Abstract
Introduction
Enzalutamide, a second-generation androgen receptor inhibitor, is indicated for the treatment of metastatic disease, as well as in the treatment of non-metastatic castration resistant prostate cancer (PCa). This systematic review aims to determine outcomes and toxicity in patients with non-metastatic castration sensitive prostate cancer (nmCSPC) treated with enzalutamide in the primary or salvage settings.
Method
We performed a systematic review focusing on the role of Enzalutamide in the treatment of nmCSPC, using the PubMed/Medline database. Articles focusing on androgen receptor inhibitors in nmCSPC were included, while articles discussing exclusively metastatic or castration-resistant PCa were excluded.
Results
The initial search retrieved 401 articles, of which 15 underwent a thorough assessment for relevance. Ultimately, 12 studies with pertinent outcomes were meticulously examined. Among these, seven studies were dedicated to the investigation of enzalutamide in the primary setting, while the remaining five publications specifically addressed its use in salvage settings. Regardless of the treatment setting, our data revealed two distinct therapeutic strategies. The first advocates for the substitution of enzalutamide for androgen deprivation therapy (ADT), based on the premise of achieving equivalent, if not superior, oncological outcomes while minimizing treatment-related toxicity. The second, adopting a more conventional approach, entails augmenting the effectiveness of ADT by incorporating enzalutamide.
Conclusion
Enzalutamide has considerable potential as a therapeutic strategy for nmCSPC, either used alone or in combination with ADT in the primary or in the salvage settings. The use of enzalutamide instead of ADT is an appealing strategy. However, more trials will be required to further understand the efficacy and side-effect profile of enzalutamide monotherapy.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is the third cause of cancer deaths in men from the European Union with 78,800 deaths in 2020. The 2020 PCa mortality rate was 10.0/100,000, declining by 7.1% since 2015 [1]. These favorable trends reflect improvements in treatment strategies. Androgen deprivation therapy (ADT) has been a cornerstone of PCa treatment for several decades. More recently, androgen receptor pathway inhibitors (ARpI), including androgen receptors targeted agents (ARTA) such as Enzalutamide, Apalutamide, and Darolutamide, and steroidogenesis inhibitors such as Abiraterone, have profoundly impacted the management of advanced prostate cancer. They have been shown to delay progression, increase overall survival (OS), and improved quality of life in patients with metastatic castration sensitive PCa (mCSPC) [2], metastatic (mCRPC) [3], or non-metastatic castration resistant PCa (nmCRPC) [4]. Combined with ADT, they have become standard of care in these indications.
After the AFFIRM study results, enzalutamide was initially approved by the United States Food and Drug Administration (U.S. FDA) in 2012 for patients with mCRPC who had previously received docetaxel treatment [5]. Enzalutamide’s indication for treatment was expanded to include mCRPC patients who had never received chemotherapy in 2014 [6]. Enzalutamide was licensed by the U.S. FDA in 2018 for use in patients with nmCRPC as a result of the promising findings of the PROSPER trial [7, 8]. Following the findings of the ARCHES trial, the FDA approved enzalutamide for patients with mCSPC in December 2019 [9]. The benefit of enzalutamide in the mCSPC setting was subsequently confirmed in the ENZAMET trial [10].
The next generation of trials is exploring the benefit of ARpI in non-metastatic PCa, especially in high-risk localized PCa in combination with localized treatment and in patients with biochemical recurrence after local treatment, alone or in combination with androgen suppression or other targeted therapies. This systematic review aims to provide an overview of the available evidence for enzalutamide in the management of non-metastatic hormone sensitive PCa (nmCSPC).
Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Review and meta-analyses (PRISMA) statement [11]. The review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42023402738).
Study selection
For the bibliographic search, the electronic database Pubmed was used with no time restriction. The final search equation was (prostate cancer[Title/Abstract]) AND ((hormone naive[Title/Abstract] OR hormone sensitive[Title/Abstract] OR castration sensitive[Title/Abstract] OR localized[Title/Abstract] OR non metastatic[Title/Abstract] OR low risk[Title/Abstract] OR intermediate risk[Title/Abstract] OR high risk[Title/Abstract] OR salvage[Title/Abstract] OR recurrent[Title/Abstract] OR relapse[Title/Abstract] OR recurrence[Title/Abstract] OR rising psa[Title/Abstract] OR rising prostate specific antigen[Title/Abstract])) AND ((enzalutamide[Title/Abstract] OR xtandi[Title/Abstract] OR mdv3100[Title/Abstract])) NOT (review[Publication Type]).
Publications were included for review if they were prospective trials exploring outcomes and toxicity of hormone sensitive PCa patients with no evidence of distant metastases treated with enzalutamide. Publications were excluded if they were not written in English; exclusively related to castration resistant PCa; exclusively related to metastatic PCa; or were published as editorials, commentaries, letters, case reports, or trial protocols. If multiple publications were identified describing similar results from the same data set, only the most recent publication was retrieved.
