Abstract
Background
Examine the real-world clinical impact of adopting less invasive surfactant administration (LISA) as the primary surfactant administration method in extremely preterm infants.
Methods
Single-center pre-post cohort study conducted over a 4-year period comparing outcomes of spontaneously breathing inborn infants 24+0–28+6 weeks gestational age (GA) receiving surfactant via endotracheal tube (pre-cohort, n = 154) or LISA via thin catheter (post-cohort, n = 70). Primary outcome was need for invasive mechanical ventilation (IMV, ≥2 h) ≤72 h of age. Secondary outcomes were a composite of mortality, bronchopulmonary dysplasia, intraventricular hemorrhage ≥grade 3 or necrotizing enterocolitis, and its individual components. Groups were compared using propensity score methods, including covariates: GA, birth weight, sex, small for GA, SNAP II ≥20, premature rupture of membranes, maternal hypertension/diabetes, and C-section.
Results
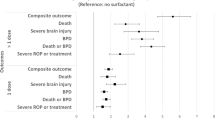
GA and birth weight were 27.1 (26, 28.1) weeks and 914 (230) g, and 27.1 (26.1, 28.1) weeks and 920 (236) g for pre- and post-cohorts, respectively. Pre-cohort had higher C-section rates, (67% vs. 51%, p = 0.03). After adjustment for covariates, LISA was associated with reduced IMV exposure [AOR (95% CI) 0.07 (0.04, 0.11)], lower odds of the composite clinical outcome [0.49 (0.33, 0.73)], and most of its individual components.
Conclusion
Real-world experience favors LISA as the primary method in extremely preterm infants with established spontaneous respiration.
Impact
-
Less invasive surfactant administration (LISA) is associated with a reduction in respiratory morbidity, but real-world data of routine use among extremely preterm infants are limited.
-
LISA is associated with reduced frequency of exposure to and duration of IMV in both ≤72 h after birth and during hospital stay.
-
LISA is associated with a reduction in mortality, and most other major morbidities including bronchopulmonary dysplasia, and interventricular hemorrhage.
-
Data from a large North American center providing real-world clinical outcomes following LISA as the primary method of surfactant administration.
Similar content being viewed by others
Background
Surfactant deficiency leading to respiratory distress syndrome (RDS) is an important cause of morbidity and mortality in preterm infants.1 Administration of exogenous surfactant for RDS is a lifesaving intervention that until recently required insertion of an endotracheal tube (ETT), often resulting in exposure to invasive mechanical ventilation (IMV, commonly defined as invasive positive pressure ventilation ≥2 h).2 Exposure to IMV is a well-known risk factor for bronchopulmonary dysplasia (BPD) among preterm infants, in particular for those born <29 weeks gestational age (GA).3,4 Less invasive surfactant administration (LISA) is a recently established method of surfactant administration, whereby a small bore soft catheter is placed in the trachea and surfactant is instilled while the infant remains spontaneously breathing on non-invasive respiratory support.5 Several randomized control trials and subsequent meta-analyses have confirmed LISA to be feasible, safe, and associated with lower mortality, BPD, and severe intraventricular hemorrhage (IVH) among preterm infants when compared to other traditional methods of surfactant administration such as INSURE (INtubate, SURfactant and Extubate) or IMV.6,7,8,9,10,11,12,13,14,15
While LISA is increasingly being adopted by neonatal intensive care units (NICUs), data regarding its clinical impact in the real-world setting are just beginning to emerge, and need further systematic evaluation. This is relevant as patient populations participating in trials are not always fully reflective of real-world clinical practice. In neonatology, the most immature and sickest patients who are also at the highest risk of complications are often under-represented in clinicals trials. Furthermore, clinical adoption of a new procedural technique is not without its challenges, for example, the need for staff training, success/failure rates of a new procedure, and uptake of the new method by clinical staff. Recently a number of studies have emerged highlighting LISA implementation strategies, using a range of materials such as direct or video laryngoscopy, and intratracheal placement of a thin catheter maneuvered using Magill forceps or rigid angiocatheter or specifically designed LISAcath (Chiesi, France).16,17,18 While these pre-post cohort studies suggest improved outcomes among infants who received surfactant via LISA, the majority were limited by possible selection bias on account of a fraction of the eligible cohort receiving LISA being included during the post-period, underreporting of LISA failure resulting in the use of alternative methods, and studying relatively more mature infants. In October 2019, the tertiary NICU at Mount Sinai Hospital, Toronto, Canada, adopted LISA using C-MAC video laryngoscopy (Karl Storz, Germany) and multi-access catheter (MAC, Avanos Medical Inc., Alpharetta, GA) as the primary method of surfactant administration for infants born >24+0 GA. This study was designed to examine the real-world impact of this practice change specifically on the clinical outcomes of extremely preterm infants, i.e., those between 24+0 and 28+6 weeks GA, born at our center. Our primary objective was to evaluate the utilization of LISA as the primary method of surfactant administration and its impact on exposure to IMV among extreme preterm infants. Our secondary objective was to evaluate its impact on mortality and common neonatal morbidities.
