision making, and care options. J Urol. 2018;199:683–90.
Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7.
Tosoian JJ, Trock BJ, Landis P, Feng Z, Epstein JI, Partin AW, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185–90.
Bul M, Zhu X, Valdagni R, Pickles T, Kakehi Y, Rannikko A, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013;63:597–603.
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–24.
Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990-2013. JAMA. 2015;314:80–2.
Eggener SE, Mueller A, Berglund RK, Ayyathurai R, Soloway C, Soloway MS, et al. A multi-institutional evaluation of active surveillance for low risk prostate cancer. J Urol. 2013;189:S19–25.
Loppenberg B, Friedlander DF, Krasnova A, Tam A, Leow JJ, Nguyen PL, et al. Variation in the use of active surveillance for low-risk prostate cancer. Cancer Cytopathol. 2018;124:55–64.
Maurice MJ, Abouassaly R, Kim SP, Zhu H. Contemporary nationwide patterns of active surveillance use for prostate cancer. JAMA Intern Med. 2015;175:1569–71.
Moschini M, Fossati N, Sood A, Lee JK, Sammon J, Sun M, et al. Contemporary management of prostate cancer patients suitable for active surveillance: a North American population-based study. Eur Urol Focus. 2018;4:68–74.
Ritch CR, Graves AJ, Keegan KA, Ni S, Bassett JC, Chang SS, et al. Increasing use of observation among men at low risk for prostate cancer mortality. J Urol. 2015;193:801–6.
Weiner AB, Patel SG, Etzioni R, Eggener SE. National trends in the management of low and intermediate risk prostate cancer in the United States. J Urol 2015;193:95–102.
Womble PR, Montie JE, Ye Z, Linsell SM, Lane BR, Miller DC, et al. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol. 2015;67:44–50.
Loeb S, Walter D, Curnyn C, Gold HT, Lepor H, Makarov DV. How active is active surveillance? Intensity of followup during active surveillance for prostate cancer in the United States. J Urol. 2016;196:721–6.
Luckenbaugh AN, Auffenberg GB, Hawken SR, Dhir A, Linsell S, Kaul S, et al. Variation in guideline concordant active surveillance followup in diverse urology practices. J Urol. 2017;197:621–6.
Bokhorst LP, Alberts AR, Rannikko A, Valdagni R, Pickles T, Kakehi Y, et al. Compliance rates with the prostate cancer research international active surveillance (PRIAS) protocol and disease reclassification in noncompliers. Eur Urol. 2015;68:814–21.
National Cancer Institute. Surveillance, epidemiology, and end results (SEER). U.S. Department of Health & Human Services; 2018. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf.
Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Cancer Netw 2010;8:145.
Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–31.
American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. Chicago, IL: Springer; 2017.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9.
Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67.
Social Security Administration. Actuarial life tables. 2021. https://www.ssa.gov/oact/STATS/table4c6.html.
Hoffman RM, Lobo T, Van Den Eeden SK, Davis KM, Luta G, Leimpeter AD, et al. Selecting active surveillance: decision making factors for men with a low-risk prostate cancer. Med Decis Mak. 2019;39:962–74.
Loppenberg B, Sood A, Dalela D, Karabon P, Sammon JD, Vetterlein MW, et al. Variation in locoregional prostate cancer care and treatment trends at commission on cancer designated facilities: a national cancer data base analysis 2004 to 2013. Clin Genitourin Cancer. 2017;15:e955–68.
Mahal BA, Butler S, Franco I, Spratt DE, Rebbeck TR, D’Amico AV, et al. Use of active surveillance or watchful waiting for low-risk prostate cancer and management trends across risk groups in the United States, 2010-2015. JAMA. 2019;321:704–6.
Burt LM, Shrieve DC, Tward JD. Factors influencing prostate cancer patterns of care: an analysis of treatment variation using the SEER database. Adv Radiat Oncol. 2018;3:170–80.
