Abstract
Background
We assessed variability of analgesic use across three tertiary neonatal intensive care units (NICUs) accounting for early-life pain, quantified as number of invasive procedures. We also determined whether analgesia exposure modifies associations between early-life pain and neurodevelopment.
Methods
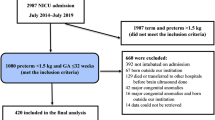
Multicenter prospective study of 276 very preterm infants (born <24–32 weeks’ gestational age [GA]). Detailed data of number of invasive procedures and duration of analgesia exposure were collected in initial weeks after birth. Eighteen-month neurodevelopmental assessments were completed in 215 children with Bayley Scales for Infant Development—Third edition.
Results
Multivariable linear regressions revealed significant differences in morphine use across sites, for a given exposure to early-life pain (interaction p < 0.001). Associations between early-life pain and motor scores differed by duration of morphine exposure (interaction p = 0.01); greater early-life pain was associated with poorer motor scores in infants with no or long (>7 days) exposure, but not short exposure (≤7 days).
Conclusions
Striking cross-site differences in morphine exposure in very preterm infants are observed even when accounting for early-life pain. Negative associations between greater early-life pain and adverse motor outcomes were attenuated in infants with short morphine exposure. These findings emphasize the need for further studies of optimal analgesic approaches in preterm infants.
Impact
-
In very preterm neonates, both early-life exposure to pain and analgesia are associated with adverse neurodevelopment and altered brain maturation, with no clear guidelines for neonatal pain management in this population.
-
We found significant cross-site variability in morphine use across three tertiary neonatal intensive care units in Canada.
-
Morphine use modified associations between early-life pain and motor outcomes. In infants with no or long durations of morphine exposure, greater early-life pain was associated with lower motor scores, this relationship was attenuated in those with short morphine exposure.
-
Further trials of optimal treatment approaches with morphine in preterm infants are warranted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to conditions of patient consent and research ethics board approvals but are available from the corresponding author on reasonable request and appropriate patient consent and data transfer agreements.
References
Carbajal, R. et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 300, 60 (2008).
Grunau, R. E. et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain 143, 138–146 (2009).
Vinall, J. et al. Invasive procedures in preterm children: brain and cognitive development at school age. Pediatrics 133, 412–421 (2014).
Smith, G. C. et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann. Neurol. 70, 541–549 (2011).
Ranger, M. et al. Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLoS ONE 8, e76702 (2013).
Ranger, M. & Grunau, R. E. Early repetitive pain in preterm infants in relation to the developing brain. Pain. Manag. 4, 57–67 (2014).
Brummelte, S. et al. Procedural pain and brain development in premature newborns. Ann. Neurol. 71, 385–396 (2012).
Duerden, E. G. et al. Early procedural pain is associated with regionally-specific alterations in thalamic development in preterm neonates. J. Neurosci. 38, 878–886 (2018).
Duerden, E. G. et al. Association of early skin breaks and neonatal thalamic maturation: a modifiable risk? Neurology 95, e3420–e3427 (2020).
Puia-Dumitrescu, M. et al. Assessment of 2-year neurodevelopmental outcomes in extremely preterm infants receiving opioids and benzodiazepines. JAMA Netw. Open 4, e2115998 (2021).
Borenstein-Levin, L. et al. Narcotics and sedative use in preterm neonates. J. Pediatr. 180, 92.e1–98.e1 (2017).
Carbajal, R. et al. Sedation and analgesia practices in neonatal intensive care units (Europain): results from a prospective cohort study. Lancet Respir. Med. 3, 796–812 (2015).
Lago, P. et al. Sedation and analgesia practices at italian neonatal intensive care units: results from the Europain Study. Ital. J. Pediatr. 43, 26 (2017).
Duerden, E. G. et al. Midazolam dose correlates with abnormal hippocampal growth and neurodevelopmental outcome in preterm infants. Ann. Neurol. 79, 548–559 (2016).
Zwicker, J. G. et al. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. J. Pediatr. 172, 81.e2–87.e2 (2016).
McPherson, C. et al. Brain injury and development in preterm infants exposed to fentanyl. Ann. Pharmacother. 49, 1291–1297 (2015).
Mcpherson, C., Miller, S. P., El-Dib, M., Massaro, A. N. & Inder, T. E. The influence of pain, agitation, and their management on the immature brain. Pediatr. Res. 88, 168–175 (2020).
McPherson, C. & Grunau, R. E. Pharmacologic analgesia and sedation in neonates. Clin. Perinatol. 49, 243–265 (2022).
Chau, C. M. Y. et al. Morphine biotransformation genes and neonatal clinical factors predicted behaviour problems in very preterm children at 18 months. EBioMedicine 40, 655–662 (2019).
Nunes, A. S. et al. Atypical neuromagnetic resting activity associated with thalamic volume and cognitive outcome in very preterm children. Neuroimage Clin. 27, 102275 (2020).
Papile, L.-A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500gm. J. Pediatr. 92, 529–534 (1978).