Two authors performed electronic searches of the online MEDLINE database in August 2023. The titles and abstracts were assessed by the same two authors and all potentially relevant articles were identified for full-text review. The final selection from the set of full-text articles was independently performed by two authors with disagreements resolved via discussion with two senior authors.
Data extraction
Information on each study characteristic was extracted by two authors (V.A and F.A) and included the article information (e.g., first author, publication year), population demographics, study methodology, and key findings. Oncological outcomes as well as enzalutamide-related toxicity were summarized.
Analysis of results
Studies were analyzed using a narrative synthesis approach. A meta-analysis of OS rates and toxicity was not performed because of the variation in study populations and intervention types in the studies.
Evidence synthesis
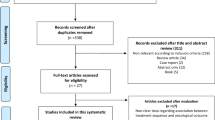
Our initial search identified 401 studies. The titles and abstracts identified via this search were screened and 15 papers were selected for full text review according to the eligibility and exclusion criteria. Of these, 3 publications were excluded (two due to redundancy in the dataset and one did not report outcomes and/or toxicity results). The study selection steps are presented in Fig. 1.
Results
Table 1 summarizes the characteristics of the 12 publications. Most trials are phase 2 trials (n = 10) except for 2 being phase 3 trials [12, 13]. The primary and the salvage setting are approximately equally represented in these publications (n = 7 [13,14,15,16,17,18,19] versus n = 6 [12, 13, 20,21,22,23] respectively). The therapeutic combinations evaluated frequently were enzalutamide and radiotherapy (RT) (n = 5 [14, 15, 18, 20, 23]), and enzalutamide + ADT ± RT ± Abiraterone (n = 5 [12, 13, 16,17,18]). Finally, 3 studies had at least one arm evaluating enzalutamide only [19, 21, 22].
Primary setting
As an alternative to Active Surveillance
The ENACT trial is an open-label phase 2 trial which randomized patients with low-risk or intermediate-risk PCa between active surveillance (AS) and AS plus enzalutamide monotherapy given for 1 year [19]. The primary endpoint was time to pathological or therapeutic PCa progression (pathological, ≥1 increase in primary or secondary Gleason pattern or ≥15% increased cancer-positive cores; therapeutic, earliest occurrence of primary therapy for PCa). Approximately half of the patients had low-risk disease, the other half had favorable intermediate-risk disease. With a median follow-up of 16.2 months for patients receiving enzalutamide and 8.8 months for patients undergoing AS, treatment with enzalutamide significantly reduced the risk of PCa progression by 46% vs AS (28.1% vs 37.2% respectively, HR, 0.54; 95% CI: 0.33–0.89; P = 0.02). During the 1-year treatment period, 92.0% of patients reported any grade toxicity in the enzalutamide group versus 54.9% in the AS group. The most frequent reported toxicity in the enzalutamide group was fatigue in more than half of the patients, gynecomastia and other-related symptoms in one third of the patients, and erectile dysfunction in 18% of patients.
When published, this study was heavily criticized, for several reasons. First, current guidelines clearly state that all active treatment options present a risk of over-treatment for the management of low-risk disease and that AS or watchful waiting (based on life expectancy) is standard of care in this setting [24]. Second, the relevance of the chosen endpoint was questioned. Giving a drug that blocks PCa growth and PSA production could delay grade progression. The fact that it could translate to benefit with stronger endpoints as metastasis-free survival (MFS) or OS is very unlikely. Based on these 2 remarks, it was deemed very questionable to provide a treatment with a high toxicity and a significant financial burden on healthcare systems without any effect on survival or quality of life, especially for patients who do not need treatment in the first place [25].
Neoadjuvant to surgery
Radical prostatectomy (RP) can be curative for high-risk PCa patients. However, a significant subset of high-risk PCa patients experience biochemical relapse (BCR) with an increased risk of PCa mortality [26]. In view of this, several studies have evaluated the potential benefit of adding neoadjuvant treatment such as ADT to RP. However, these studies fail to show any benefit in prostate cancer-specific survival (PCSS) or PSA relapse-free survival [27, 28], and therefore neoadjuvant ADT before RP is not recommended by the EAU guidelines [24]. Combination neoadjuvant therapy including the addition of docetaxel or ARPIs to ADT could bring benefit to high-risk PCa patients where ADT alone has failed. Two randomized phase 2 trials have investigated the role of enzalutamide alone or in combination with leuprolide and abiraterone in the neoadjuvant setting [16, 17]. In a non-comparative study, Montgomery et al. randomized 52 patients with localized PCa with Gleason Score of 7 or above or PSA > 10 ng/mL between 6 months of neoadjuvant enzalutamide in combination with dutasteride and leuprolide, and enzalutamide alone. The primary objective was to assess the pathologic complete response (pCR) rate [17]. Baseline characteristics were well balanced between the 2 treatment arms with approximately 79% of patients with a high-risk disease in each arm. In the enzalutamide alone arm, none of the 25 patients achieved pCR whereas in the enzalutamide/dutasteride/leuprolide arm, 4.3% achieved pCR. Grade ≥ 3 toxicity occurred in 11.1% of patients in the enzalutamide alone arm versus 24.0% of patients in the combination arm. Gynecomastia and mastodynia were more frequent in the enzalutamide arm than in the combination arm (63% vs. 12 and 59% vs. 8%, respectively, all grades). On the contrary, patients in the enzalutamide arm experienced fewer hot flashes than those in the combination arm (26% vs. 96%, respectively, all grades). Though non-castrating therapy alone seems ineffective in producing pathological response, combining ADT and ARPIs may represent a promising therapeutic approach. That is why, the same group conducted subsequently a randomized phase 2 trial evaluating 6 months of neoadjuvant enzalutamide and leuprolide with or without abiraterone and prednisone before RP. Patients mainly had high-risk disease (86.7%). Addition of abiraterone and prednisone increased the pCR rate by 2% (8% versus 10%, respectively), this difference being not statistically significant. No grade 4 toxicity was observed. Grade 3 toxicity was higher in the abiraterone and prednisone arm (26% vs 4%) driven by increased hypertension, and liver toxicity (increased liver enzymes). In conclusion, the benefit of combining enzalutamide with or without ADT or other ARPIs in the neoadjuvant setting prior to RP is not very clear.
