Abstract
Relieving neonatal pain is essential for the management of premature infants. Morphine is the most frequently used analgesic in neonatal intensive care. Here we report the relationship between early morphine infusion and the composite outcome of intraventricular hemorrhage and/or death in intubated premature infants. Infants (gestational age ≤ 32 weeks and birth weight < 1,500 g) intubated on admission were retrospectively evaluated in a large tertiary neonatal intensive care unit. Modified log-Poisson regression with robust variance estimator and Cox regression was applied to adjust the relative risk for infants’ outcomes. Of 420 premature infants, 230 (54.7%) received continuous morphine infusion in the first 72 h. Of these, 153 were < 28 gestational weeks; of the 190 patients who did not receive morphine, 63 were < 28 gestational weeks. The analysis revealed that infants < 28 gestational weeks who received morphine were significantly associated with an increased risk for IVH and/or death [adjusted relative risk (aRR) 1.37, 95% confidence interval (CI) 1.1–1.71)], and mortality (aRR 1.83, 95% CI 1.17–2.89). Moreover, in infants < 28 gestational weeks, survival was low in those infants who were exposed to morphine infusion in the first 72 h (hazard ratio 2.11; 95% CI 1.19–3.73). Early morphine infusion is associated with an increased risk for IVH and/or death; however, further studies are required to verify our findings.
Similar content being viewed by others
Introduction
Once premature infants are born and admitted in the neonatal intensive care unit (NICU), they are subjected to several invasive procedures, such as intubation, ventilation, blood extraction, and umbilical or central line insertion1,2. It has been proven that these procedures are painful and stressful and are associated with an increase in stress hormone levels3.
Neonatal pain can impact the clinical picture of patients. It alters the heart rate, blood pressure, and oxygen saturation, and consequently, it can cause intraventricular hemorrhage (IVH) in premature infants4,5. Increasing research has demonstrated that neonatal pain plays a vital role in the short- and long-term neurodevelopmental outcomes of premature infants6,7,8,9.
Therefore, theoretically, relieving pain in the early period of life will reduce the complications associated with neonatal pain and improve the potential neurodevelopmental outcome. Neonatologists have attempted to minimize the severity of pain, particularly in premature patients who are connected to mechanical ventilation, by administering sedative and analgesic medications once they are admitted. Morphine is the most commonly used analgesic medication in the NICU10,11.
Studies have reported that administration of morphine infusion can decrease the secretion of catecholamines and reduce the pain score12,13,14. Moreover, it can improve the synchronicity of patients’ respiration with the mechanical ventilator15. In contrast, other studies have demonstrated that morphine infusion can cause systemic hypotension16,17 and does not improve the respiratory outcome of intubated infants18. Therefore, as morphine is the most common analgesic used in the NICU, its safety and efficacy in premature infants remains disputable. This study tests the hypothesis that arbitrary initiation of morphine infusion in intubated premature infants is independently associated with an increase in the incidence of a combined outcome of IVH and/or death.
We conducted this study to investigate the relationship between initiating morphine infusion in the first 72 h after birth in intubated premature infants and the composite outcome of IVH and/or death.
Results
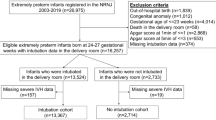
During the study period, of 1,080 preterm infants with ≤ 32 gestational weeks and birth weight > < 1,500 g admitted to the NICU (level 3), 420 met the inclusion criteria and were eligible for inclusion in the final analysis (Fig. 1).
A total of 230 of the 420 infants (54.7%) were exposed to morphine infusion in the first 72 h after birth. Of 230 patients, 153 were aged < 28 gestational weeks. Of 190 patients who did not receive morphine, 63 were aged < 28 gestational weeks. The demographic characteristics of the mothers and infants stratified according to morphine exposure are presented in Table 1. Infants who were aged < 28 gestational weeks and received early morphine infusion had significantly lower birth weight, lower gestational age, and lower Apgar score at 1 and 5 min (P < 0.001, < 0.001, < 0.001, and 0.04, respectively).
