Abstract
Background
A sealed abdominal interface was positioned below the diaphragm (the “NeoVest”) to apply synchronized and proportional negative pressure ventilation (NPV) and was compared to positive pressure ventilation (PPV) using neurally adjusted ventilatory assist (NAVA). Both modes were controlled by the diaphragm electrical activity (Edi).
Methods
Eleven rabbits (mean weight 2.9 kg) were instrumented, tracheotomized, and ventilated with either NPV or PPV (sequentially) with different loads (resistive, dead space, acute lung injury). Assist with either PPV or NPV was titrated to reduce Edi by 50%.
Results
In order to achieve a 50% reduction in Edi, NPV required slightly more negative pressure (−8 to −12 cm H2O) than observed in PPV (+6 to +10 cm H2O). The efficiency of pressure transmission from the NeoVest into gastric pressure was 69.6% (range 61.3–77.4%). Swings in esophageal pressure were more negative during NPV than PPV, for all conditions, due to transmission of negative pressure. Transpulmonary pressure was lower during NPV. Transdiaphragmatic pressure swings were reduced similarly for PPV and NPV, suggesting equivalent unloading of the diaphragm. NPV did not affect hemodynamics.
Conclusions
It is feasible to apply NPV sub-diaphragmatically in synchrony and in proportion to Edi in an animal model of respiratory distress.
Impact
-
Negative pressure ventilation (NPV), for example, the “Iron Lung,” may offer advantages over positive pressure ventilation.
-
In the present work, we describe the “NeoVest,” a system consisting of a sealed abdominal interface and a ventilator that applies NPV in synchrony and in proportion to the diaphragm electrical activity (Edi).
Similar content being viewed by others
Introduction
In premature infants, application of negative pressure ventilation (NPV) may offer an advantage to traditional non-invasive positive pressure ventilation (PPV) and may avoid such side effects as nasal skin breakdown.1 Negative pressure ventilators were used widely in infants during the polio epidemics from the 1930s to 1970s.2 The underlying principle about these “iron lungs” is that negative pressure applied outside of the thorax and abdomen (like a vacuum) leads to expansion of the lungs and drawing of air into them. However, these devices are cumbersome and are not synchronized to patient’s breathing, thus ineffective compared to traditional PPV devices.2
We have recently shown that it is feasible to apply synchronized NPV in a very small animal model (~400 g) with respiratory distress.3 In rodents placed in a sealed “body box,” the diaphragm electrical activity (Edi) was successfully used to control the timing and amount of negative pressure application, and the physiological responses were similar to those observed during Edi-controlled PPV.3 Despite the potential for improved synchrony and increased comfort, synchronized whole-body NPV in a “body box” does not allow easy physical access to the entire patient. In addition, preterm infants are mainly abdominal breathers and may not need application of negative pressure to the entire chest wall. In fact, chest wall distortion may occur in preterm infants when they breathe in (inward movement of thorax with outward movement of abdomen up to 180 degrees out-of-phase) and therefore, tidal volume is reduced, and the diaphragm needs to descend further with every breath to compensate for the lost volume. Under these conditions, diaphragmatic work increases which could lead to diaphragmatic fatigue and respiratory failure.4,5
We hypothesized that it would be sufficient to use an abdominal interface alone to apply synchronized and proportional NPV, allowing more specific unloading of the diaphragm. We sought to assess synchronized sub-diaphragmatic unloading using NPV compared to synchronized PPV delivered by the neurally adjusted ventilatory assist (NAVA) mode in rabbits undergoing different loaded-breathing conditions.
Methods
The study was approved by St. Michael’s Hospital Animal Care and Use Committee (ACC585). Care and handling of the animals were performed according to the Canadian Council on Animal Care.
