Abstract
Background
Antiandrogen withdrawal (AAW) response is the paradoxical decrease in prostate-specific antigen (PSA) following the withdrawal of antiandrogen in patients with advanced prostate cancer. Currently, the reported literature on the proportion of patients exhibiting AAW response and the differences in PSA response between the types of antiandrogens is unclear.
Methods
This review aimed to explore the PSA response to AAW and to identify if the response depends on the type of antiandrogens. A literature search was performed using databases PubMed, Cochrane and EMBASE with a cut-off date of 23rd of November 2020. Studies reporting on outcomes of AAW and prostate cancer were included. Studies were screened by two reviewers and relevant data extracted. Meta-analysis of outcomes was reported using random-effects and fixed-effects model. A subgroup analysis was performed for type of antiandrogen.
Results
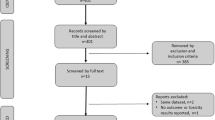
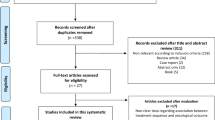
From 450 studies, 23 were included with a total of 1474 patients with advanced prostate cancer were available for further analysis. Overall, 395 (26%) patients had any reduction in PSA levels (95% CI: 20–32%) and 183 (11%) patients had a ≥50% reduction in PSA levels (95% CI: 6–16%). Among the 1212 patients on first-generation antiandrogens, 30% (95% CI: 23–38%) had any PSA decline with 15% patients having a ≥50% PSA decline (95% CI: 8–22%). In contrast, among the 108 patients on second-generation antiandrogens, 7% (95% CI: 0–13%) had any PSA decline and only 1% (95% CI: 0–5%) had a ≥50% PSA decline. Also, among the 154 patients on androgen synthesis inhibitors, 26% (95% CI: 19–33%) had any PSA decline and only 4% (95% CI: 0–13%) had a ≥50% PSA decline.
Conclusions
One-fourth of patients treated with AAW show a PSA response. However, PSA response to AAW is uncommon with second-generation antiandrogens and androgen synthesis inhibitors. Further research is required to understand the differences in response between the types of antiandrogen.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol. 2004;22:1025–33.
Rice MA, Malhotra SV, Stoyanova T. Second-generation antiandrogens: from discovery to standard of care in castration resistant prostate cancer. Front Oncol. 2019;9:801.
Griend DJV, d’Antonio JM, Isaacs JT. Hormonal Regulation of the Prostate. In: Berges R, Tombal B, editors. Androgens and prostate cancer. Belgium: Ismar Healthcare Publishing; 2009. p. 11–34.
Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308.
Miyamoto H, Rahman MM, Chang C. Molecular basis for the antiandrogen withdrawal syndrome. J Cell Biochem. 2004;91:3–12.
Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34.
Scher HI, Kelly WK. Flutamide withdrawal syndrome: its impact on clinical trials in hormone-refractory prostate cancer. J Clin Oncol. 1993;11:1566–72.
von Klot CA, Kramer MW, Boker A, Herrmann TR, Peters I, Kuczyk MA, et al. Is there an anti-androgen withdrawal syndrome for enzalutamide? World J Urol. 2014;32:1171–6.
Dupont A, Gomez J-L, Cusan L, Koutsilieris M, Labrie F. Response to flutamide withdrawal in advanced prostate cancer in progression under combination therapy. J Urol. 1993;150:908–13.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41.
MIMS Online [Internet]. MIMS Australia. 2020. Available from: http://www.mims.com.au.
Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation.
Higgins JPT, Green S. Cochrane handbook for systematic review of interventions. The Cochrane Collaboration; 2011. Available from: www.cochrane-handbook.org.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48.
Vatandoust S, Kichenadasse G, O’Callaghan ME, Kopsaftis T, Walsh S, Borg M, et al. Antiandrogen withdrawal (AAWD) in patients (pts) with prostate cancer (PCa): Retrospective analysis of data from the South Australian Prostate Cancer Clinical Outcome Collaborative (SA-PCCOC). J Clin Oncol. 2015;33 7_suppl:240.
Albiges L, Auclin E, Rousseau B, Boughalem E, Levy A, Loriot Y, et al. Is there a withdrawal syndrome with abiraterone acetate (AA)? J Clin Oncol. 2013;31 (6_suppl):89.
Poole A, Gill D, Hahn AW, Johnson E, Carroll E, Boucher K, et al. Incidence and characterization of antiandrogen withdrawal syndrome after discontinuation of treatment with enzalutamide in castration-resistant prostate cancer. Clin Genitourin Cancer. 2018;16:e169–72.
Small EJ, Srinivas S. The antiandrogen withdrawal syndrome. Experience in a large cohort of unselected patients with advanced prostate cancer. Cancer. 1995;76:1428–34.
Figg WD, Sartor O, Cooper MR, Thibault A, Bergan RC, Dawson N, et al. Prostate specific antigen decline following the discontinuation of flutamide in patients with stage D2 prostate cancer. Am J Med. 1995;98:412–4.
Rodriguez-Vida A, Bianchini D, Van Hemelrijck M, Hughes S, Malik Z, Powles T, et al. Is there an antiandrogen withdrawal syndrome with enzalutamide? BJU Int. 2015;115:373–80.
Sella A, Flex D, Sulkes A, Baniel J. Antiandrogen withdrawal syndrome with cyproterone acetate. Urology. 1998;52:1091–3.