Methods
Study design, site, and patient population
This was a single-center retrospective pre-post cohort study conducted at the tertiary NICU Mount Sinai Hospital in Canada over a 4-year period: pre-cohort (January 2017 to September 2019), and post-cohort (October 2019 to October 2020). Inclusion criteria were inborn infants between 24+0 and 28+6 weeks GA at birth who did not require endotracheal intubation as a part of resuscitation, and required surfactant replacement therapy for RDS. Exclusion criteria were infants transferred to another tertiary facility prior to 72 h of age, and infants with major genetic disorders or congenital anomalies. Potentially eligible patients were identified from our unit’s local electronic database that is maintained prospectively, and the list was then cross-checked using a prospectively collected list of all infants receiving surfactant maintained separately by the Respiratory Therapy department. The heath records of all identified infants were then reviewed to confirm eligibility for inclusion. The study was approved by the institutional Research Ethics Board and need for informed consent was waived.
Data collection
For all included patients, in addition to demographic details, initial stabilization notes were reviewed in detail to collect information on the method of surfactant administration, attempt of LISA and its success or failure, and use of direct versus video laryngoscopy for LISA by operators. Furthermore, for outcomes, we collected details on mortality and common prematurity-related morbidities experienced by included infants. These included, significant brain injury, defined as IVH ≥grade 3 as per Papile’s classification19 or periventricular leukomalacia (PVL) noted on the final head ultrasound scan performed during NICU stay, BPD defined as need for supplemental oxygen or any respiratory support at 36 weeks post menstrual age,20 necrotizing enterocolitis (NEC) ≥stage 2a as per modified Bell criteria,21,22 and any exposure to patent ductus arteriosus treatments. Specific ventilation outcomes collected for this study included any exposure of IMV during first 3 days and overall NICU stay, number of surfactant doses received, and hours of IMV during the first 72 h.
Study groups and outcomes
For comparison, the overall study cohort was divided into two groups: “pre-cohort” when surfactant via ETT was the standard of care, and “post-cohort” when LISA was available and was the standard of care for primary surfactant administration. The primary outcome was the frequency of exposure to IMV during the first 72 h after birth, defined as IMV ≥2 h duration. Secondary outcomes included duration of IMV in hours during the first 72 h after birth; frequency of exposure to IMV at any time during NICU stay; a composite of mortality and major relevant morbidities (IVH ≥grade 3 or PVL, BPD or NEC ≥stage 2a) and the individual components; and receipt of treatment for patent ductus arteriosus. Additional outcomes that were evaluated in survivors only included total duration of IMV in days, days on oxygen therapy, and length of NICU stay.