Experimental set-up
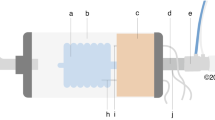
Eleven adult male New Zealand white rabbits (Charles River Labs, St Constant, Quebec, Canada) with a mean body weight of 2.9 kg (range 2.7–3.2) were studied. A schematic of the experimental set-up is provided in Fig. 1a. Animals were initially anesthetized by an intramuscular bolus of ketamine hydrochloride (35 mg/Kg) and xylazine (10 mg/Kg), followed by continuous intravenous infusion of ketamine hydrochloride (10 ml/Kg per hour), xylazine (2 mg/Kg per hour). Lactated Ringer’s solution (5 mL/Kg per hour) was continuously infused intravenously with a volumetric infusion pump. Arterial blood pressure (Pd 23, Gould Inc. Cleveland, OH) and arterial blood gas measurements (RADIOMETER ABL800 FLEX, Mississaugua, Canada) were obtained from an ear artery with an indwelling arterial line. Transcutaneous oxygen saturation was measured with pulse oximetry (NONIN 8600 VTM, Nonin Medical Inc., Plymouth, MN) at the tail. Edi was measured with sensors placed on an 8 F oro-gastric catheter, with a balloon mounted for measurement of esophageal pressure (Pes) and gastric balloon for gastric pressure (Pga) (Neurovent Research Inc. Toronto, Canada). Proper positioning of the catheter was confirmed using a dedicated window on a Servo-i ventilator (Maquet, Sweden), as well as inspiratory occlusion maneuvers.A tracheotomy was performed, and an endotracheal (ET) tube (size 4.0) was inserted, as previously described.6
a Experimental set-up for both positive pressure ventilation (PPV) delivered by endotracheal tube and negative pressure ventilation (NPV) applied to the abdomen via a sealed Neovest (gray shaded box). Note ET tube in place but open to atmosphere during NPV. Electrical activity of the diaphragm (Edi) was measured with an array of sensors placed on an oro-gastric tube containing esophageal and gastric balloons (Pes and Pga). The processed Edi waveform was used to control the positive pressure delivery during PPV, like “conventional” NAVA (Edi = triggered and Edi-cycled off). Or, during NPV, the Edi was used to control the application of negative pressure in the sealed Neovest, using continuous NAVA,6 i.e., no triggering or cycling-off criteria. During both PPV and NPV, the NAVA level was used to determine the level of assist (NAVA level = cm H2O/μV). b Example of experimental data (raw tracings) from one individual subject breathing at baseline (BL, far left), with negative pressure ventilation (NPV, middle) and positive pressure ventilation (PPV, right). Gray vertical shadowed-bars demonstrate the synchrony between the diaphragm electrical activity (Edi) and pressure applied (Pvent or Pvest), and represents neural inspiration. The arrows indicate the proportionality increase in pressure that is applied when the Edi breath is larger (green arrows for NPV, red arrows for PPV). See “Methods” for details on both modes.
Method for PPV
The Servo-I ventilator was used in the NAVA mode and connected to the endo-tracheal tube. During PPV (i.e., the NAVA mode), the Edi waveform is used to trigger on and cycle-off the ventilator, but also controls proportional assist on inspiration. Triggering occurs when the Edi exceeds a threshold increment in Edi (0.5 μV in the present study). After triggering, pressure is delivered in proportion to the Edi throughout inspiration (the proportionality factor between Edi and pressure is the “NAVA level,” in cm H2O per μV, sometimes referred to as “NAVA gain” in the literature). The breath is cycled-off when the Edi drops to 70% of peak, to a user-defined arbitrary positive end-expiratory pressure (PEEP; 0 cm H2O in the present study) during neural expiration.
Method for NPV
A custom-made negative pressure ventilator was used. Using the Edi signal, the NeoVest ventilator system applies negative pressure, that is both synchronized and proportional to the Edi. The applied negative pressure is continuous, meaning there is no triggering to define inspiration and expiration, and can be thought of as a variable CPAP, but with negative pressure.6,7 More specifically, the processed Edi signal was acquired from the Servoi Edi module and multiplied by the NAVA level to generate a target negative pressure. This target value is converted to a voltage and sent to the stand-alone, custom-made negative pressure ventilator, which continuously adjusts the negative pressure inside the sealed NeoVest (see below) to match the “target pressure.” If tonic Edi is present, the negative end-expiratory pressure (NEEP) is also “neurally” adjusted. Details about continuous NAVA and neurally adjusted PEEP have been described previously in rodents,3 rabbits,6,7 and preterm neonates.8
The “NeoVest” interface was applied around the abdomen (Figs. 1a and E1A). The NeoVest is a stiff wearable device, designed as two interlocking parts, made from 3D-printed PLA (polylactic acid) plastic with a dorsal viewing window fashioned from 6 mm acrylic sheet. Standard respiratory circuit and tubing connectors (the latter serving as a sensor port) were incorporated into the window. Once placed over the abdomen, the interface was wrapped with non-stick plastic film and secured and sealed by elastic bands. NPV was applied at the center top (10 mm OD connector) and measured/monitored via a luer placed next to the connector.
Ventilator pressure was measured at the y-piece (Pvent) for PPV, and inside the NeoVest interface (Pvest) for NPV. The endo-tracheal tube remained in situ during NPV, but open to atmosphere. For both PPV and NPV runs, additional loads were added to the ET tube: either a resistor (RES) or an added dead space (DS) (Fig. E1B). See below for more details. Oxygen was supplied as usual during PPV (i.e., from ventilator) at 50%, whereas during NPV oxygen was supplied at 100% with a face mask at the ET tube opening.
