Abstract
Background
The role of cytokines in the pathogenesis of febrile seizures (FSs) is unclear, and information regarding cytokine production outside of FS episodes is scarce.
Methods
In our controlled follow-up study of patients with FSs, we compared the levels of 12 serum cytokines after the patients’ first FSs, during febrile episodes without FSs, after recurrent FSs, during healthy periods after FSs, and between patients and controls.
Results
Two-hundred fifty-one patients with first FS participated in the study, of whom 17 (mean age 1.6 years, SD 0.7) with recurrent FSs completed the protocol as required by the sample size calculations. The mean IL-1RA level was higher after the first FSs (2580 pg/mL, SD 1516) than during febrile episodes without FSs (1336 pg/mL, SD 1364, P = 0.006) and healthy periods after FSs (474 pg/mL, SD 901, P = 0.001). IL-1RA levels were also higher during first (2580 pg/mL) and recurrent FSs (2666 pg/mL, SD 1747) in comparison with febrile controls (746 pg/mL, SD 551) (P < 0.001 and P = 0.001, respectively), but there was no difference in the IL-1RA between febrile episodes without FSs and febrile controls.
Conclusions
Patients with FSs produce stronger inflammatory reactions during febrile episodes with FSs compared with febrile episodes without FSs and febrile controls.
Impact
-
In patients with FSs, IL-1RA was higher following first FS than during febrile episodes without FSs and healthy periods after FSs.
-
IL-1RA was higher in patients with FSs following first and recurrent FSs than in febrile controls.
-
There was no significant difference in IL-1RA between febrile episodes of patients without FSs and febrile controls.
-
Using IL-1RA as a surrogate marker of IL-1 axis activity, our results indicate that patients with FSs produced stronger inflammatory reactions during FS episodes but not during other febrile episodes or healthy periods after FSs.
-
Cytokines may play a role in pathogenesis of FSs.
Similar content being viewed by others
Introduction
Febrile seizures (FSs) occur in 2–5% of children under 6 years of age and recur in 20–30% of the patients in subsequent febrile episodes.1 FSs cause significant emotional and economic burdens. The exact pathogenesis of FSs is unknown, but during a specific developmental stage, certain genetically determined features of the central nervous and inflammatory systems may induce responses that cause seizures during febrile infections. This notion is supported by the recent identification of new genetic loci that are functionally related to fever responses and neuronal excitability.2
Prostaglandins and cytokines mediate a host’s inflammatory response to an infection. It has been suggested that virus-induced release of cytokines in the central nervous system is one of the factors that causes neuronal hyperexcitability, which can lead to seizures.3,4 In animal studies, various cytokines have been found to either enhance or suppress provoked convulsions.5,6 Skotte et al.2 found an association between FSs and single nucleotide polymorphisms in the anti-inflammatory cytokine interleukin (IL)-10 genes. Altered levels of the pro-inflammatory cytokines IL-1β, IL-6, tumor necrosis factor (TNF)-α and the anti-inflammatory cytokines IL-1RA and IL-10 have been found in sera and cerebrospinal fluid from patients with FSs immediately following seizures,7,8,9,10 but information regarding cytokine levels in patients with FSs outside of febrile episodes with FSs is scarce.
Previously, we showed that antipyretics were ineffective in lowering temperatures during infections that led to seizures.11 Considering this persistent fever response, we hypothesized that patients’ cytokine levels differed between febrile infections without seizures and infections that led to FS. In addition, we hypothesized that cytokine levels differed in patients with FSs compared to other febrile children and healthy adults without FSs.
Methods
Study population and study design
This was a controlled follow-up study. The Ethics Committee (Institutional Review Board) of Northern Ostrobothnia Hospital District, Finland, found the study plan acceptable (diary number 6/2011). Informed consent was obtained from the guardians of the participating children and from the adult study participants. The study was conducted from January 2012 to May 2019 in Oulu University Hospital, Finland. This hospital is the only hospital in the area with a pediatric emergency room (ER) and pediatric wards. The hospital’s primary catchment area has a population of 85,000 children aged <16 years, and the secondary catchment area has a population of 148,000 in the same age group. The study cohort was most likely representative of typical FSs among the population, as the current Finnish guidelines recommend that all patients having their first FS should be referred to a pediatric ER.