In combination with RT
As an alternative to ADT in intermediate-risk PCa
Several studies showed an improved OS and PCSS with the addition of short-term ADT to RT in patients with intermediate-risk PCa compared to RT alone [29,30,31]. However, ADT is associated with several side effects including hot flashes, loss of libido, metabolic syndrome, and erectile dysfunction [32]. Anti-androgen monotherapy does not suppress serum testosterone production, and may thus avoid several of ADT side effects at the expense of inducing others such as painful gynecomastia. Two very similar phase 2 single arm trials have investigated PSA response and side-effects after RT and 6 months of enzalutamide in intermediate-risk PCa. Kaplan et al. included 64 patients of whom 84% had unfavorable intermediate-risk disease as defined by the National Comprehensive Cancer Network guidelines while the ENZART study conducted by Lara at al included 62 patients of whom 73.7% with unfavorable intermediate-risk disease [14, 15]. Patients in the Kaplan study received 79.2 Gy in 44 fractions, whereas those in the ENZART study received 70 Gy in 25 fractions. Defining PSA response as a PSA nadir below 0.2 ng/ml during the 6 months of enzalutamide, 79% of patients achieved a PSA response in the Kaplan trial while 100% of patients did so in the ENZART study [15]. Six months after the end of enzalutamide, 56.8% of patients continued to have PSA response (<0.2 ng/ml) in the Lara et al. study (no data is available after enzalutamide cessation in the Kaplan et al. study). Up to 48% of patients had a grade ≥3 toxicity in the Lara et al. study, mainly hypertension (33.9%) with 2 patients experiencing a grade 4 toxicity (hypertensive and liver enzyme elevation). On the contrary, no grade 4 toxicity was observed in the Kaplan et al. study and 19% of patients had a grade 3 toxicity, mainly in the form of hypertension (12%). Interestingly, toxicities associated with ADT, such as hot flashes and loss of libido, were low and predominantly grade 1 in both trials. Furthermore, Kaplan et al. documented grade 1–3 toxicity rates for erectile dysfunction at 20.3%, 12.5%, and 1.5%, respectively. Finally, there is an increase in testosterone levels compared to baseline after initiation of enzalutamide with a decrease to pretreatment level 6 months after the end of therapy.
These 2 studies showed that combining enzalutamide with RT for intermediate risk cancer is safe and effective in terms of PSA response. However, PSA response is not a surrogate for OS or PCSS and thus the real long-term impact of enzalutamide on PCa patients receiving RT remains unknown [33]. Thus, the comparison of enzalutamide with ADT in combination with RT in terms of efficacy and safety still needs to be confirmed in a randomized phase III study. The only comparison that we have so far between antiandrogen and ADT in combination with RT in terms of efficacy and side effects comes from an exploratory analysis from the CHHiP trial [34]. This non-randomized comparison showed no difference in efficacy and patient-reported outcomes according to the type of hormonal treatment, though clinician-assessed erectile function at 2 years was better in the antiandrogen group. The antiandrogen used in the CHHiP trial was bicalutamide. Since Enzalutamide is a second-generation antiandrogen and has a higher affinity to androgen receptor than bicalutamide, it might be postulated that it could be more efficacious, but this still need to be demonstrated [14].