Experimental protocol
Before and after acute lung injury (Pre and Post ALI (see below for details)), the load (RES or DS) was initially implemented until the Edi had reached ~10 μV; then animals were ventilated with either PPV or NPV, with the NAVA level titrated until the Edi was reduced by 50%. Animals were ventilated at the titrated NAVA level for 10 min, under the following non-randomized order of conditions:
-
1.
Resistive load on with no assist for baseline measures (BL RES)
-
2.
Negative pressure ventilation with added resistive load (NPV RES)
-
3.
Positive pressure ventilation with added resistive load (PPV RES)
-
4.
Dead space on with no assist for baseline measures (BL DS)
-
5.
Negative pressure ventilation with added dead space (NPV DS)
-
6.
Positive pressure ventilation with added dead space (PPV DS)
-
7.
No load and no assist after acute lung injury (BL ALI)
-
8.
Negative pressure ventilation after acute lung injury (NPV ALI)
-
9.
Positive pressure ventilation after acute lung injury (PPV ALI)
-
10.
Resistive load on with no assist after acute lung injury (BL ALI RES)
-
11.
Negative pressure ventilation after acute lung injury with added resistive load (NPV ALI RES)
-
12.
Positive pressure ventilation after acute lung injury with added resistive load (PPV ALI RES)
-
13.
Dead space on with no assist after acute lung injury (BL ALI DS)
-
14.
Negative pressure ventilation after acute lung injury with added dead space (NPV ALI DS)
-
15.
Positive pressure ventilation after acute lung injury with added dead space (PPV ALI DS)
Eleven animals were studied before ALI, and eight after ALI (three animals passed away during the ALI). An arterial blood gas was obtained at the end of each 10-min period.
Description of respiratory loads
Resistive breathing (RES)
Animal was breathing through a resistor inserted at the ET tube in order to increase respiratory drive to 10 μV, with supplemental O2.
Dead space (DS)
Animal was breathing with an additional physical (instrumental) dead space (no resistive load) in order to increase respiratory drive to 10 μV.
Acute lung injury (ALI)
ALI was induced by instilling 1.0 ml/kg of hydrochloric acid, adjusted to pH 1.5, into each lung with the animal in the lateral position using a cannula passed to the tracheal bifurcation through the ET tube, during neuro-muscular paralysis (Pancuronium, 0.03 mg/kg). The Servo-I ventilator was used to deliver 6 ml/kg at a rate of 40 breaths/min and zero PEEP (at the tracheotomy) in the volume control mode, until Edi recovered from the paralysis. Once recovered, the animal was ventilated without assist (as described in step 7), and the protocol continued.
Data analysis
Off-line breath-by-breath analysis was performed on the acquired data for Edi, Pes, Pga, flow, volume, and pressure waveforms for both PPV and NPV periods. Pdi was calculated from a mathematically constructed waveform of Pga–Pes. Transpulmonary pressure (PL) was calculated as mean inspiratory Pvent − mean inspiratory Pes. Note for NPV, the mean inspiratory Pvent is zero cm H2O since the airway is open to atmosphere. The last 3 min of each condition was used in the analysis, and averages (SD) were obtained, and reported.
Statistical analysis
The statistics were designed to compare BL, NPV, and PPV. A two-way repeated-measures analysis of variance was used with Student–Newman–Keuls method for all pairwise multiple comparison procedures (SigmaPlot v.12.0). The Pre-ALI conditions (n = 11) were analyzed separately from the Post-ALI conditions (n = 8) because 3 animals were lost due to the lung injury procedure. There was no intention to statistically compare the impact of the independent loads. Statistics for applied pressures were performed on absolute values (i.e., NPV pressures were turned positive for statistical comparisons).
Results
During both Pre- and Post-ALI, the added DS and RES loads increased respiratory drive up to ~10 μV (Fig. 2a, blue bars). The average resistance applied was 100 cm H2O/l/s before lung injury and 58 cm H2O/l/s after lung injury. The average instrumental dead space volume was 26 ml before ALI and 25 ml post-ALI. In the 8 animals surviving the ALI procedure, the mean reduction in respiratory system compliance was 44% (p < 0.001) and the mean P/F ratio was 84.4, 56.9, and 72.6 after 1, 5 and 15 min, respectively (all significantly lower than Pre-ALI, p < 0.001).
a Evidence that delta Edi (y axis) was reduced by 50% during both NPV (green bars) and PPV (red bars), compared to baseline (BL, blue bars). Shown for both before acute lung injury (PRE-ALI, left) and after acute lung injury (POST-ALI, right). *Significantly different from baseline. b Swings in Pes are demonstrated for loaded baseline (BL) conditions (blue bars), and after application of NPV (green bars), or PPV (red bars). Pes swings were consistently less with PPV than NPV. This is not to be interpreted as less work of breathing or less inspiratory effort because the more negative Pes during NPV is due to the -ve pressure applied. #Significant for NPV or PPV vs. BL. *Significant for NPV vs. PPV.