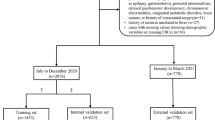
All patients visiting the hospital’s pediatric ER with their first FS were invited to join the study. We chose to exclude patients younger than 6 months and older than 6 years. The exclusion criteria for patients with FSs were a history of previous afebrile seizures or epilepsy, any syndrome or cerebral malformation that markedly increased the risk of epilepsy, an evident central nervous system infection, a significant electrolyte imbalance, a body temperature <38 °C, and psoriasis. We only included patients with at least one native Finnish-speaking parent to avoid communication problems. The study clinician/nurse contacted the family of each patient after the first seizure. The study protocol included four visits for each patient with FSs: one for the first FS, one for a febrile infection without FSs, one for a recurrent FS, and one while the patient was healthy after a seizure (Fig. 1). The order of the revisits varied because some patients had several FS episodes before having a febrile episode without FSs. Samples taken during healthy periods after seizures were programmed to be collected 3 weeks after the recurrent FS; however, in some cases, they were postponed due to recurrent infections.
We selected one febrile control patient for each patient with FSs after the index patient had had a recurrent FS. We recruited the controls from hospital patients with febrile infections and body temperatures >38 °C. These controls were matched by age (±6 months) and sex. We also selected unrelated healthy adult control patients, matched by sex. The exclusion criteria for the controls were a history of FS, epilepsy, any syndrome or cerebral malformation that markedly increased the risk of epilepsy, an evident central nervous system infection, and psoriasis. Exclusion of patients with psoriasis was needed for another part of the study. No patients were excluded because of this criterion.
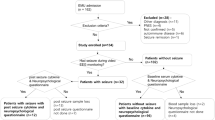
By comparing patients with FSs across different time points and to febrile children without FSs, we aimed to determine whether the cytokine production in patients with FSs varied in different situations and whether there was a fundamental difference in cytokine responses between these two groups (Fig. 2). We specifically chose to use healthy adults as controls in addition to the febrile children. It is unethical to draw blood samples from healthy infants only for study purposes. In addition, the cytokine levels that are measured from clinical samples vary among different laboratories. As there are more available data regarding the baseline cytokine values in healthy adults, we wanted to give the reader of the article the possibility to compare our values to the values of their own. Thus, we had to compare the cytokine levels of patients with FSs during healthy periods with those of healthy adults.
For c-reactive protein analysis and cytokine measurement purposes, we collected a blood sample from a peripheral vessel from each patient at each study visit. The blood samples were centrifugated, and the sera were stored at –80 °C until the analysis. For the FS-associated visits, we obtained the samples within 24 h post-seizure. A nasopharyngeal respiratory virus sample was taken during each febrile study visit for multiplex polymerase chain reaction assay purposes. Each of these samples was either a nasopharyngeal aspirate or swab.
Patient data were systematically collected from the medical records and through interviews with the parents using a structured questionnaire. For each patient, these data included demographic features; family history of FSs; clinical diagnosis; symptoms of the infection; starting date and time of the infection and the fever; primary focus of the infection; date, time, type, and duration of FS; body temperature on arrival to the ER; highest body temperature during the period measured by the parents, paramedics, or hospital staff; and the use of antipyretics or antiepileptic emergency medications. Complex FSs were defined as focal seizures, seizures lasting more than 15 min, or recurrent seizures during a 24-h period.