Guo, T. et al. Quantitative assessment of white matter injury in preterm neonates. Neurology 88, 614–622 (2017).
Benavente-Fernandez, I. et al. Association of socioeconomic status and brain injury with neurodevelopmental outcomes of very preterm children. JAMA Netw. Open 2, e192914 (2019).
Cayam-Rand, D. et al. Predicting developmental outcomes in preterm infants: a simple white matter injury imaging rule. Neurology 93, e1231–e1240 (2019).
Keels, E. et al. Prevention and management of procedural pain in the neonate: an update. Pediatrics 137, e20154271 (2016).
Ng, E., Taddio, A. & Ohlsson, A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst. Rev. 1, CD002052 (2017).
Steinbauer, P. et al. Long-term impact of systematic pain and sedation management on cognitive, motor, and behavioral outcomes of extremely preterm infants at preschool age. Pediatr. Res. 89, 540–548 (2021).
Giordano, V. et al. Effect of increased opiate exposure on three years neurodevelopmental outcome in extremely preterm infants. Early Hum. Dev. 123, 1–5 (2018).
Steinhorn, R. et al. Neonatal morphine exposure in very preterm infants—cerebral development and outcomes. J. Pediatr. 166, 1200.e4–1207.e4 (2015).
Bellu, R. et al. Opioids for newborn infants receiving mechanical ventilation. Cochrane Database Syst. Rev. 3, CD013732 (2021).
Lammers, E. M. et al. Association of fentanyl with neurodevelopmental outcomes in very-low-birth-weight infants. Ann. Pharmacother. 48, 335–342 (2014).
Simons, S. H. P. et al. Routine morphine infusion in preterm newborns who received ventilatory support. JAMA 290, 2419 (2003).
de Graaf, J. et al. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children’s functioning: five-year follow-up of a randomized controlled trial. Pain 152, 1391–1397 (2011).
de Graaf, J. et al. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age? Pain 154, 449–458 (2013).
Nandi, R. & Fitzgerald, M. Opioid analgesia in the newborn. Eur. J. Pain 9, 105–108 (2005).
Fitzgerald, M. & Walker, S. M. Infant pain management: a developmental neurobiological approach. Nat. Clin. Pract. Neurol. 5, 35–50 (2009).
Durrmeyer, X., Vutskits, L., Anand, K. J. S. & Rimensberger, P. C. Use of analgesic and sedative drugs in the NICU: integrating clinical trials and laboratory data. Pediatr. Res. 67, 117–127 (2010).
Ranger, M. et al. Adverse behavioral changes in adult mice following neonatal repeated exposure to pain and sucrose. Front. Psychol. 9, 2394 (2018).
Tremblay, S. et al. Repeated exposure to sucrose for procedural pain in mouse pups leads to long-term widespread brain alterations. Pain 158, 1586–1598 (2017).
Acknowledgements
We would like to thank the child and families who participated in this longitudinal study. We also thank Giselle Da Rocha, Janet Rigney, Mark LePine, Steven Ufkes, Mary Beckingham, Poala Zen, and the staff in the Neonatal Follow-up Programs at BC Women’s & Children’s Hospital, the Hospital for Sick Children, and Mount Sinai Hospital for their assistance with this study.
Funding
The study was supported by the Canadian Institutes of Health Research (CIHR MOP-136966 and PJT-168894), CP Alliance (PG-016817), and Brain Canada (The Canadian Neonatal Brain Platform). S.P.M. received support from the Bloorview Children’s Hospital Chair in Paediatric Neuroscience, and now from the Hudson Family Hospital Chair in Pediatric Medicine and the James & Annabel McCreary Chair in Pediatrics. R.E.G. holds a senior scientist salary award from the BC Children’s Hospital Research Institute. T.S. is supported by CIHR Canada Graduate Scholarship – Master’s and Doctoral Awards, Ontario Ministry of Health & University of Toronto Clinician Investigator Program, and the SickKids Research Institute Clinician Scientist Training Program. M.A.M. is supported by a CIHR Postdoctoral Research Fellowship and Michael Smith for Health Research Foundation Trainee Award.
Author information
Authors and Affiliations
Contributions
T.S., R.E.G. and S.P.M. made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critically reviewing the manuscript for important intellectual content. C.M.Y.C., A.R.S., L.G.L., and E.K. contributed to the conception and design, acquisition of data, and critically reviewing the manuscript for important intellectual content. P.Z. contributed to acquisition of data, analysis and interpretation of data, drafting of the manuscript and critically reviewing the manuscript for important intellectual content. M.A.M., S.H.A.-Y., and C.M.Y.C. contributed to acquisition of data and critically reviewing the manuscript for important intellectual content. All authors approved the final version of the manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written informed consent was obtained from a parent/guardian of each participant in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Selvanathan, T., Zaki, P., McLean, M.A. et al. Early-life exposure to analgesia and 18-month neurodevelopmental outcomes in very preterm infants. Pediatr Res 94, 738–746 (2023). https://doi.org/10.1038/s41390-023-02536-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02536-y