Filson CP, Marks LS, Litwin MS. Expectant management for men with early stage prostate cancer. CA Cancer J Clin. 2015;65:265–82.
Al Hussein Al Awamlh B, Ma X, Christos P, Hu JC, Shoag JE. Active surveillance for black men with low-risk prostate cancer in the United States. N Engl J Med. 2019;381:2581–2.
Butler S, Muralidhar V, Chavez J, Fullerton Z, Mahal A, Nezolosky M, et al. Active surveillance for low-risk prostate cancer in black patients. N Engl J Med. 2019;380:2070–2.
Washington SL 3rd, Jeong CW, Lonergan PE, Herlemann A, Gomez SL, Carroll PR, et al. Regional variation in active surveillance for low-risk prostate cancer in the US. JAMA Netw Open. 2020;3:e2031349.
Sundi D, Faisal FA, Trock BJ, Landis PK, Feng Z, Ross AE, et al. Reclassification rates are higher among African American men than Caucasians on active surveillance. Urology. 2015;85:155–60.
Gokce MI, Sundi D, Schaeffer E, Pettaway C. Is active surveillance a suitable option for African American men with prostate cancer? A systemic literature review. Prostate Cancer Prostatic Dis. 2017;20:127–36.
Deka R, Parsons JK, Simpson DR, Riviere P, Nalawade V, Vitzthum LK, et al. African-American men with low-risk prostate cancer treated with radical prostatectomy in an equal-access health care system: implications for active surveillance. Prostate Cancer Prostatic Dis. 2020;23:581–8.
Deka R, Courtney PT, Parsons JK, Nelson TJ, Nalawade V, Luterstein E, et al. Association between African American race and clinical outcomes in men treated for low-risk prostate cancer with active surveillance. JAMA. 2020;324:1747–54.
Mahal BA, Chen YW, Muralidhar V, Mahal AR, Choueiri TK, Hoffman KE, et al. Racial disparities in prostate cancer outcome among prostate-specific antigen screening eligible populations in the United States. Ann Oncol. 2017;28:1098–104.
Distler FA, Radtke JP, Bonekamp D, Kesch C, Schlemmer HP, Wieczorek K, et al. The value of PSA density in combination with PI-RADS for the accuracy of prostate cancer prediction. J Urol. 2017;198:575–82.
Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part II: recommended approaches and details of specific care options. J Urol. 2018;199:990–7.
Simpkin AJ, Tilling K, Martin RM, Lane JA, Hamdy FC, Holmberg L, et al. Systematic review and meta-analysis of factors determining change to radical treatment in active surveillance for localized prostate cancer. Eur Urol. 2015;67:993–1005.
Jeong CW, Washington SL 3rd, Herlemann A, Gomez SL, Carroll PR, Cooperberg MR. The new surveillance, epidemiology, and end results prostate with watchful waiting database: opportunities and limitations. Eur Urol. 2020;78:335–44.
Acknowledgements
This work was supported by the University of Iowa Holden Comprehensive Cancer Center Population Research and Biostatistics Cores and the National Cancer Institute (P30 CA086862; R50 CA243692). This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement # U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The research project was approved by the University of Iowa Human Subjects Review Board, IRB number 01. The study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hoffman, R.M., Mott, S.L., McDowell, B.D. et al. Trends and practices for managing low-risk prostate cancer: a SEER-Medicare study. Prostate Cancer Prostatic Dis 25, 100–108 (2022). https://doi.org/10.1038/s41391-021-00393-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00393-6
This article is cited by
-
Intensity of observation with active surveillance or watchful waiting in men with prostate cancer in the United States
Prostate Cancer and Prostatic Diseases (2023)
-
Patient and physician perspectives on treatments for low-risk prostate cancer: a qualitative study
BMC Cancer (2023)
-
Re: Trends and practices for managing low-risk prostate cancer: A SEER-Medicare study
Prostate Cancer and Prostatic Diseases (2022)