Cytokine measurement
The concentrations of pro-inflammatory cytokines (IL-1β, IL-2, IL-5, IL-6, IL-8, IL-12p70, TNF-α, TNF-β, and IFN-γ) and anti-inflammatory cytokines (IL-4, IL-10, and IL-1RA) were measured using a commercially available bead-based FlowCytomix™ Multiple Analyte Detection System according to the manufacturer’s instructions (eBioscience, Thermo Fisher Scientific, Waltham, Massachusetts). The detection was carried out with a BD LSRFortessa™ Flow Cytometer (BD Biosciences, San Jose, California). For IL-8, the lower limit of quantification (LLQ) was 13.72 pg/mL; for the rest of the cytokines, the LLQ was 27.43 pg/mL. For statistical purposes, we expressed serum cytokine levels below the LLQ as the midpoint between zero and the LLQ of the analyte. If the program gave a specific value below the LLQ, we left it unchanged.
Ideally, cytokine measurements are performed immediately after samples are taken. However, to execute our follow-up protocol, we were required to choose between a long storage time or several cytokine analyses performed at different time points. We concluded that allowing a longer storage time would cause less bias because the storage times of samples from patients and controls would be similar and, thus, comparable. The samples were stored at –80 °C to slow the degradation process.
Outcomes
We compared the cytokine levels of patients with FSs in four different situations: within 24 h after the first FS, during febrile infection without FSs, within 24 h after a recurrent FS, and while a patient was healthy after FS. This way, patients with FS served as controls for themselves. We also compared the cytokine levels of patients with FSs with those of two control populations: febrile control children without FSs (matched by age and sex) and healthy adult controls (matched by sex).
Statistical analyses
We calculated the sample size using an α error of 5% and a β error of 20%. We chose IL-1β as the primary cytokine based on its central role in the formation of febrile response, the results of animal studies regarding provoked convulsions, and the results of genetic and clinical studies examining cytokines in FSs.5,8,12 We selected the 25 pg/mL standard deviation (SD) of IL-1β concentration from an experimental study that evaluated the effect of priming macrophages with interferon-γ on the cytokine production of the cells.13 We estimated that this change of one SD could be regarded as the smallest clinically significant change. To our knowledge, earlier studies regarding cytokine production in patients with FSs have not used sample size calculations, and there are no clinical studies defining the smallest clinically significant change in IL-1β concentration. Our estimates resulted in a sample size of 16 patients for each group.
The background characteristics of the patients with FSs and control patients were tested a priori using the Friedman test, which is a generally acknowledged way to measure differences between repeated measurements among multiple groups. If the Friedman test gave a statistically significant P-value, we used the Wilcoxon-signed rank test as a post-hoc test for continuous variables. For dichotomous variables, we used a sign test. The cytokine levels were expressed as means and SDs. Differences in serum cytokine levels between visits of patients with FSs and between patients with FSs and controls were compared a priori using the Friedman test. If the Friedman test gave a significant P-value, a post-hoc Wilcoxon-signed rank test was used. Cytokine levels were given as medians and interquartile ranges (IQRs). All analyses were performed using IBM Statistics for Windows version 27 (IBM Corp., Armonk, New York) and StatsDirect statistical software version 3 (StatsDirect Ltd., Birkenhead, UK). Figures were drawn using GraphPad Prism version 9 (Graphpad, San Diego, California) and SmartDraw version 2008 (SmartDraw Software, LLC, The Woodlands, Texas).
Results
Patient characteristics
In total, 599 patients with FSs were treated at the pediatric ER during the study period. Of them, 307 patients were invited to join the study; 251 patients accepted. Of the 251 patients with FSs, 60 (24%) had recurrent seizures. Altogether, 18 patients with recurrent seizures completed the study protocol; however, the cytokine analysis failed for 1 patient. Therefore, 17 patients were included in the final analysis (Fig. 1).
Overall, 10 (59%) of the 17 patients with FSs were boys (Table 1). The mean age of the patients at the first FS episode was 1.6 years (SD 0.7). The mean duration of the first FS was 5 min and 45 s (median 3:00 min, range 01:00 to 25:00 min). The mean duration of recurrent FSs was 5 min and 57 s (median 3:00 min, range 0:30 to 20:00 min). Overall, 8 (47%) patients with recurrent FSs had complex FSs (Table 1).