In the univariate analysis, we found that early initiation of morphine infusion was significantly associated with neonatal morbidity and mortality in both subgroups (Table 2). A significant association was found between infants aged < 28 gestational weeks and 28–32 gestational weeks who received morphine infusion and the composite outcome (P < 0.001 and P = 0.005, respectively). The multivariable regression analysis performed after adjusting the variables that were significant in the univariate analysis revealed that early initiation of morphine infusion remained significantly associated with the composite outcome in infants aged < 28 gestational weeks [adjusted relative risk (aRR) 1.37; 95% confidence interval (CI) 1.1–1.71)]. However, aRR revealed that there was no association between early morphine infusion and composite outcome in infants aged 28–32 gestational weeks (Table 3).
We took further steps to ensure that the gestational age and birth weight do not affect the primary outcome. We performed 1:1 matching for a group of infants aged < 28 gestational weeks and who received morphine in the first 72 h of life with another group who did not received morphine. We found that early morphine infusion remained significantly associated with composite outcome and mortality (P = 0.008 and P = 0.009, respectively) (Table 4).
Table 5 shows the length of hospital stay, length of total parenteral nutrition (TPN), and invasive ventilator days among the admitted premature infants in both groups.
Infant aged < 28 gestational weeks and who did not receive morphine stayed longer at hospital [median 63; interquartile range (IQR) 37–84] compared with those who received morphine infusion [median 32; IQR (9–81)] (P = 0.01) (Fig. 2).
Boxplot of median hospital stay, TPN days and invasive ventilator days among infants < 28 weeks who did not receive early morphine infusion versus those who received early morphine infusion. Median values are indicated by bold horizontal lines. P = 0.01 between early morphine infusion and hospital stay. P = 0.7 and P = 0.51 respectively in both groups between early morphine infusion and TPN days and invasive ventilator days. TPN total parenteral nutrition.
However, after excluding the dead infants in both groups, the length of hospital stay in patients who received early morphine infusion was higher [median 85; IQR 61.75–111] compared with that in who did not receive morphine infusion [median 75; IQR (54–88.75)]. Thus, the length of hospital stay was not significantly different between the groups (P = 0.29). Moreover, there was no relationship between early exposure to morphine infusion and length of hospital stay in infants aged 28–32 gestational weeks. The median length of hospital stay in infants exposed to morphine was (median 48; IQR 23–62) and in those who were not exposed to morphine was (median 44.5; IQR 21.75–61) (P = 0.48).
In the infants aged < 28 gestational weeks, there was no significant difference in the length of invasive ventilator use and TPN among those who received morphine infusion and those who did not. Patients who received early morphine infusion stayed on invasive ventilators and TPN (median 16; IQR 7–27.25) (median 34.5; IQR 19.75–53) compared with those who did not receive morphine infusion [median 8.75; IQR (1–22.75)] (median 26; IQR 10.5–51.75) (P = 0.3 and P = 0.08, respectively). Furthermore, there was no significant difference in the length of invasive ventilator use and TPN in infants aged 28–32 gestational weeks. Patients who received early morphine infusion stayed on the invasive ventilator and TPN (median 7; IQR 3.5–10) (median 18; IQR 8–30) compared with those who did not receive morphine infusion (median 2; IQR 1–5.75) (median 13.5; IQR 7–25.75) (P = 0.17 and P = 0.56, respectively).
Multivariable negative binomial regression analysis was performed to predict the impact of several independent significant risk factors [gestational age, birth weight, 1-min and 5-min Apgar score, expressed breast milk (EBM) use, patent ductus arteriosus (PDA), and including early exposure to morphine infusion] on the length of hospital stay.
The adjusted model showed no relationship between morphine exposure and length of hospital stay among admitted infants < 28 gestational weeks (aRR 0.83, 95% CI 0.63–1.08, P = 0.16).
Figure 3 shows the Kaplan–Meier curves of the relationship between early exposure to morphine and survival among all infants (log-rank test, P < 0.001). Multivariable Cox regression curve adjusted for significant potential confounders (gestational age, birth weight, 1-min and 5-min Apgar scores, IVH, severe IVH, EBM use, PDA, and PET) showed a significant association between infants who received morphine infusion in the first 72 h after birth and survival, and the hazard ratios (HRs) for death increased by 1.97-fold (95% CI 1.27–3.08) compared with that of infants who did not receive early morphine infusion.
