Abstract
Background
Experiences of African/Afro-Caribbean men on active surveillance (AS) for prostate cancer (PCa) in the United Kingdom (UK) are not well documented. We compared follow-up appointments, adherence, and clinical outcomes among African/Afro-Caribbean men on AS at a high-volume UK hospital with other ethnicities.
Methods
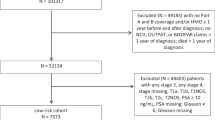
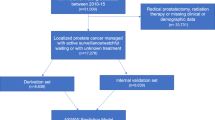
Men with confirmed low-intermediate risk Pca who attended the AS clinic (2005–2016) and had undergone ≥1 follow-up biopsy (n = 458) were included. Non-adherence (defined as >20% missed appointments), suspicion of disease progression (any upgrading, >30% positive cores, cT-stage > 3, PIRADS > 3), any upgrading from diagnostic biopsy and conversion to active treatment (prostatectomy, radiotherapy or hormone therapy) according to ethnicity (African/Afro-Caribbean versus other ethnicities) were assessed using multivariable regression analysis.
Results
Twenty-three percent of eligible men were recorded as African/Afro-Caribbean, while the remainder were predominantly Caucasian. African/Afro-Caribbean men had slightly lower PSA at diagnosis (median 5.0 vs. 6.0 ng/mL) and more positive cores at diagnosis (median 2 vs. 1). They had a substantially higher rate of non-attendance at scheduled follow-up visits (24% vs. 10%, p < 0.001). Adjusted analyses suggest African/Afro-Caribbean men may be at increased risk of disease progression (hazard ratio [HR]: 1.38; 95% confidence interval [CI] 0.99–1.91, P = 0.054) and upgrading (HR: 1.29; 95% CI 0.87–1.92, P = 0.305), though neither reached statistical significance. No difference in risk of conversion to treatment was observed between ethnic groups (HR: 1.03; 95% CI 0.64–1.47, P = 0.873).
Conclusions
African/Afro-Caribbean men on AS for PCa in the UK are less likely to adhere to scheduled appointments, suggesting a more tailored service addressing their specific needs may be required. While African/Afro-Caribbean men were no more likely to convert to treatment than Caucasian/other men, findings of a potentially higher risk of disease progression signal the need for careful selection and monitoring of African/Afro-Caribbean men on AS. Larger prospective, multicentre studies with longer follow-up are required to provide more definitive conclusions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Johns LE, Houlston RS. A systematic review and meta-analysis of familial prostate cancer risk. BJU Int. 2003;91:789–94.
U.S. Cancer Statistics Working Group. Prostate cancer rates by race and ethnicity 1999–2014. 2014. Available from: https://www.cdc.gov/cancer/prostate/statistics/race.htm.
Ben-Shlomo Y, Evans S, Ibrahim F, Patel B, Anson K, Chinegwundoh F, et al. The risk of prostate cancer amongst black men in the United Kingdom: the PROCESS cohort study. Eur Urol. 2008;53:99–105.
Chinegwundoh F, Enver M, Lee A, Nargund V, Oliver T, Ben-Shlomo Y. Risk and presenting features of prostate cancer amongst African-Caribbean, South Asian and European men in North-east London. BJU Int. 2006;98:1216–20.
Jack RH, Davies EA, Moller H. Prostate cancer incidence, stage at diagnosis, treatment and survival in ethnic groups in South–East England. BJU Int. 2010;105:1226–30.
Mahal BA, Berman RA, Taplin ME, Huang FW. Prostate cancer-specific mortality across Gleason scores in black vs nonblack men. JAMA. 2018;320:2479–81.
Karami S, Young HA, Henson DE. Earlier age at diagnosis: another dimension in cancer disparity? Cancer Detect Prev. 2007;31:29–34.
Metcalfe C, Evans S, Ibrahim F, Patel B, Anson K, Chinegwundoh F, et al. Pathways to diagnosis for Black men and White men found to have prostate cancer: the PROCESS cohort study. Br J Cancer. 2008;99:1040–5.
Kim HS, Moreira DM, Jayachandran J, Gerber L, Banez LL, Vollmer RT, et al. Prostate biopsies from black men express higher levels of aggressive disease biomarkers than prostate biopsies from white men. Prostate Cancer Prostatic Dis. 2011;14:262–5.
Powell IJ, Bock CH, Ruterbusch JJ, Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol. 2010;183:1792–6.
Evans S, Metcalfe C, Ibrahim F, Persad R, Ben-Shlomo Y. Investigating Black–White differences in prostate cancer prognosis: a systematic review and meta-analysis. Int J Cancer. 2008;123:430–5.
Evans S, Metcalfe C, Patel B, Ibrahim F, Anson K, Chinegwundoh F, et al. Clinical presentation and initial management of black men and white men with prostate cancer in the United Kingdom: the PROCESS cohort study. Br J Cancer. 2010;102:249–54.