Cytokine concentrations in patients with FSs
Anti-inflammatory cytokines
The IL-1RA levels measured after first FSs (mean 2580 pg/mL [SD 1516]; median 2640 [IQR 1238–3713]) were significantly higher than those measured during febrile episodes without FS (mean 1336 pg/mL [SD 1364]; median 867 [IQR 344–1674]; P = 0.006) and those measured during healthy periods after FSs (mean 474 pg/mL [SD 901]; median 134 [IQR 97–345]; P = 0.002; Table 2, Fig. 3). The IL-1RA levels were somewhat higher after recurrent FSs (mean 2666 pg/mL [SD 1747]; median 2843 [IQR 1375–4130]) than they were during febrile episodes without FSs (mean 1336 pg/mL); however, this difference was not statistically significant (P = 0.124). The IL-1RA levels were also significantly higher during febrile episodes without FSs (mean 1336 pg/mL) than they were during healthy periods after FSs (mean 474 pg/mL). There was no difference in the IL-1RA levels between the first FSs (mean 2580 pg/mL) and recurrent FSs (mean 2666 pg/mL; Table 2, Fig. 3). The levels of IL-10 were significantly higher during healthy periods after FSs than they were after first FS episodes (mean 1317 pg/mL [SD 5184] vs. 66 pg/mL [SD 86]; P = 0.0039; Table 2).
Serum (a) IL-1RA and (b) IL-1β levels, and (c) IL-1RA/IL-6 and (d) IL-1RA/IL-1β ratios in patients with FSs during the first FS, during febrile episodes without FSs, during recurrent FSs and during a healthy period after FSs and in controls. For IL-1β and the IL-1RA/IL-1β ratio, the y-axis illustrated in logarithmic scale. FS febrile seizure.
Pro-inflammatory cytokines
The IL-6 levels were significantly higher during healthy periods after FSs than they were during febrile episodes without FSs, with mean values of 1630 pg/mL (SD 6181) and 350 pg/mL (SD 1010), respectively (P = 0.005). TNF-α levels were significantly higher during febrile episodes without FSs than after the first FS episodes (mean 194 pg/mL [SD 519] vs. 26 pg/mL [SD 37]; P = 0.05) and during healthy periods after FSs compared to after first FS episodes (mean 903 pg/mL [SD 3427] vs. 26 pg/mL [SD 37]; P = 0.037; Table 2).
Ratios of anti-inflammatory cytokines to pro-inflammatory cytokines
The mean IL-1RA:IL-1β ratio was higher after the first FSs than during febrile episodes without FSs (250 [SD 325] vs. 78 [SD 102]; P = 0.01; Table 2, Fig. 3). The mean IL-1RA:IL-1β ratio was also somewhat higher after recurrent FS episodes than during febrile episodes without FSs (665 [SD 2168] vs. 78 [SD 102]), but this difference was not statistically significant (P = 0.149). There was no significant difference in the IL-1RA:IL-6 ratio between the first and recurrent FS episodes. The mean IL-1RA:IL-6 ratio was significantly higher after the first FS episodes (35 [SD 23]) than during febrile episodes without FSs (23 [SD 29]; P = 0.039; Table 2, Fig. 3).
Comparisons of cytokine concentrations between patients with FSs and controls
Anti-inflammatory cytokines
The IL-1RA levels were significantly higher following both first FSs (mean 2580 pg/mL [SD 1516]; median 2640 [IQR 1238–3713]) and recurrent FSs (mean 2666 pg/mL [SD 1747]; median 2843 [IQR 1375–4130]) compared with the levels in febrile control children (mean 746 pg/mL [SD 551]; median 652 [IQR 368–946]; P < 0.001 and P = 0.001, respectively). The IL-1RA levels of patients with FS during febrile episodes without FSs (mean 1336 pg/mL [SD 1364]; median 867 [IQR 345–1674]) did not differ from those of febrile control children (mean 746 pg/mL; Table 3, Fig. 3). There were no significant differences between the patients with FSs and febrile control children with regard to any other anti-inflammatory cytokines (Table 3).