Figure 4 shows Kaplan–Meier curves of the relationship between early exposure to morphine and survival among infants aged < 28 gestational weeks (log-rank test, P < 0.001). In infants aged < 28 gestational weeks, the multivariable Cox regression curve adjusted for significant potential confounders (gestational age, birth weight, 1-min and 5-min Apgar scores, IVH, severe IVH, and EBM use) showed that in infants who received morphine infusion in the first 72 h after birth, the HRs for death increased by 2.11-fold (95% CI 1.19–3.73) compared with that of infants who did not receive early morphine infusion.
In addition, Kaplan–Meier curves showed the relationship between early exposure to morphine and survival among infants aged 28–32 gestational weeks (log-rank test, P = 0.02) (Fig. 5). However, the multivariable Cox regression adjusted for potential confounders (1-min Apgar scores and PDA) in infants aged 28–32 gestational weeks showed that there was no association between early morphine infusion in the first 72 h after birth and death (HR 1.92, 95% CI 0.96–3.82).
Discussion
In the present study, we found that initiation of continuous morphine infusion therapy in the first 72 h after birth in intubated premature infants aged < 28 gestational weeks increased the risk for IVH and/or death after controlling for confounder variables. Moreover, it increased the possibility of developing systemic hypotension in all premature infants who are intubated and aged ≤ 32 gestational weeks, which is a well-known side effect of morphine administration17,19 and consequently increased the use of inotropes and hydrocortisone for treating systemic hypotension. We assumed that early initiation of continuous morphine infusion in intubated premature infants compromises the systemic blood pressure, prompting the neonatologist to establish inotropes, which in turn increases the fluctuation of cerebral blood flow and causes brain injury.
A large randomized clinical trial investigated whether morphine decreases the incidence of brain injury and/or death17. The “NEOPAIN” study reported the following two findings: first, early continuous morphine infusion was associated with an increased risk for brain injury and/or death (P = 0.03) and severe IVH (P = 0.02); second, intermittent morphine boluses dramatically increased the rate of death and brain injury (P = 0.0001). Our study results of the intubated premature infants aged < 28 gestational weeks are consistent with the “NEOPAIN” trial results; therefore, continuous morphine infusion in the first 72 h after birth may increase the risk of developing brain injury and/or death.
Moreover, some studies found that assisted mechanical ventilation aggravates the pain score in premature infants3,20. Therefore, a good number of neonatologists routinely initiate morphine infusion early in premature infants who require assisted ventilation. Studies have found that morphine improves the synchronization between the intubated infants and the mechanical ventilation and, as a consequence, reduces the number of days of ventilation15,21. In these studies, the new ventilation strategies were not available, and they had a small sample size and included infants who were aged > 32 gestational weeks.
In contrast, the “NEOPAIN” trial found that morphine prolonged the duration of mechanical support in extremely premature infants22.
Our study finding is not consistent with the latter one, i.e., there was no change in the ventilator duration in the intubated premature infants’ group who received continuous morphine infusion in the first 72 h after birth. Therefore, the initiation of morphine infusion on admission has no particular pulmonary benefit, which was also the conclusion of a meta-analysis Bellu et al.23.
Another aspect in our study is that early infusion of morphine was not associated with an increased incidence of gastrointestinal pathology such as NEC and the period of TPN among the survivors. In this regard, our results are compatible with those of the “NEOPAIN” trial, in which morphine exposure did not affect NEC, either medical or surgical NEC; however, it prolonged the duration to reach the full feed status24.
Apparently, administering systemic morphine infusion to intubated premature infants in the early period of life increases the risk of mortality twofold compared with that in those who do not receive continuous morphine infusion. This observed relationship between early infusion of morphine and mortality risk corresponds with the results of studies stating that morphine enhances the apoptotic death of cells in the immune and nervous systems, which would negatively influence the developmental outcome of premature infants25,26,27,28.
In our opinion, the drawbacks of administering continuous morphine infusion outweigh the advantages; therefore, initiating early morphine infusion should be restricted and applied according to very clear guidelines. Furthermore, nonpharmacological interventions such as skin-to-skin care, music therapy, and parental attendance can be implemented. A recent systemic review has shown that kangaroo mother care can make the patient more comfortable by reducing the pain score and the episodes of crying28. Similarly, music therapy can be beneficial in minimizing the stress and pain caused during the procedures29.