In combination with ADT in high-risk and very high-risk PCa
In a meta-analysis pooling data from 2 randomized controlled phase 3 trials conducted in the Systematic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE, MRC-PR08), Attard et al. investigated the addition of abiraterone and prednisone with or without enzalutamide to ADT for nodal positive non-metastatic PCa or localized PCa with at least two high-risk features, which were defined as tumor stage T3 or T4, Gleason sum score of 8–10 and PSA concentration >40 ng/ml [13]. The first trial allocated 455 patients to the control group (ADT only) and 459 patients to combination therapy (ADT and abiraterone and prednisone), and the second trial, which added enzalutamide in the combination therapy arm, allocated 533 patients to the control group and 527 patients to combination therapy. Almost all (99%) newly diagnosed N0 and 71% of N1 patients were planned for local RT. With a median follow-up of 72 months, 6-year metastasis-free survival (MFS) improved from 69 to 82% with the addition of abiraterone and prednisone ± enzalutamide. Moreover, and though they could not rule out a small benefit from combining enzalutamide and abiraterone, the authors did not recommend this combination due to increased toxicity and costs of adding enzalutamide to abiraterone. Indeed, 37% of patients in the abiraterone trial and 57% of patients in the abiraterone and enzalutamide trial had grade ≥3 toxicity during the first 24 months. This practice changing study is responsible for the new recommendation in the EAU as well as the ASTRO ACROP guidelines to offer 24 months of abiraterone with long-term ADT and RT to cN0 cM0 patients with very high-risk features and to cN1 cM0 patients [24, 35].
The choice of an ARPI in the high-risk localized setting will likely be based on market authorization, mode of administration and convenience, side-effect profile, patient profile, drug-drug interactions, rather than based on efficacy which is expected to be pretty much the same for all these second-generation antiandrogens. The benefit of the addition of enzalutamide in the high-risk non-metastatic setting still must be formally demonstrated with a randomized phase 3 trial. The ENZARAD trial (NCT02446444) (Table 2) is currently investigating the benefit of enzalutamide in high-risk PCa treated with external beam RT (EBRT) and 2 years of ADT. Similar to abiraterone, the entry of enzalutamide in the generic drug market should broaden its use. Awaiting for these results, a small phase 2 trial investigated safety, tolerability, and efficacy (PSA complete response rate) of adding enzalutamide to ADT in high-risk localized or nodal positive non-metastatic PCa patients [18]. Definition of high-risk features (node positive or, if node negative, having at least two of the following: tumor stage T3a/b, Gleason sum score of 8–10, and PSA concentration ≥20 ng/mL, ≥33% core involvement on biopsy) was very close to the STAMPEDE criteria. Sixteen patients were enrolled, and 5 were excluded before starting treatment. Of the remaining 11 patients, all completed the 24 months follow-up and 9 of them completed the 36 months follow-up. One patient (9%) had grade 4 toxicity (seizure), and 4 patients (36.4%) had grade 3 toxicity. All patients achieved PSA complete response defined as PSA ≤ 0.3 and at 36 months, 1 out of the 9 evaluable patients presented with a biochemical recurrence as per the Phoenix criteria. This study, limited by sample size, short follow-up, and lack of control group suggests that combining enzalutamide with ADT and RT is relatively well tolerated.
Salvage setting
In combination with RT
The addition of ADT to salvage RT as well as its duration is still an open question. Three randomized controlled trials have compared short-term ADT versus no ADT in combination with salvage RT [36,37,38]. The GETUG-AFU 16 was the only one showing a benefit in MFS of the addition of short-term ADT [37]. This was not seen in the RADICALS-HD [38] and RTOG 0534 [36] trials. The DADSPORT meta-analysis pooled these 3 trials to show a statistically significant but clinically irrelevant 5-year absolute improvement in MFS of 0 vs 6 months ADT of 2% (90% vs 92%, HR 0.82; 95% CI: 0.70–0.96) [39]. The only trials assessing the benefit of a long-term (2 years) regimen compared to RT alone used the first-generation antiandrogen bicalutamide 150 mg (RTOG 9601) [40, 41]. Long-term bicalutamide improved OS and MFS in patients with high baseline PSA values (>0.6 ng/ml) but was on the contrary detrimental to patients treated with early salvage RT (PSA < 0.6 ng/ml), causing an increase of other-cause mortality and late grade ≥3 toxicity hazard. Likewise, the first results of the RADICALS-HD trial suggest that in postoperative radiotherapy patients, long-term ADT improves time to salvage ADT and MFS in comparison to 6 months ADT. However, no OS benefit was shown so far [38].
In the complex context of adding ADT to salvage RT after RP, two phase 2 trials have investigated the addition of enzalutamide to salvage RT [23] or to salvage RT with ADT [20].
-
In the SALV-ENZA trial, men with BCR after RP were enrolled into a randomized, phase 2, double‐blinded, placebo-controlled, multicenter study of RT plus enzalutamide or placebo, given for 6 months. The primary endpoint was freedom from PSA progression (FFPP). With a median follow-up of 34 months, FFPP was significantly improved with enzalutamide versus placebo (hazard ratio [HR], 0.42; 95% CI: 0.19 to 0.92; P = 0.031), and 2-year FFPP was 84% versus 66%, respectively. Apart from increased nausea and breast pain observed in the enzalutamide arm, no significant differences in grade 1 and 2 toxicity were noted between the two groups. Grade 3 toxicities were rare, with only 3 cases for the enzalutamide arm and 7 cases for the placebo arm [23].
-
In the single arm phase 2 STREAM trial, patients with BCR after RP were treated with a short course of ADT and enzalutamide combined with prostate bed RT [20]. With a median follow-up of 37.5 months, they reported a 2-year PFS of 65%. Grade 3 hypertension was the most frequent grade 3 event, occurring in 11% of patients. Other grade 3 toxicities included headache, tremor, back pain, and fatigue (each 3%).