Pro-inflammatory cytokines
Regarding pro-inflammatory cytokines, only the levels of IL-6 were significantly higher in patients with FSs during healthy periods after FSs (110 pg/mL [SD 116]) than in healthy adults (42 pg/mL [SD 67]; P = 0.034; Table 3).
Ratios of anti-inflammatory cytokines to pro-inflammatory cytokines
The mean IL-1RA:IL-1β ratio was significantly higher in the first FS episodes of patients with FSs (250 [SD 325]) than in febrile control children (58 [SD 96]; P = 0.002; Table 3, Fig. 3). The same was true for the mean IL-1RA:IL-6 ratio (35 [SD 23] and 12 [SD 13], respectively; P = 0.006). The mean IL-1RA:IL-6 ratio was also significantly higher after recurrent FSs of patients with FSs (46 [SD 51]) than in febrile control children (12 [SD 13]; P = 0.017; Table 3, Fig. 3).
Discussion
Our controlled follow-up study compared the cytokine levels in patients with FSs across different situations and to matched controls. We found that in patients with FSs, the levels of IL-1RA were significantly higher immediately following FSs than during febrile episodes without FSs and during healthy periods after FSs. In addition, IL-1RA levels were higher in patients with FS during first and recurrent FS episodes than in febrile control children, but there was no significant difference in IL-1RA levels between febrile episodes without FSs and control patients. We also found similar differences in the IL-1RA:IL-1β and IL-1RA:IL-6 ratios between these groups. Our results indicate that patients with FSs produce exaggerated inflammatory responses during FS episodes but not during other febrile episodes or during healthy periods after FSs.
It has been suggested that IL-1RA is one of the best surrogate markers of IL-1β activity, which, in turn, has been shown to cause seizures.3,4,14,15 IL-1RA acts by limiting IL-1β-mediated pro-inflammatory actions, and clinical studies have shown that IL-1RA is robustly released following IL-1β induction activity. However, the measurement of IL-1β itself may not be an ideal marker of IL-1 system activation, because very small concentrations of IL-1β are required to initiate robust inflammatory responses. In addition, IL-1β binds to large proteins, which may reduce its detection in clinical samples by 50%.14
Our results suggest that patients with FSs produce higher levels of cytokines only during infections that lead to FSs. These results confirm the findings from our previous randomized controlled trial, in which we found that the maximum body temperature measured during a febrile episode was higher in a FS episode than in a febrile episode without FSs. Furthermore, in the same study, all antipyretics studied were ineffective in lowering body temperature during FS episodes, though they were effective in febrile episodes without FSs for the same patients.11 In another recent study, we discovered that patients with FSs produced stronger febrile reactions to respiratory viruses than controls matched with the same respiratory virus.16 Such a febrile reaction may reflect the cytokine reaction of the host. These exaggerated reactions seem to be influenced by several factors; it may be that different viruses cause distinct cytokine reactions. In the current study, we were unable to match controls for the same respiratory virus; therefore, we could not evaluate the effects of different viruses on cytokine production. However, we aimed to ensure the viral etiology to be as equal as possible by recruiting the patients and the matched controls as simultaneously as possible.
Presuming that IL-1RA can be regarded as a surrogate marker of IL-1 axis activity, we demonstrated that patients with FSs produced higher inflammatory responses than febrile control patients without FSs. Furthermore, as there were no significant differences in the cytokine levels between patients with FSs during febrile episodes without FS and febrile control patients, we were able to show that the altered cytokine levels were actually linked to the seizures. This raises the question whether the increase in IL-1RA is a result of the fever and the seizure itself or whether this increase precedes the seizure. As pointed out by a recent, large, genome-wide association study of FSs, there seem to be several factors affecting the unique febrile response of patients with FSs.2 Once these factors are confirmed and the functions of the specific cytokines are thoroughly understood, it will be possible to develop targeted medications to resolve these febrile responses and prevent FSs. However, before this can become possible, there is a need for both functional and genetic studies on this subject.