Study setting
The NICU at Mount Sinai Hospital is a tertiary teaching hospital, admitting primarily inborn patients ≥22 weeks GA. Expectant preterm delivery management includes antenatal corticosteroid administration, magnesium sulfate for neuroprotection, intrapartum antibiotics when indicated, and delayed cord clamping for up to 60 s. Immediately following delivery, spontaneously breathing extremely preterm infants are initiated onto nasal continuous positive airway pressure (CPAP), with a fraction of inspired oxygen (FiO2) set to 0.30 and titrated to maintain peripheral oxygen saturation between 91 and 95%. As per Neonatal Resuscitation Program, cardiopulmonary insufficiency including apnea or heart rate below 100 beats per min is managed using positive pressure ventilation; intubation is considered when positive pressure ventilation is deemed ineffective or if apnea persists. After initial stabilization, criteria for surfactant replacement for first and subsequent doses throughout the study period was an FiO2 > 0.30 and mean airway pressure ≥7 cmH2O. In the pre-cohort period, surfactant was administered via ETT in one bolus as tolerated, together with positive pressure ventilation using the Neopuff T-piece resuscitator (Fisher & Paykel Healthcare, New Zealand), followed by ETT removal within 2 h (INSURE method), as deemed appropriate by the attending team. In October 2019, the LISA method was implemented in our NICU as the primary method of surfactant administration in spontaneously breathing preterm infants. Prior to its implementation a comprehensive protocol was developed, multidisciplinary education sessions were conducted, and staff procedural training using high-fidelity airway simulation was completed. The LISA procedure itself involved comfort measures (swaddling and sucrose administration), vocal cord visualization using C-MAC video laryngoscopy, insertion of a MAC guided with Magill forceps, surfactant instilled in small boluses coordinating with the infant’s inspiratory effort, and gastric tube aspiration post surfactant instillation to rule out MAC displacement. The entire procedure was performed with infants receiving nasal CPAP via mask or prong Flexi-trunk interfaces (Fisher & Paykel Healthcare, New Zealand). In our protocol, staff were encouraged to keep the non-invasive interface applied during the procedure, except when it interfered with vocal cord visualization. The use of video laryngoscopy was encouraged over direct laryngoscopy. Failure of LISA was considered when the patient condition or FiO2 remained unchanged or worsened, at which point intubation for surfactant administration was indicated. For patients who demonstrated clinical improvement after the first dose of surfactant using the LISA method but required further doses, the method of administration was left to the discretion of the attending team, either LISA or via ETT.
Statistical analysis
The study population was summarized descriptively. Categorical variables were presented as count (percentage), and continuous variables were presented as mean (standard deviation) or when the variables were non-normally distributed as median (interquartile range), as appropriate. In the post-cohort, those who were eligible and LISA was attempted were included for analysis independent of the success of the procedure. Patients who may have been eligible but were not given LISA were excluded from primary analysis. A secondary sensitivity analysis was also conducted by including all identified patients during the post-cohort period, even if they were not given LISA despite being eligible. Baseline characteristics and outcomes were compared between groups using χ2 test or Fisher’s exact test for categorical variables, and Student t-test or Mann–Whitney U test for continuous variables as appropriate. To examine the association between LISA and clinical outcomes, weighted logistic and quantile regressions were conducted where the weights were the inverse probability of treatment as estimated by the propensity score. The propensity score was estimated using a logistic regression model with the following covariates: GA, birth weight, sex, small for GA, Score for Neonatal Acute Physiology version II (SNAP II) ≥20, premature rupture of membranes, maternal hypertension/diabetes, and C-section. A two-tailed p value <0.05 was considered to be statistically significant. Data management and all statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
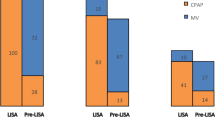
A total of 224 infants were identified as eligible for inclusion during the study (pre-cohort, n = 154; post-cohort, n = 70) (Fig. 1); 6 infants in the post-cohort were excluded from the primary analysis as LISA was not utilized despite eligibility. The median GA and birth weight of the cohort was 27.1 (26.1, 28.1) weeks and 890 (743, 1060) g, respectively. Video laryngoscopy was utilized by service providers in 63/70 (90%) of LISA procedures, direct laryngoscopy for 1/70 (1.4%), and in 6/70 (8.6%) the specific device type was not documented. On intergroup comparison both groups had similar baseline demographics, except for a higher C-section rate in the pre-cohort (Table 1). In comparison to the pre-cohort, the post-cohort had reduced frequency of exposure to and duration of IMV in both ≤72 h after birth and during the entirety of NICU stay. In addition, the post-cohort experienced a lower rate of the composite clinical outcome of mortality, IVH ≥grade 3 or PVL, BPD or NEC, as well as composite of mortality or BPD (Table 2). The regression analysis using propensity score methods demonstrated that utilization of LISA was associated with lower odds of the primary outcome, as well as the majority of studied secondary clinical outcomes (Table 3). Sensitivity analysis was conducted including the previously excluded 6 infants who were intubated for surfactant replacement despite LISA eligibility in the post-cohort. The results remained unchanged apart from differences in mortality and patent ductus arteriosus treatment that were no longer statistically significant (Table 4).
The flowchart depicts the total number of neonates identified as having received surfactant treatment, less those who were excluded due to missing data, outborn status, transferred prior to 72 hours of age, and requiring intubation during resuscitation, for the final number of neonates included in the study.