As Monotherapy or in combination with ADT
According to the EAU guidelines, ADT may be offered to patients as monotherapy in selected cases with BCR [24]. Intensification of ADT with enzalutamide or replacement of ADT by enzalutamide are 2 pathways that are currently investigated in this setting:
-
Tombal et al. were the first to investigate enzalutamide monotherapy as the front-line treatment in patients with localized PCa and mCSPC [22, 42, 43]. In a phase 2 multicenter open label single arm study, 67 patients (35 M0, 10 M1, and 22 Mx) received enzalutamide monotherapy until disease progression or unacceptable toxicity development. To assess the efficacy of enzalutamide, they used PSA response defined as 80% PSA drop over pretreatment values. Of the 42 patients who remained on enzalutamide up to the 3-year visit, 38 (90.5%) maintained a PSA response. At 3 years, there was a small decrease in bone mineral density (BMD) compared to baseline, lower than the mean BMD decrease reported for long-term ADT. Nine out of 67 patients (13.4%) had adverse events possibly related to enzalutamide which led to enzalutamide discontinuation during or after 3 years. Gynecomastia and fatigue were the 2 most frequent adverse events (49.3% and 38.8% respectively, all grades). During the 3 years, grade ≥3 toxicity was reported in 34% of patients. The open label single arm design and the small and heterogenous study population are among the limitations of this study. However, it provided rationale for further investigation of enzalutamide monotherapy in the nmCSPC setting, which was subsequently carried out with the EMBARK trial.
-
The EMBARK trial included 1068 patients with high-risk BCR (PSA doubling time (PSADT) ≤ 9 months) after local treatment and no evidence of distant metastases on conventional imaging. Patients were randomized between ADT, ADT plus enzalutamide, and enzalutamide alone [12, 44]. In this trial, after a median follow-up of 60.7 months, enzalutamide + ADT demonstrated a statistically significant improvement in 5-year MFS versus ADT + placebo (87.3% vs 71.4%, HR, 0.42; 95% CI: 0.31–0.61; P < 0.0001). Interestingly, enzalutamide monotherapy also significantly improves MFS versus ADT (HR 0.63; 95% CI: 0.46−0.87; P = 0.0049). Of all patients, 164 (46.5%) patients experienced a grade ≥3 adverse events in the combination arm, versus 151 (42.7%) in the ADT + placebo arm, and 177 (50.0%) in the enzalutamide alone arm. More patients experienced gynecomastia in the enzalutamide monotherapy arm than in the ADT ± enzalutamide arms. In conclusion, enzalutamide, either alone or in combination with ADT, may be beneficial for high-risk BCR patients without metastasis. However, it’s important to note that the Embark study only utilized conventional imaging, leaving the effectiveness of PSMA PET/CT unaddressed, despite its emerging role in detecting prostate cancer recurrence, even in low levels of PSA [45].
Discussion
Enzalutamide was the first second-generation ARTA to receive FDA approval and is now approved in combination with ADT for the treatment of castration-resistant PCa, regardless of the presence of metastases, and as first-line therapy in mCSPC [5, 6, 8,9,10]. A growing number of studies have examined the effectiveness and safety of enzalutamide when used in earlier stage of the disease. In this systematic review, we summarize the use of enzalutamide in non-metastatic hormone sensitive prostate cancer.
Conventionally, enzalutamide is used in addition to ADT, in populations where ADT alone or in combination with RT would be the SOC. The idea is to reinforce the systemic treatment, a strategy which has been conducted in other settings resulting in better oncological outcomes [5, 6, 8,9,10]. In the primary setting, this idea is particularly relevant for high-risk and very high-risk PCa patients treated with a combination of long-term ADT and RT. Attard et al. changed the SOC treatment for this group of patient with the results of the STAMPEDE trial [13] and now the addition of abiraterone to ADT is recommended for N0 patients with high risk features or N1 patients [24]. We expect to have the same benefit demonstrated in the ENZARAD trial (NCT02446444) with the addition of enzalutamide to long-term ADT and RT for this group of patients.
In the same primary setting, combining ADT and enzalutamide before RP is being explored in some studies [16, 17]. However, this approach remains investigational and is very unlikely to be practice changing. In the salvage setting, the benefit of adding enzalutamide on top of SOC treatment needs to be further investigated. For patients relapsing after RP treated at a low PSA level (<0.5 ng/ml) with salvage prostate bed RT, it is already very debatable to add ADT. Indeed, the DADSPORT meta-analysis specifically investigated the impact of the addition of ADT to salvage RT in terms of OS and showed no evidence of an OS benefit with ADT vs none, irrespective of whether 6 months or 24 months of ADT but only a modest MFS benefit on the long-term FU (2% with short-term ADT at 5-yr and 6% with long-term ADT at 10-y) [39]. This meta-analysis also underlines the good prognosis of PCa patients treated with salvage RT alone with MFS rates exceeding 70% at 10 years. In a situation when adding ADT does not bring any survival benefit, a combination of ADT and enzalutamide is therefore highly questionable. However, for patients with a high PSA level at relapse and a short PSADT (≤9 months), the benefit of intensifying ADT with enzalutamide compared to ADT alone has been proven in the EMBARK trial with an increased MFS in the combination arm compared to the leuprolide arm. These patients did not have any metastases based on conventional imaging. However, with the advance of molecular imaging, it is expected a high proportion of these patients would exhibit metastatic or pelvic lesions on PSMA PET/CT and may thus benefit from radiotherapy [45].