Rather than a single, specific cytokine, the balance between pro- and anti-inflammatory cytokines might be essential in the multifactorial pathogenesis of FSs. We found significant differences in the ratios of the anti-inflammatory IL-1RA and the pro-inflammatory IL-1β and IL-6 when comparing patients’ first FS episodes with their febrile episodes without FSs. There were also significant differences between first FS episodes and febrile control patients. These results are consistent with previous findings.8 The levels of cytokines are highly co-dependent, so reporting isolated values can be misleading, and we recommend that future studies assess cytokine activity as a whole—for example, with network analysis. Mathematical network analysis is a computer-based method of modeling the relationships between several co-dependent factors; therefore, it is suitable for studying the function of cytokine networks.
Cytokines are highly unstable substances, and the timing of sample collection is complicated because cytokines have short half-lives and peak at different points of time. The technical difficulty of measuring cytokines may, in part, explain the contradictions between the results of the current study and those of earlier studies of cytokine levels in patients with FSs.7,8,9,10,17,18,19,20,21 In the present study, IL-1RA and IL-6 were the only cytokines that demonstrated significant differences between study patients and control patients. The level of IL-1RA, which is described as a reliable indicator of IL-1 axis activity, rises several hours after pro-inflammatory cytokines, reaching its peak about 24 h after a seizure; therefore, we chose a sampling time limit of 24 h after a seizure. This time limit allowed us to standardize the sampling times between febrile cases and controls. Furthermore, it would have been impossible to acquire samples from control patients immediately after the rise of fever. In general, the cytokine levels were likely lowered by the degradation caused by the long storage time. This may mean that the differences in the cytokine levels between groups are actually larger than what we found.
The strengths of the present study include the use of sample size calculations and the versatile study setting that included follow-ups of patients with FSs as well as comparisons between patients’ multiple visits and with matched controls. A limitation of the study is that the cytokine levels may have been affected by the long storage time due to the long time the study lasted. Also, we did not have healthy children as controls.
Conclusions
According to our results, the levels of IL-1RA were significantly higher in patients with FSs following their first FS episodes than during febrile episodes without FSs and healthy periods after FSs in the same patients. In addition, IL-1RA levels were higher in patients with FSs during first and recurrent FS episodes than in febrile control children, but there was no significant difference in the IL-1RA level between the patients’ febrile episodes without FSs and the febrile control patients. Our results indicate that patients with FSs produce exaggerated inflammatory reactions during FS episodes but not during other febrile episodes or healthy periods after FSs.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Nelson, K. B. & Ellenberg, J. H. Prognosis in children with febrile seizures. Pediatrics 61, 720–727 (1978).
Skotte, L. et al. Genome-wide association study of febrile seizures implicates fever response and neuronal excitability genes. Brain 145, 555–568 (2022).
Viviani, B. et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 23, 8692–8700 (2003).
Wang, S., Cheng, Q., Malik, S. & Yang, J. Interleukin-1beta inhibits gamma-aminobutyric acid type A (GABA(A)) receptor current in cultured hippocampal neurons. J. Pharm. Exp. Ther. 292, 497–504 (2002).
Heida, J. G., Moshé, S. L. & Pittman, Q. J. The role of interleukin-1beta in febrile seizures. Brain Dev. 31, 388–393 (2009).
Vezzani, A. et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc. Natl Acad. Sci. USA 97, 11534–11539 (2000).
Kwon, A. et al. Cytokine levels in febrile seizure patients: a systematic review and meta-analysis. Seizure 59, 5–10 (2018).
Virta, M., Hurme, M. & Helminen, M. Increased plasma levels of pro- and anti-inflammatory cytokines in patients with febrile seizures. Epilepsia 43, 920–923 (2002).
Ha, J. et al. Interleukin-4 and tumor necrosis factor-alpha levels in children with febrile seizures. Seizure 58, 156–162 (2018).