Discussion
While several controlled studies and meta-analyses comparing LISA to traditional methods of surfactant administration have established its association with a reduction in respiratory morbidity and mortality, real-world data of its routine use are still limited, particularly for extremely preterm infants. In this single-center retrospective pre-post cohort study including preterm infants born at GA 24+0 and 28+6 weeks who otherwise did not require intubation as part of initial resuscitation, we identified a significant reduction in exposure to IMV, overall mortality, as well as the majority of prematurity-related in-hospital morbidities in association with LISA utilization as the primary method for surfactant administration.
In the last few years, two large meta-analyses have reported compiled data from several randomized control trials and observational cohort studies to establish LISA methods as safe and effective compared to other traditional methods of surfactant administration, even in the most immature infants. The first was a systematic review and meta-analysis comparing several common ventilation strategies for preterm infants <33 weeks GA. Among the most salient findings, compared to IMV, LISA was associated with the lowest likelihood of the composite outcome BPD or mortality at 36 weeks post menstrual age, BPD alone, and severe IVH.15 In a subgroup analysis including only infants ≤28 weeks GA, compared to IMV, LISA-treated infants demonstrated lower odds of the composite outcome of BPD or mortality [OR 0.55 (95% CI: 0.31–0.94)], while other analyzed outcomes were not different. Neither primary nor subgroup analyses comparing LISA to INSURE demonstrated differences in clinical outcomes. However, more recently, in a network meta-analysis comparing different methods of surfactant administration among preterm infants <37 weeks GA, compared to INSURE, surfactant administered via thin catheter was associated with significantly lower rates of mortality, frequency of IMV exposure, BPD, PVL, and NEC.23 Analysis to examine clinical outcomes (mortality, NEC, and patent ductus arteriosus) of infants <28 weeks GA demonstrated lower odds of mortality [OR 0.56 (95% CI: 0.46–0.67)] in favor of thin catheter surfactant administration compared to INSURE. This recent evidence showing overall benefit of LISA methods has substantiated its addition to RDS management guidelines,24 and prompted increased implementation in favor of traditional methods of surfactant administration in NICUs worldwide.16,17,18,25,26
With the rise in adoption and incorporation of LISA as part of routine care for preterm infants, the interest in evaluating its real-world impact on important prematurity-related clinical outcomes has grown. This is important as findings from research settings may not always translate exactly to clinical practice. On the contrary, the real-life impact may be greater owing to the well-known under-representation of the most vulnerable infants in clinical trials. Most of the literature describing the clinical impact of LISA has been published from European units, where LISA appears to have been incorporated earlier than the rest of the world.7,10,14,24,26 An epidemiological network-level evaluation of surfactant administration practices from Germany, including infants <29 weeks GA over a 7-year period, demonstrated an increase in the use of LISA from 28.7% in 2009 to 50.1% in 2016.12 In comparison to surfactant administration via ETT, after adjusting for the variables collected in the database, LISA was associated with lower odds of mortality, BPD, severe IVH or PVL, and ROP. However, amongst infants <26 weeks GA, it was reported to be associated with higher risk of focal intestinal perforation requiring surgical intervention [OR 1.49 (95% CI: 1.14–1.95)]. Over the last few years, several unit-level reports have also been published.27,28,29,30,31,32 While these studies support the trial findings of superiority of LISA vs. ETT for surfactant administration in preterm infants, most evaluated outcomes of relatively more mature infants, and their conclusion were significantly limited by the possibility of selection bias. Many apparently eligible preterm infants born during the LISA period in these studies either were excluded on account of not receiving the intended treatment or their numbers were not included in the results. The reasons for underutilization of LISA, such as care giver’s discretion or whether it was attempted without success (failure rate), or comparisons between those who received LISA vs. not to confirm the representative nature of the included cohort, were not provided.