Figure 1b demonstrates that both NPV and PPV were synchronized and proportional to the Edi signal (example taken from one subject breathing during the RES condition PRE_ALI). During PPV, synchrony is possible to quantify by comparing the onset of Edi to the onset of positive pressure. During PPV, the average trigger delay was 32 ms (SD 13 ms). Note that during NPV, the NeoVest (negative) pressure matches the Edi continuously with very little time difference. Because there are no triggers during NPV, it is not possible to report trigger delays. For visual appreciation however, we have indicated the inspiratory time with vertical gray bars in Fig. 1b, and arrows to demonstrate proportionality.
Our goal was to apply NPV or PPV in order to standardize the reduction in Edi for all conditions by 50%. This was achieved as per protocol (Fig. 2a), where the average Edi decrease was 52 ± 4% for all conditions and modes. In order to achieve the reduction in Edi by 50%, the NAVA gain levels that were used in NPV vs. PPV are shown in Tables 1 and 2, for each condition. Consistently, the NAVA gain levels required for NPV ventilation were higher than for PPV (the largest difference being e.g., 0.8 cm H2O/μV during ALI alone), in order to achieve the 50% reduction in Edi from baseline (Fig. 2, green and red bars). The peak Δ pressures (Δ pressure = pressure swing above/below PEEP and NEEP) required to achieve the 50% reduction are presented in Fig. 3 and demonstrate that negative pressure swings applied to the abdomen needed to be greater than PPV, during the resistive loading condition. During dead space loading and after ALI, the same negative pressure application was required. Of note, the range of negative pressure required to reduce Edi by 50% ranged between −8 and −12 cm H2O (Fig. 3, green bars). In Fig. 3, the values applied during NPV are presented as absolute values for better comparison. The mean inspiratory transpulmonary pressure (delta PL) was statistically higher for PPV than for NPV.
a Delta applied pressures required to reduce diaphragm electrical activity by 50%. Of the 5 conditions studied, the two resistive (RES) conditions required more negative pressure application (green bars) than PPV (red bars). Note that the negative pressure has been “flipped” to positive values to allow for comparisons. For the remaining conditions, there were no significant differences between applied pressures. #Significantly different from baseline. b Mean inspiratory transpulmonary pressure was significantly higher for PPV (red bars) than NPV (green bars) for all conditions. *PPV vs. NPV significantly different.
Esophageal pressure swings (Fig. 2b) during NPV (green bars) were consistently more negative than during PPV (red bars), for all conditions. This was expected due to the sum of the pressure from the inspiratory muscles and the transmitted negative pressure during NPV. During PPV, compared to baseline (blue bars), Pes swings were reduced. The mean inspiratory Pes was close to 0 cm H2O (albeit always negative) during PPV, and hence, the mean inspiratory transpulmonary pressure (PLmean) was mainly caused by the positive pressure delivered by the ventilator. Note that the negative Pes swings observed during NPV were not statistically different from the spontaneous, negative Pes swings the subjects generated on their own (blue bars). Tidal volume was higher during PPV (by 4–5 ml) compared to NPV during DS conditions, but otherwise not significant (Table 1).
Pga swings (Fig. 4) were negligible during BL and PPV (<1 cm H2O) but were affected by the NPV applied to the abdomen during NPV (greatest Pga swing observed was -9.7 cm H2O during the RES load before ALI). The transmission of applied NPV pressure to Pga is demonstrated in Fig. 5. The efficiency of the PVest pressure being transmitted to the abdomen was 69.6% (range 61.3% during ALI DS to 77.4% during RES).
Mean values +/− SD are plotted for pressure in the NeoVest (Pvest, x axis), vs. gastric pressure in abdomen (Pga, y axis), for the different loaded conditions. The slope of the line is described as y = 0.9787x + 2.8428, with R2 of 0.97. (Blue = ALI RES; Red = RES; Green = ALI DS; Gray = DS; Orange = ALI). The mean loss in pressure from NeoVest to gastric is 30.4 ± 6.6%.
Transdiaphragmatic pressure (Pdi) swings were reduced similarly for PPV and NPV, compared to BL, suggesting equivalent unloading of the diaphragm for the two modes (Fig. 6). The percentage reduction in Pdi ranged from 50 to 65%, slightly higher than the reduction in Edi (52%).