Though the efficacy of enzalutamide seems to be equal if not superior to ADT in the EMBARK trial [12], the toxicity may not be decreased. It is indeed very tempting to believe that because enzalutamide does not lower testosterone level, it is better tolerated than ADT. However, in the EMBARK trial, grade ≥3 adverse events were present in 50.0% of patients in the enzalutamide monotherapy arm versus 42.7% in the leuprolide arm. If we investigate the type of adverse events by treatment arm, we observe more frequent hot flashes in the leuprolide arm than in the enzalutamide arm (57.3% vs 21.8%). On the contrary, fatigue was higher in the enzalutamide arm than in the leuprolide arm (46.6% vs 32.8%), as well as gynecomastia (44.9% vs 9.0%), and ischemic heart disease (9.0% vs 5.6%). Despite enzalutamide being more effective than ADT in the EMBARK trial, the use of enzalutamide in place of ADT in populations where ADT use is the standard of care needs to be further investigated.
Conclusions
In summary, our results highlight the different therapeutic strategies trying to involve enzalutamide at an earlier stage of Pca treatment. In the nmCSPC setting, using enzalutamide instead of ADT or in combination with ADT to intensify systemic treatment are 2 options that are currently investigated. For the first option using enzalutamide instead of ADT, the equivalence or superior efficacy of enzalutamide compared to ADT still needs to be demonstrated. Moreover, the toxicity of enzalutamide may not be lower than that of ADT but just different. For the second option, adding enzalutamide to ADT in order to intensify the systemic treatment should be reserved for very high-risk PCa patients, as per the STAMPEDE population [13], or relapsing disease following primary treatment with high-risk features including high PSA level at relapse and short PSADT [12]. ARPIs have drastically changed the treatment landscape of mCSPC and both metastatic and nmCRPC. Their incorporation earlier on in the disease stage was just a matter of time and STAMPEDE [13] and EMBARK [12] trials are for now the most successful examples in this regard. However, one must be very careful about the risk of overtreatment given the unnecessary toxicity it brings to patients. In the metastatic setting, de-intensification is currently being studied, notably with the EORTC-2238 GUCG (De-ESCALATE) NCT05974774, revisiting the concept of intermittent ADT in patients achieving a good PSA response after 6–12 months of ADT and one of the ARPIs. To avoid de-intensification trials in the non-metastatic setting, indications for treatment intensification should be carefully thought. Several studies are ongoing or have been recently completed further investigating the efficacy and safety of Enzalutamide in the definitive or salvage setting (Table 2). The results of these studies will further inform clinical practice in the next decade.
References
Carioli G, Bertuccio P, Boffetta P, Levi F, La Vecchia C, Negri E, et al. European cancer mortality predictions for the year 2020 with a focus on prostate cancer. Ann Oncol. 2020;31:650–8.
Hamid AA, Sayegh N, Tombal B, Hussain M, Sweeney CJ, Graff JN, et al. Metastatic hormone-sensitive prostate cancer: toward an era of adaptive and personalized treatment. Am Soc Clin Oncol Educ Book. 2023;43:e390166.
Ferretti S, Mercinelli C, Marandino L, Litterio G, Marchioni M, Schips L. Metastatic castration-resistant prostate cancer: insights on current therapy and promising experimental drugs. Res Rep Urol. 2023;15:243–59.
Berruti A, Bracarda S, Caffo O, Cortesi E, D’Angelillo R, Del Re M, et al. nmCRPC, a look in the continuous care of prostate cancer patients: state of art and future perspectives. Cancer Treat Rev. 2023;115:102525.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33.
Sternberg CN, Fizazi K, Saad F, Shore ND, De Giorgi U, Penson DF, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197–206.
Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378:2465–74.
Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37:2974–86.
Sweeney CJ, Martin AJ, Stockler MR, Begbie S, Cheung L, Chi KN, et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24:323–34.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021;372:n71.
Freedland SJ, de Almeida Luz M, De Giorgi U, Gleave M, Gotto GT, Pieczonka CM, et al. Improved outcomes with enzalutamide in biochemically recurrent prostate cancer. N Engl J Med. 2023;389:1453–65.
Attard G, Murphy L, Clarke NW, Cross W, Jones RJ, Parker CC, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2022;399:447–60.
Kaplan I, Bubley GJ, Bhatt RS, Taplin ME, Dowling S, Mahoney K, et al. Enzalutamide with radiation therapy for intermediate-risk prostate cancer: a phase 2 study. Int J Radiat Oncol Biol Phys. 2021;110:1416–22.