One of the largest such pre-post cohort studies, which included all patients and had population like ours, was published by Klebermass-Schrehof et al. from a unit in Vienna.10 The authors compared the frequency of various morbidities among 224 infants <28 weeks GA managed using a standardized less invasive respiratory management protocol between 2009 and 2011 with 182 historical controls between 2005 and 2007, and data for the same time period from the Vermont-Oxford Neonatal Network. The post-cohort demonstrated a lower frequency of receipt of IMV (23% on day 3, 59% at any time during NICU stay) as well as lower rates of mortality, IVH, PVL, and death/BPD, but higher rates of PDA treatment and ROP. These results, however, were limited by the lack of regression analysis as there were several important and potentially confounding differences in baseline characteristics between groups (higher rate of preterm premature rupture of membranes, lower rate of antenatal steroids, and C-section in the control group). Furthermore, the management protocol included a combination of interventions such as use of “high flow CPAP” (20 l/min) via nasopharyngeal tube, pre-surfactant administration of caffeine citrate and LISA via intratracheal placement of a gastric tube, making it difficult to isolate the impact of LISA. On the contrary, we included all eligible infants in our analysis minimizing the potential of selection bias, and the studied periods immediately before and after LISA implementation, reducing the effect of time on our results. Furthermore, no other clinical practice change was implemented for stabilization after birth of preterm infants; during both periods spontaneously breathing infants received mask CPAP with a MAP ≤7 cmH2O. Finally, we incorporated a robust regression analysis using a propensity score method to account for the potential confounding effect of important observed baseline characteristics.
Limitations
Our study results should be interpreted in the context of some key limitations. Although we included all potentially eligible infants born during both the study periods, due to the retrospective design we cannot rule out some degree of misclassification as few spontaneously breathing preterm infants may have been intubated electively during early resuscitation at the clinician’s discretion. We did identify six infants who despite LISA eligibility were intubated for surfactant administration and conducted a sensitivity analysis by including these, which did not affect the majority of study findings. Another important limitation of our study design is the possibility of aggregate bias. While there was no other formal clinical change implemented during the LISA period related to respiratory care of preterm infants immediately after birth, the potential interaction between staff education pertaining to lung protection, standardization of practice, and clinical outcomes evaluated may not be accounted for. And while using the study periods immediately before and after the practice change minimizes confounding by longitudinal secular trends, we are not able to comment on whether the improvement in clinical outcomes observed with the practice change to LISA may be sustained over a longer period of time. Future studies should evaluate the impact of LISA utilization on long term post-discharge outcomes, and its clinical effectiveness in the care of peri viable infants born at 22 and 23 weeks GA, as to date these data are very limited. Lastly, while the majority of LISA procedures were performed with the use of video laryngoscopy, we cannot remark on whether its use compared to direct laryngoscopy provided any additional benefit. However, this would be an interesting research question that could be examined in a future study.
Conclusion
This systematic and comprehensive evaluation of data from a large North American perinatal center provides support in favor of LISA compared to ETT as the primary method of surfactant administration in spontaneously breathing extreme preterm infants. These data may substantiate LISA as the standard of care for surfactant administration whenever feasible for infants born between 24+0 and 28+6 weeks GA.
Data availability
The dataset generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rojas-Reyes, M. X., Morley, C. J. & Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. CD000510 (2012).
Owen, L. S., Manley, B. J., Davis, P. G. & Doyle, L. W. The evolution of modern respiratory care for preterm infants. Lancet 389, 1649–1659 (2017).
Kalikkot Thekkeveedu, R., Guaman, M. C. & Shivanna, B. Bronchopulmonary dysplasia: a review of pathogenesis and pathophysiology. Respir. Med. 132, 170–177 (2017).
Stevens, T. P., Harrington, E. W., Blennow, M. & Soll, R. F. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst. Rev. CD003063 (2007).
Herting, E., Hartel, C. & Gopel, W. Less invasive surfactant administration (LISA): chances and limitations. Arch. Dis. Child. Fetal Neonatal Ed. 104, F655–F659 (2019).
Dargaville, P. A. et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch. Dis. Child. Fetal Neonatal Ed. 98, F122–F126 (2013).
Kribs, A. et al. Surfactant without intubation in preterm infants with respiratory distress: first multi-center data. Klinische Padiatrie. 222, 13–17 (2010).
Gopel, W. et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet 378, 1627–1634 (2011).
Kanmaz, H. G., Erdeve, O., Canpolat, F. E., Mutlu, B. & Dilmen, U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics 131, e502–e509 (2013).
Klebermass-Schrehof, K. et al. Less invasive surfactant administration in extremely preterm infants: impact on mortality and morbidity. Neonatology 103, 252–258 (2013).
Kribs, A. et al. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 169, 723–730 (2015).
Hartel, C. et al. Less invasive surfactant administration and complications of preterm birth. Sci. Rep. 8, 8333 (2018).
Aldana-Aguirre, J. C., Pinto, M., Featherstone, R. M. & Kumar, M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 102, F17–F23 (2017).