National Institute for Health and Care Excellence. Prostate cancer: diagnosis and management, NICE Guideline [NG131]. 2019. Available from: https://www.nice.org.uk/guidance/ng131/chapter/Recommendations#assessment-and-diagnosis.
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22.
Dall’Era MA, Albertsen PC, Bangma C, Carroll PR, Carter HB, Cooperberg MR, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62:976–83.
Moses KA, Paciorek AT, Penson DF, Carroll PR, Master VA. Impact of ethnicity on primary treatment choice and mortality in men with prostate cancer: data from CaPSURE. J Clin Oncol. 2010;28:1069–74.
Lewisham PH. Lewisham’s joint strategic needs assessment: index of multiple deprivation. 2010. Available from: http://www.lewishamjsna.org.uk/health-inequities/index-of-multiple-deprivation.
Statistics DoCaLGOoN. The English indices of deprivation. 2015. Available from: http://www.gov.uk/government/statistics/english-indices-of-deprivation-2015.
Trust of London. Poverty and inequity data for Southwark. 2020. Available from: https://www.trustforlondon.org.uk/data/boroughs/southwark-poverty-and-inequaty-indicators/.
Du XL, Fang S, Coker AL, Sanderson M, Aragaki C, Cormier JN, et al. Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma: findings from a large community-based cohort. Cancer. 2006;106:1276–85.
Kheirandish P, Chinegwundoh F. Ethnic differences in prostate cancer. Br J Cancer. 2011;105:481–5.
Graham-Steed T, Uchio E, Wells CK, Aslan M, Ko J, Concato J. ‘Race’ and prostate cancer mortality in equal-access healthcare systems. Am J Med. 2013;126:1084–8.
Exarchakou A, Rachet B, Belot A, Maringe C, Coleman MP. Impact of national cancer policies on cancer survival trends and socioeconomic inequalities in England, 1996–2013: population based study. BMJ. 2018;360:k764.
Veugelers PJ, Yip AM. Socioeconomic disparities in health care use: does universal coverage reduce inequalities in health? J Epidemiol Community Health. 2003;57:424–8.
Fazil Q. Better health briefing 47: cancer and black and ethnic communities. A race equality foundation briefing paper. 2018. Available from: http://raceequalityfoundation.org.uk/wp-content/uploads/2018/07/REF-better-health-471-1.pdf.
Prostate Cancer UK. ‘Check it out’ prostate cancer support group for African and African Caribbean men. 2019. Available from: https://prostatecanceruk.org./get-support/find-local-support/check-it-out-prostate-cancer-support-group.
Casey RG, Powell L, Braithwaite M, Booth CM, Sizer B, Corr JG. Nurse-led phone call follow-up clinics are effective for patients with prostate cancer. J Patient Exp. 2017;4:114–20.
Anderson B. The benefits to nurse-led telephone follow-up for prostate cancer. Br J Nurs. 2010;19:1085–90.
Wasson J, Gaudette C, Whaley F, Sauvigne A, Baribeau P, Welch HG. Telephone care as a substitute for routine clinic follow-up. JAMA. 1992;267:1788–93.
Kinsella N, Beckmann K, Cahill D, Elhage O, Popert R, Cathcart P, et al. A single educational seminar increases confidence and decreases dropout from active surveillance by 5 years after diagnosis of prostate cancer. Eur. Urol Oncol. 2019;2:464–70.
Kinsella N, Stattin P, Cahill D, Brown C, Bill-Axelson A, Bratt O, et al. Factors influencing men’s choice of and adherence to active surveillance for low-risk prostate cancer: a mixed-method systematic review. Eur Urol. 2018;74:261–80.
Formica MK, Wason S, Seigne JD, Stewart TM. Impact of a decision aid on newly diagnosed prostate cancer patients’ understanding of the rationale for active surveillance. Patient Educ Couns. 2017;100:812–7.
Kinsella N, Helleman J, Bruinsma S, Carlsson S, Cahill D, Brown C, et al. Active surveillance for prostate cancer: a systematic review of contemporary worldwide practices. Transl Androl Urol. 2018;7:83–97.
Acknowledgements
The authors thank Milad Golsharifi, Hannah Yonis, and Anisah Khan for their assistance in collecting and collating the data used in this study.
Funding
KB was supported by an NHMRC Early Career Research Fellowship (GNT1124210).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kum, F., Beckmann, K., Aya, H. et al. Presentation, follow-up, and outcomes among African/Afro-Caribbean men on active surveillance for prostate cancer: experiences of a high-volume UK centre. Prostate Cancer Prostatic Dis 24, 549–557 (2021). https://doi.org/10.1038/s41391-020-00313-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-020-00313-0