Lara PC, Rodríguez-Melcón JI, Palacios-Eito A, Lozano A, Hervás-Morón A, Villafranca E, et al. Phase II study of ENZAlutamide combined with hypofractionated radiation therapy (ENZART) for localized intermediate risk prostate cancer. Front Oncol. 2022;12:891886.
McKay RR, Ye H, Xie W, Lis R, Calagua C, Zhang Z, et al. Evaluation of intense androgen deprivation before prostatectomy: a randomized phase II trial of enzalutamide and leuprolide with or without abiraterone. J Clin Oncol. 2019;37:923–31.
Montgomery B, Tretiakova MS, Joshua AM, Gleave ME, Fleshner N, Bubley GJ, et al. Neoadjuvant enzalutamide prior to prostatectomy. Clin Cancer Res. 2017;23:2169–76.
Shee K, de la Calle CM, Chang AJ, Wong AC, Feng FY, Gottschalk AR, et al. Addition of enzalutamide to leuprolide and definitive radiation therapy is tolerable and effective in high-risk localized or regional nonmetastatic prostate cancer: results from a phase 2 trial. Adv Radiat Oncol. 2022;7:100941.
Shore ND, Renzulli J, Fleshner NE, Hollowell CMP, Vourganti S, Silberstein J, et al. Enzalutamide monotherapy vs active surveillance in patients with low-risk or intermediate-risk localized prostate cancer: the ENACT randomized clinical trial. JAMA Oncol. 2022;8:1128–36.
Bitting RL, Healy P, George DJ, Anand M, Kim S, Mayer T, et al. Phase II trial of enzalutamide and androgen deprivation therapy with salvage radiation in men with high-risk prostate-specific antigen recurrent prostate cancer: the STREAM trial. Eur Urol Oncol. 2021;4:948–54.
Madan RA, Karzai F, Donahue RN, Al-Harthy M, Bilusic M, Rosner II, et al. Clinical and immunologic impact of short-course enzalutamide alone and with immunotherapy in non-metastatic castration sensitive prostate cancer. J Immunother Cancer. 2021;9:e001556.
Tombal B, Borre M, Rathenborg P, Werbrouck P, Van Poppel H, Heidenreich A, et al. Long-term antitumor activity and safety of enzalutamide monotherapy in hormone Naïve prostate cancer: 3-year open label followup results. J Urol. 2018;199:459–64.
Tran PT, Lowe K, Tsai HL, Song DY, Hung AY, Hearn JWD, et al. Phase II randomized study of salvage radiation therapy plus enzalutamide or placebo for high-risk prostate-specific antigen recurrent prostate cancer after radical prostatectomy: the SALV-ENZA trial. J Clin Oncol. 2023;41:1307–17.
Mottet NCP, van den Bergh RCN, et al. EAU guidelines 2023 [Available from: https://uroweb.org/guidelines/prostate-cancer/chapter/treatment.
Gómez Rivas J, Gandaglia G, Montorsi F. Re: Neal D. Shore, Joseph Renzulli, N E. Fleshner, et al. Active Surveillance plus Enzalutamide Monotherapy vs Active Surveillance Alone in Patients with Low-risk or Intermediate-risk Localized Prostate Cancer: The ENACT Randomized Clinical Trial. JAMA Oncol 2022;8:1128-36: Let’s Not Go Back in Time! What Have We Learned in the Treatment of Localized Prostate Cancer? Applicability of the ENACT Randomized Clinical Trial in Clinical Practice. Eur Urol Open Sci. 2022;46:135–6.
Stephenson AJ, Kattan MW, Eastham JA, Bianco FJ Jr, Yossepowitch O, Vickers AJ, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27:4300–5.
Liu W, Yao Y, Liu X, Liu Y, Zhang GM. Neoadjuvant hormone therapy for patients with high-risk prostate cancer: a systematic review and meta-analysis. Asian J Androl. 2021;23:429–36.
Kumar S, Shelley M, Harrison C, Coles B, Wilt TJ, Mason MD. Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst Rev. 2006;2006:Cd006019.
Krauss DJ, Karrison T, Martinez AA, Morton G, Yan D, Bruner DW, et al. Dose-escalated radiotherapy alone or in combination with short-term androgen deprivation for intermediate-risk prostate cancer: results of a phase III multi-institutional trial. J Clin Oncol. 2023;41:3203–16.
Bolla M, Maingon P, Carrie C, Villa S, Kitsios P, Poortmans PM, et al. Short androgen suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: results of EORTC trial 22991. J Clin Oncol. 2016;34:1748–56.
Jones CU, Hunt D, McGowan DG, Amin MB, Chetner MP, Bruner DW, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–18.
Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115:2388–99.
Roy S, Romero T, Michalski JM, Feng FY, Efstathiou JA, Lawton CAF, et al. Biochemical recurrence surrogacy for clinical outcomes after radiotherapy for adenocarcinoma of the prostate. J Clin Oncol. 2023;41:5005–14.