Murakami T, Obata H, Akitake N, Shiota M, Takeuchi A, Kashiwagi E, et al. Prognostic and predictive factors for anti-androgen withdrawal in castration-resistant prostate cancer. Anticancer Res. 2018;38:4115–21.
Sartor AO, Tangen CM, Hussain MHA, Eisenberger MA, Parab M, Fontana JA, et al. Antiandrogen withdrawal in castrate-refractory prostate cancer. Cancer. 2008;112:2393–400.
Caffo O, Palermo A, Veccia A, Maines F, Chierichetti F, Berruti A, et al. Biochemical and objective response to abiraterone acetate withdrawal: incidence and clinical relevance of a new scenario for castration-resistant prostate cancer. Urology. 2013;82:1090–3.
Herrada J, Dieringer P, Logothetis CJ. Characterization of patients with androgen-independent prostatic carcinoma whose serum prostate specific antigen decreased following flutamide withdrawal. J Urol. 1996;155:620–3.
Matsumoto K, Tanaka N, Hayakawa N, Ezaki T, Suzuki K, Maeda T, et al. The type of patients who would benefit from anti-androgen withdrawal therapy: could it be performed safely for aggressive prostate cancer? Med Oncol. 2013;30:647.
Higgins J, Altman D, Sterne JE. Chapter 8: Assessing risk of bias in included studies. In: Higgins J, Churchill R, Chandler J, Cumpston ME, editors. Cochrane handbook for systematic reviews of interventions. Cochrane; Chichester, UK, 2017.
Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90.
Giatromanolaki A, Fasoulaki V, Kalamida D, Mitrakas A, Kakouratos C, Lialiaris T, et al. CYP17A1 and androgen-receptor expression in prostate carcinoma tissues and cancer cell lines. Cur Urol. 2019;13:157–65.
Richards J, Lim AC, Hay CW, Taylor AE, Wingate A, Nowakowska K, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 2012;72:2176–82.
Davies A, Conteduca V, Zoubeidi A, Beltran H. Biological evolution of castration-resistant prostate cancer. Eur Urol Focus. 2019;5:147–54.
Lorenzin F, Demichelis F. Evolution of the prostate cancer genome towards resistance. J Transl Genet Genom. 2019;3:5. https://doi.org/10.20517/jtgg.2019.01.
Yamamoto Y, Akashi Y, Minami T, Nozawa M, Kiba K, Yoshikawa M, et al. Serious hypokalemia associated with abiraterone acetate in patients with castration-resistant prostate cancer. Case Rep Urol. 2018;2018:1414395.
Sartor O, Parker CC, De Bono J. Reappraisal of glucocorticoids in castrate-resistant prostate cancer. Asian J Androl. 2014;16:666.
Veldscholte J, Berrevoets C, Ris-Stalpers C, Kuiper G, Jenster G, Trapman J, et al. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol. 1992;41:665–9.
Azad AA, Eigl BJ. Evaluation of prostate-specific antigen response following cessation of abiraterone acetate: is there evidence for a withdrawal syndrome? Eur Urol. 2014;65:504–5.
Lau YK, Chadha MK, Litwin A, Trump DL. A dramatic, objective antiandrogen withdrawal response: case report and review of the literature. J Hematol Oncol. 2008;1:21.
Leone G, Tucci M, Buttigliero C, Zichi C, Pignataro D, Bironzo P, et al. Antiandrogen withdrawal syndrome (AAWS) in the treatment of patients with prostate cancer. Endocr Relat Cancer. 2018;25:R1–9.
Bracarda S, Procopio G, Alesini D, Grillone F, Massari F, Zaniboni A, et al. Enzalutamide activity in patients with metastatic castration resistant prostate cancer (mCRPC) previously responding to antiandrogen withdrawal syndrome (AWN): a preliminary report. J Clin Oncol. 2014;32:e16508.
El Geneidy MM, Lewis G, Dainer P, Terris MK, Brown J, Coleman T. Factors predicting a response to anti-androgen withdrawal maneuvers in prostate cancer patients failing combined androgen blockade. J Clin Oncol. 2008;26:e16116.
Momozono H, Miyake H, Tei H, Harada KI, Fujisawa M. Clinical outcomes of anti-androgen withdrawal and subsequent alternative anti-androgen therapy for advanced prostate cancer following failure of initial maximum androgen blockade. Mol Clin Oncol. 2016;4:839–44.
Morote J, Bellmunt J. Bone alkaline phosphatase serum level predicts the response to antiandrogen withdrawal. Eur Urol. 2002;41:257–61.
Schellhammer PF, Venner P, Haas GP, Small EJ, Nieh PT, Seabaugh DR, et al. Prostate specific antigen decreases after withdrawal of antiandrogen therapy with bicalutamide or flutamide in patients receiving combined androgen blockade. J Urol. 1997;157:1731–5.
Yoneyama S, Miyoshi Y, Yasui M, Uemura K, Kawahara T, Hattori Y, et al. The evaluation of antiandrogen withdrawal syndrome after discontinuation of bicalutamide in metastatic castration-resistant prostate cancer. Int J Urol. 2017;24:38.
Figg WD, McCall NA, Reed E, Sartor O. The in-vitro response of 4 antisteroid receptor agents on the hormone-responsive prostate-cancer cell-line lncap. Oncol Rep. 1995;2:295–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soo, A., O’Callaghan, M.E., Kopsaftis, T. et al. PSA response to antiandrogen withdrawal: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 24, 826–836 (2021). https://doi.org/10.1038/s41391-021-00337-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00337-0