There are some limitations to our study. First, because this study was retrospective, it was difficult to control for all clinical variables using statistical methods. However, those variables that were related to disease severity were adjusted. Furthermore, by applying the Cox regression model, early initiation of morphine infusion was found to be associated with increased risk of mortality and developing IVH in premature infants aged < 28 gestational weeks. Second, the attending neonatologists who ordered continuous morphine infusion did not look at the pain score and did not use it as a score to finalize or control the dose of morphine infusion. Third, we did not focus on the dose of morphine infusion as it was variable and ranged between 10 and 20 µg/kg/h without using morphine boluses; however, some studies have reported the lack of relationship between the clinical efficacy and the concentration of morphine infusion31,32,32. We do not use other analgesics or sedative medications such as fentanyl or midazolam on admission, and therefore, a discussion about their efficacy in terms of outcomes is not in the scope of this study.
Our study has several strong aspects. First, we included only those premature inborn infants who required intubation within the first 72 h of life. Second, head ultrasound findings were reviewed by a paediatric radiologist who was not aware of the aim of the study. Third, because we had a good number of patients in each group, we attempted to stratify the outcomes using the gestational age, making the results more specific. Fourth, our study findings can serve as a reminder to NICU practitioners regarding the serious complications of early use of systemic analgesia as there are limited data regarding this aspect. In conclusion, establishing early morphine infusion in intubated premature infants is associated with an increased incidence of the composite outcome of IVH and/or death in infants who are aged < 28 gestational weeks. Large, well-designed, randomized controlled trials are required to address the sequelae of early initiation of sedation and analgesia in intubated premature infants.
Methods
Study design
This study was a retrospective cohort review of the charts of premature infants (gestational age ≤ 32 weeks; birth weight < 1,500 g) who were intubated and admitted to the NICU of King Saud Medical City (KSMC) tertiary referral center from July 2014 to July 2019.
The NICU has an average annual admission of 1,100 patients, including NICU levels 2 and 3. This study was conducted in accordance with the Declaration of Helsinki and Good Pharmacoepidemiology Practices guidelines and was approved by the medical ethical review committee of KSMC with a waiver of consent (reference number H1RI-25-Feb19-01).
Definitions
Intraventricular hemorrhage
IVH was classified into grades I–IV according to the IVH classification described by Papile et al. IVH was diagnosed based on the findings of head ultrasound performed between days 5 and 7 of life after birth33,34. All ultrasound findings were reviewed by an expert pediatric radiologist. Transducers of 7.5 and 10 MHz (LOGIQ e; GE Medical Systems Co., Ltd., Jiangsu, China) were used to perform ultrasound in the sagittal and coronal planes.
Bronchopulmonary dysplasia (BPD) was defined as the need for respiratory support at 36 weeks of corrected gestational age35.
Necrotizing enterocolitis (NEC)
This includes all Bell Staging Criteria36.
Inclusion and exclusion criteria
Infants included in this study were born at KSMC at ≤ 32 gestational weeks with a birth weight of < 1,500 g, admitted to the NICU, and intubated and received continuous infusion of morphine within the first 72 h of life. In the control group, intubated patients did not receive morphine within the first 72 h of life during the same study period. Gestational age was categorized into two groups: < 28 gestational weeks and 28–32 gestational weeks.
We excluded all non-intubated patients on admission, those with major congenital anomalies, those who were not born at KSMC, those who had congenital infection, those who developed early onset of sepsis, or those for whom data could not be retrieved.
A sample size of 420 premature infants was analysed to detect differences in the short-term outcome.
Data collection and follow-up
Patients’ charts from NICU admission until discharge or death were reviewed. Demographic and clinical data and outcome data of all infants were obtained. Maternal data, including the presence of gestational diabetes mellitus, maternal hypertension, antenatal steroid treatment, and mode of delivery, were also obtained.
Study outcomes
The primary outcome of this study was the composite outcome, comprising IVH and/or mortality. The secondary outcomes were any grade of IVH, severe IVH (grade III or IV), NEC (medical or surgical), BPD, and infant mortality.
Statistical analysis
Before starting the analysis, the dataset was checked for missing variables. Data were analyzed using a statistical software package (Statistical Package for the Social Sciences, version 25.0, SPSS Inc., Chicago, IL, USA).
Descriptive statistics, including median, (IQR), frequency, and percentages, were used to describe the maternal and neonatal variables.