Tree A, Griffin C, Syndikus I, Birtle A, Choudhury A, Graham J, et al. Nonrandomized Comparison of Efficacy and Side Effects of Bicalutamide Compared With Luteinizing Hormone-Releasing Hormone (LHRH) analogs in combination with radiation therapy in the CHHiP trial. Int J Radiat Oncol Biol Phys. 2022;113:305–15.
Schmidt-Hegemann N-S, Zamboglou C, Mason M, Mottet N, Hinnen K, De Meerleer G, et al. ESTRO-ACROP recommendations for evidence-based use of androgen deprivation therapy in combination with external-beam radiotherapy in prostate cancer. Radiother Oncol. 2023;183:109544.
Pollack A, Karrison TG, Balogh AG, Gomella LG, Low DA, Bruner DW, et al. The addition of androgen deprivation therapy and pelvic lymph node treatment to prostate bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): an international, multicentre, randomised phase 3 trial. Lancet. 2022;399:1886–901.
Carrie C, Magne N, Burban-Provost P, Sargos P, Latorzeff I, Lagrange JL, et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): a 112-month follow-up of a phase 3, randomised trial. Lancet Oncol. 2019;20:1740–9.
Parker CC, Clarke N, Cook A, Catton C, Cross WR, Kynaston H, et al. LBA9 Duration of androgen deprivation therapy (ADT) with post-operative radiotherapy (RT) for prostate cancer: first results of the RADICALS-HD trial (ISRCTN40814031). Ann Oncol. 2022;33:S1427.
Burdett S, Fisher D, Parker CC, Sydes MR, Pommier P, Sargos P, et al. LBA64 Duration of androgen suppression with post-operative radiotherapy (DADSPORT): A collaborative meta-analysis of aggregate data. Ann Oncol. 2022;33:S1428–S9.
Dess RT, Sun Y, Jackson WC, Jairath NK, Kishan AU, Wallington DG, et al. Association of presalvage radiotherapy PSA levels after prostatectomy with outcomes of long-term antiandrogen therapy in men with prostate cancer. JAMA Oncol. 2020;6:735–43.
Shipley WU, Seiferheld W, Lukka HR, Major PP, Heney NM, Grignon DJ, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376:417–28.
Tombal B, Borre M, Rathenborg P, Werbrouck P, Van Poppel H, Heidenreich A, et al. Long-term efficacy and safety of enzalutamide monotherapy in hormone-naïve prostate cancer: 1- and 2-year open-label follow-up results. Eur Urol. 2015;68:787–94.
Tombal B, Borre M, Rathenborg P, Werbrouck P, Van Poppel H, Heidenreich A, et al. Enzalutamide monotherapy in hormone-naive prostate cancer: primary analysis of an open-label, single-arm, phase 2 study. Lancet Oncol. 2014;15:592–600.
Freedland SJ, De Giorgi U, Gleave M, Rosbrook B, Shen Q, Sugg J, et al. A phase 3 randomised study of enzalutamide plus leuprolide and enzalutamide monotherapy in high-risk non-metastatic hormone-sensitive prostate cancer with rising PSA after local therapy: EMBARK study design. BMJ Open. 2021;11:e046588.
Eissa A, Elsherbiny A, Coelho RF, Rassweiler J, Davis JW, Porpiglia F, et al. The role of 68Ga-PSMA PET/CT scan in biochemical recurrence after primary treatment for prostate cancer: a systematic review of the literature. Minerva Urol Nefrol. 2018;70:462–78.
Acknowledgements
We thank Ahmed Shaheen, Data scientist at Alexandria University, Egypt for developing a search strategy and performing the literature search.
Funding
Open access funding provided by University of Bern.
Author information
Authors and Affiliations
Contributions
MS conceived the design of the study. MS and FA developed and submitted the protocol. Together with VA, FA, and LM jointly developed the search strategy and performed the literature search. MS, VA, and FA independently screened reports, assessed them for eligibility and selected reports for inclusion by consensus. MS jointly wrote the manuscript with VA while FA, SF, CZ, TZ, OM, DA, & RC critically reviewed it.
Corresponding author
Ethics declarations
Competing interests
MS received honoraria and travel costs (institutional) from Janssen and Debiopharm, along with research grants from Debiopharm. Additionally, MS served on advisory boards for Astellas. VA served as a speaker for Janssen and Accor Healthcare and provided consultancy for Astellas. TZ disclosed receiving honoraria and travel costs (institutional) from Janssen, Amgen, Ferring, Debiopharm, Bayer, Astellas, Telix, and MVison, research grants from Varian Medical Systems, and advisory board roles with Janssen, Accord, and Astellas. RC declared an advisory role (institutional) with Astellas, Bayer, Janssen, Sanofi, Debiopharm, BMS, MSD, Roche, Novartis, Pfizer, Merck, and Ipsen, along with honoraria (institutional) from Astellas, Janssen, and Novartis. All other authors reported no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shelan, M., Achard, V., Appiagyei, F. et al. Role of enzalutamide in primary and recurrent non-metastatic hormone sensitive prostate cancer: a systematic review of prospective clinical trials. Prostate Cancer Prostatic Dis (2024). https://doi.org/10.1038/s41391-024-00829-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-024-00829-9