Gopel, W. et al. Less invasive surfactant administration is associated with improved pulmonary outcomes in spontaneously breathing preterm infants. Acta Paediatr. 104, 241–246 (2015).
Isayama, T., Iwami, H., McDonald, S. & Beyene, J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA 316, 611–624 (2016).
Williamson, S. L., McDermott, H. & Gowda H. Implementing less invasive surfactant administration on a neonatal unit. Arch. Dis. Child Educ. Pract. Ed. edpract-2020-320574 (2021).
Roberts, C. T. et al. Outcomes after introduction of minimally invasive surfactant therapy in two Australian tertiary neonatal units. J. Pediatr. 229, 141–146 (2021).
Conlon, S. M. et al. Introducing less-invasive surfactant administration into a level IV NICU: a quality improvement initiative. Children 8, 580 (2021).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–534 (1978).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. care Med. 163, 1723–1729 (2001).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7 (1978).
Walsh, M. C. & Kliegman, R. M. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr. Clin. North Am. 33, 179–201 (1986).
Bellos, I., Fitrou, G., Panza, R. & Pandita, A. Comparative efficacy of methods for surfactant administration: a network meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 106, 474–487 (2021).
Sweet, D. G. et al. European consensus guidelines on the management of respiratory distress syndrome – 2019 update. Neonatology 115, 432–450 (2019).
Szczapa, T., Hozejowski, R., Krajewski, P. & Study, G. Implementation of less invasive surfactant administration in clinical practice-Experience of a mid-sized country. PLoS One 15, e0235363 (2020).
Berneau, P., Nguyen Phuc Thu, T., Pladys, P. & Beuchee, A. Impact of surfactant administration through a thin catheter in the delivery room: a quality control chart analysis coupled with a propensity score matched cohort study in preterm infants. PLoS One 13, e0208252 (2018).
Elbaz, Y., Portnov, I., Lurie-Marcu, B. & Shinwell, E. S. Minimally invasive surfactant therapy versus intubation for surfactant delivery in preterm infant with RDS: evaluation of safety and efficacy. J. Matern. Fetal Neonatal Med. 1–5 (2021).
Shetty, S. et al. Less invasive surfactant administration in very prematurely born infants. AJP Rep. 11, e119–e122 (2021).
Perez-Iranzo, A., Jarque, A., Toledo, J. D. & Tosca, R. Less invasive surfactant administration reduces incidence of severe intraventricular haemorrage in preterms with respiratory distress syndrome: a cohort study. J. Perinatol. 40, 1185–1192 (2020).
Bugter, I. A. L. et al. Introduction of less invasive surfactant administration (LISA), impact on diagnostic and therapeutic procedures in early life: a historical cohort study. BMC Pediatr. 20, 421 (2020).
Ramos-Navarro, C., Sanchez-Luna, M., Zeballos-Sarrato, S. & Gonzalez-Pacheco, N. Three-year perinatal outcomes of less invasive beractant administration in preterm infants with respiratory distress syndrome. J. Matern. Fetal Neonatal Med. 33, 2704–2710 (2020).
Dargaville, P. A., Ali, S. K. M., Jackson, H. D., Williams, C. & De Paoli, A. G. Impact of minimally invasive surfactant therapy in preterm infants at 29-32 weeks gestation. Neonatology 113, 7–14 (2018).
Author information
Authors and Affiliations
Contributions
A.J. conceived the research question, conceptualized the initial study design and data collection forms, and revised the final draft of the manuscript. M.B. participated in the initial study design and data collection forms, collected in-hospital data for the study cohort, and produced the first draft of the manuscript. V.D. reviewed the study protocol, collected in-hospital data for the study cohort, reviewed the manuscript, and provided critical feedback. L.H. reviewed the study protocol, collected in-hospital data for the study cohort, reviewed the manuscript, and provided critical feedback. B.L. reviewed the study protocol, collected in-hospital data for the study cohort, reviewed the manuscript, and provided critical feedback. X.Y.Y. performed all statistical analysis for the study, reviewed the manuscript, and provided critical feedback. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baczynski, M., Deekonda, V., Hamilton, L. et al. Clinical impact of less invasive surfactant administration using video laryngoscopy in extremely preterm infants. Pediatr Res 93, 990–995 (2023). https://doi.org/10.1038/s41390-022-02197-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02197-3