The Fisher’s exact test was used to determine the association between categorical variables. The Mann–Whitney U test was used for ordinal qualitative variables [gestational age, birth weight, Apgar score, and the Score for Neonatal Acute Physiology with Perinatal Extension-II (SNAPP-II)]. For continuous variables, the unpaired Student’s t-test was used; when data were not normally distributed, the Mann–Whitney U test was performed. The Kolmogorov–Smirnov test and a visual inspection of histograms were performed to verify the normality of distribution of the quantitative variables.
To analyse the association between early exposure to morphine infusion and outcomes, a univariate relative risk analysis was first conducted on the recorded variables [gestational age, birth weight, gender, 1-min Apgar, 5-min Apgar, SNAPPE-II, surfactant use, maternal hypertension, antenatal steroid treatment, mode of delivery, (PDA), and (EBM)] because we considered those to be potential confounders38,39,39. All factors with a P value of < 0.05 in the univariate analysis were considered for inclusion in the final multivariable regression model. Modified log-Poisson regression in generalized linear models with robust variance estimator (Huber-White) was applied for univariate relative risk analysis and to the models to adjust the relative risk for infants’ morbidity and mortality outcomes.
A negative binomial regression analysis was conducted to compare the effect of early morphine infusion exposure on dependent variables [days on ventilator, days of (TPN), and length of hospital stay].
Kaplan–Meier survival curve with log rank test was used to compare survival among those who received morphine versus those who did not receive morphine. Multivariable Cox regression analysis was used to analyse the HRs for death with 95% CIs. Independent variables for the models were selected from variables significantly related to death in a univariate analysis. All statistical tests were 2-tailed, and P values of < 0.05 were considered to be statistically significant.
Data availability
The datasets analysed during this study are available from the corresponding author on reasonable request.
References
Carbajal, R. et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 300, 60–70 (2008).
Cruz, M. D., Fernandes, A. M. & Oliveira, C. R. Epidemiology of painful procedures performed in neonates: A systematic review of observational studies. Eur. J. Pain 20, 489–498 (2016).
Barker, D. P. & Rutter, N. Stress, severity of illness, and outcome in ventilated preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 75, F187–F190 (1996).
Anand, K. J. Clinical importance of pain and stress in preterm neonates. Biol. Neonate 73, 1–9 (1998).
Pineles, B. L., Sandman, C. A., Waffarn, F., Uy, C. & Davis, E. P. Sensitization of cardiac responses to pain in preterm infants. Neonatology 91, 190–195 (2007).
Bouza, H. The impact of pain in the immature brain. J. Matern. Fetal Neonatal Med. 22, 722–732 (2009).
Walker, S. M. Translational studies identify long-term impact of prior neonatal pain experience. Pain 158, S29–S42 (2017).
Smith, G. C. et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann. Neurol. 70, 541–549 (2011).
Steinhorn, R. et al. Neonatal morphine exposure in very preterm infants-cerebral development and outcomes. J. Pediatr. 166, 1200–1207 (2015).
Clark, R. H., Bloom, B. T., Spitzer, A. R. & Gerstmann, D. R. Reported medication use in the neonatal intensive care unit: Data from a large national data set. Pediatrics 117, 1979–1987 (2006).
Kumar, P. et al. Medication use in the neonatal intensive care unit: Current patterns and off-label use of parenteral medications. J. Pediatr. 152, 412–415 (2008).
Quinn, M. W. et al. Effect of morphine and pancuronium on the stress response in ventilated preterm infants. Early Hum. Dev. 30, 241–248 (1992).
Quinn, M. W. et al. Randomised double-blind controlled trial of effect of morphine on catecholamine concentrations in ventilated pre-term babies. Lancet 342, 324–327 (1993).
Simons, S. H. et al. Randomised controlled trial evaluating effects of morphine on plasma adrenaline/noradrenaline concentrations in newborns. Arch. Dis. Child Fetal Neonatal Ed. 90, F36–F40 (2005).
Dyke, M. P., Kohan, R. & Evans, S. Morphine increases synchronous ventilation in preterm infants. J. Paediatr. Child Health 31, 176–179 (1995).
Carbajal, R. et al. Morphine does not provide adequate analgesia for acute procedural pain among preterm neonates. Pediatrics 115, 1494–1500 (2005).
Anand, K. J. et al. Effects of morphine analgesia in ventilated preterm neonates: Primary outcomes from the NEOPAIN randomised trial. Lancet 363, 1673–1682 (2004).