Kim, K. et al. Analysis of plasma multiplex cytokines and increased level of IL-10 and IL-1Ra cytokines in febrile seizures. J. Neuroinflammation. 14, 200 (2017); erratum 14, 226 (2017).
Strengell, T. et al. Antipyretic agents for preventing recurrences of febrile seizures: randomized controlled trial. Arch. Pediatr. Adolesc. Med. 163, 799–804 (2009).
Virta, M., Hurme, M. & Helminen, M. Increased frequency of interleukin-1beta (-511) allele 2 in febrile seizures. Pediatr. Neurol. 26, 192–195 (2002).
Wallet, M. A., Wallet, S. M., Guiulfo, G., Sleasman, J. W. & Goodenow, M. M. IFNgamma primes macrophages for inflammatory activation by high molecular weight hyaluronan. Cell Immunol. 262, 84–88 (2010).
Dinarello, C. A. Biologic basis for interleukin-1 in disease. Blood 87, 2095–2147 (1996).
Dubé, C., Vezzani, A., Behrens, M., Bartfai, T. & Baram, T. Z. Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann. Neurol. 57, 152–155 (2005); erratum 57, 609 (2005).
Hautala, M. et al. Respiratory viruses and febrile response in children with febrile seizures: a cohort study and embedded case-control study. Seizure 84, 69–77 (2020).
Choi, J., Min, H. J. & Shin, J. S. Increased levels of HMGB1 and pro-inflammatory cytokines in children with febrile seizures. J. Neuroinflammation 8, 135 (2011).
Haspolat, S. et al. Interleukin-1beta, tumor necrosis factor-alpha, and nitrite levels in febrile seizures. J. Child Neurol. 17, 749–751 (2002); erratum 18, 118 (2003).
Azab, S. F. et al. Serum and CSF adiponectin, leptin, and interleukin 6 levels as adipocytokines in Egyptian children with febrile seizures: a cross-sectional study. Ital. J. Pediatr. 42, 38 (2016).
Şahin, S., Uysal, S., Yentür, S. P. & Kaçar, A. Reduced cerebrospinal fluid levels of interleukin-10 in children with febrile seizures. Seizure 65, 94–97 (2019).
Mahyar, A. et al. Serum interleukin-1beta and tumor necrosis factor-alpha in febrile seizures: is there a link? Korean J. Pediatr. 57, 440–444 (2014).
Acknowledgements
We would like to thank Emma Pirilä, Ph.D, Mirka Säkkinen, the study nurse, and Helena Moilanen; Nordlab, for their contributions to the study.
Funding
M.H. and K.M. have received a grant from Arvo and Lea Ylppö Foundation and Alma och K.A. Snellman Foundation. H.H. has received a grant from the Finnish National State Grants (VTR Funding), Alma och K.A. Snellman Foundation, and Eemil Aaltonen Foundation. Open Access funding provided by University of Oulu including Oulu University Hospital.
Author information
Authors and Affiliations
Contributions
M.H.: acquisition of data, data-analysis and interpretation and drafting the article. H.H.: acquisition of data, data interpretation and drafting the article. Biostatistician T.P.: data-analysis and interpretation, drafting the article. U.K.: acquisition of data, drafting the article. H.R.: conception and design of the study, data-analysis and interpretation, drafting the article and revising it critically for important intellectual content. M.U.: conception and design of the study, data-analysis and interpretation, drafting the article and revising it critically for important intellectual content. T.K.: conception and design of the study and revising the article critically for important intellectual content. K.M.: conception and design of the study, data-analysis and interpretation, drafting the article and revising it critically for important intellectual content. All authors have given their final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Informed consent was obtained from the guardians of the participating children and from the adult study participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hautala, M.K., Helander, H.M., Pokka, T.ML. et al. Recurrent febrile seizures and serum cytokines: a controlled follow-up study. Pediatr Res 93, 1574–1581 (2023). https://doi.org/10.1038/s41390-022-02282-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02282-7