Simons, S. H. et al. Routine morphine infusion in preterm newborns who received ventilatory support: A randomized controlled trial. JAMA 290, 2419–2427 (2003).
Hall, R. W. et al. Morphine, hypotension, and adverse outcomes among preterm neonates: Who’s to blame? Secondary results from the NEOPAIN trial. Pediatrics 115, 1351–1359 (2005).
Hall, R. W., Boyle, E. & Young, T. Do ventilated neonates require pain management?. Semin. Perinatol. 31, 289–297 (2007).
Jiang, H. H. et al. Clinical evaluation of the effects of morphine in mechanical ventilation of neonates. Zhonghua Er Ke Za Zhi 50, 350–355 (2012).
Bhandari, V. et al. Morphine administration and short-term pulmonary outcomes among ventilated preterm infants. Pediatrics 116, 352–359 (2005).
Bellu, R., de Waal, K. & Zanini, R. Opioids for neonates receiving mechanical ventilation: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 95, F241–F251 (2010).
Menon, G. et al. Morphine analgesia and gastrointestinal morbidity in preterm infants: Secondary results from the NEOPAIN trial. Arch. Dis. Child. Fetal Neonatal Ed. 93, F362–F367 (2008).
Singhal, P. C. et al. Role of p38 mitogen-activated protein kinase phosphorylation and Fas-Fas ligand interaction in morphine-induced macrophage apoptosis. J. Immunol. 168, 4025–4033 (2002).
Goswami, R., Dawson, S. A. & Dawson, G. Cyclic AMP protects against staurosporine and wortmannin-induced apoptosis and opioid-enhanced apoptosis in both embryonic and immortalized (F-11kappa7) neurons. J. Neurochem. 70, 1376–1382 (1998).
Kocek, M., Wilcox, R., Crank, C. & Patra, K. Evaluation of the relationship between opioid exposure in extremely low birth weight infants in the neonatal intensive care unit and neurodevelopmental outcome at 2 years. Early Hum. Dev. 92, 29–32 (2016).
Johnston, C. et al. Skin-to-skin care for procedural pain in neonates. Cochrane Database Syst. Rev. 2, CD008435 (2017).
Hartling, L. et al. Music for medical indications in the neonatal period: A systematic review of randomised controlled trials. Arch. Dis. Child. Fetal Neonatal Ed. 94, F349–F354 (2009).
Scott, C. S. et al. Morphine pharmacokinetics and pain assessment in premature newborns. J. Pediatr. 135, 423–429 (1999).
Saarenmaa, E., Neuvonen, P. J., Rosenberg, P. & Fellman, V. Morphine clearance and effects in newborn infants in relation to gestational age. Clin. Pharmacol. Ther. 68, 160–166 (2000).
Anand, K. J. et al. Morphine pharmacokinetics and pharmacodynamics in preterm and term neonates: Secondary results from the NEOPAIN trial. Br. J. Anaesth. 101, 680–689 (2008).
Ballabh, P. Intraventricular hemorrhage in premature infants: Mechanism of disease. Pediatr. Res. 67, 1–8 (2010).
Huvanandana, J. et al. Prediction of intraventricular haemorrhage in preterm infants using time series analysis of blood pressure and respiratory signals. Sci. Rep. 7, 46538 (2017).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7 (1978).
Hintz, S. R. et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 115, 696–703 (2005).
Kurlat, I., Stoll, B. J. & McGowan, J. E. Jr. Time to positivity for detection of bacteremia in neonates. J. Clin. Microbiol. 27, 1068–1071 (1989).
Kristof, K., Kocsis, E. & Nagy, K. Clinical microbiology of early-onset and late-onset neonatal sepsis, particularly among preterm babies. Acta Microbiol. Immunol. Hung. 56, 21–51 (2009).
Author information
Authors and Affiliations
Contributions
M.M.A. designed the study, analysed the data, and wrote the manuscript. S.S.A. collected the data, contributed to data analysis, created the figures and tables, and wrote the manuscript. T.M.K. collected the data and wrote the manuscript. All authors critically revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Mouqdad, M.M., Khalil, T.M. & Asfour, S.S. Retrospective study of short-term complications associated with early morphine use in intubated premature infants. Sci Rep 10, 10874 (2020). https://doi.org/10.1038/s41598-020-67891-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-67891-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.