Tables 1 and 2 provides a summary of the remaining variables analyzed for each of the runs (p values provided for NPV vs. PPV). There were no significant differences in Tonic Edi across all conditions. Before ALI, PEEP during PPV was set to 0 cm H2O (PEEP is fixed during PPV), and after ALI was set on average to 5.9 (2.6) cm H2O. NEEP during NPV was neurally adjusted and significantly higher than PPV for RES and DS before lung injury, but not after ALI. Neural respiratory rate was not different between NPV and PPV for any condition. Minute ventilation was significantly higher during PPV during the DS conditions only. Mean inspiratory flow was always higher during PPV compared to NPV.
Application of NPV did not affect BP (continuously measured), HR, PCO2, or Ph. It is difficult to interpret the FIO2 values since 100% O2 was applied with a leaky interface during NPV.
Discussion
This is the first study to show that it possible to provide sub-diaphragmatic unloading, which is synchronized and proportional to the Edi, during different conditions of respiratory distress. It should be clarified that the interface and ventilator used in the present study were designed to specifically apply intermittent (fluctuating) negative pressure below the diaphragm, different from previous studies applying continuous (constant) negative pressure to both the thorax and the abdomen,9,10,11 or the abdomen alone.12
The concept of applying respiratory support in the form of NPV is not new, but this is the first report of synchronized (intermittent) NPV, with an interface that is sub-diaphragmatic. In humans, a variety of pumps13 and interfaces have been described for children ranging from whole-body iron lungs14 to cuirass devices that enclose the entire chest wall15 or the Air Shields isolette.16 A recent review17 about the use of NPV in pediatrics reported that negative pressure devices were mainly used to apply continuous negative expiratory pressure (“CNEP”), with pressures ranging between −10 and −14 cm H2O.17 Our system is not to be confused with the “intermittent abdominal pressure ventilator” (which increases tidal volume through the raising of the diaphragm with positive pressure, and the diaphragm’s passive descent, sometimes known as a “pneumobelt”).13 Very few studies describe intermittent NPV delivered to assist inspiration, and even fewer have attempted to provide synchronized and proportional NPV. We recently demonstrated in a very small animal model the feasibility of applying chest wall NPV (animal in a sealed “body box”) and that the physiologic responses to changing synchronized NPV assist levels were similar to PPV.3 In the current study, we demonstrate that by only applying intermittent negative pressure to the abdomen, we can unload the diaphragm on inspiration, as evidenced by the reduced Edi and Pdi (by ~50%). This could occur almost as efficiently as traditional PPV with NAVA, where NPV was “missing” a small amount of pressure and required at the most −3 cm H2O extra for the RES condition after ALI.
Our NeoVest interface was designed to apply unloading of the abdomen below the diaphragm, although the negative pressure could still be transmitted somewhat to the Pes balloon, as demonstrated in Fig. 2b, where we observed more-or-less similar ΔPes for BL and NPV conditions. In earlier pilot work with the same model, we observed changes in the baseline of the Pes tracing as we introduced more and more negative values of constant NEEP (data not shown). In the present study, we did not measure the displacements of the rib cage or abdomen with Respiratory Inductance Plethysmography, and therefore we are not able to determine if more inward “paradoxing” occurred for the rib cage during NPV. This would be an important consideration for some human preterm infants, who can demonstrate significant paradoxing and inward movement of the rib cage during inspiration due to increased chest wall compliance. In that case, one solution would be to apply synchronized positive pressure at the airways simultaneously with the negative pressure,3 which could be done invasively or non-invasively with nasal prongs, thereby stabilizing the chest wall during inspiration.
The data from the present study suggest we are both unloading and ventilating during NPV. PCO2 was similar during PPV and NPV, suggesting ventilation was similar between the 2 types of pressure applications. Tidal volume was only higher during PPV during the DS conditions. Respiratory rate was not affected by NPV or PPV in any of the conditions. Mean inspiratory flow was higher with PPV, likely because it is the patient flow and ventilator flow combined, whereas during NPV flow is delivered by the action of the respiratory muscles and the negative pressure applied on the abdomen (no flow is being delivered at the trachea from an external source), and there is no bias flow from the Servo-ventilator. With NPV there is no additional help to increase volume, however, it likely facilitates diaphragm unloading. On the same topic, we observed a greater reduction in relative Pdi than the reduction in Edi, suggesting slight uncoupling of the Edi-Pdi relationship, a further indication that we are unloading the diaphragm (normally a 1:1 relation). The mechanisms of this uncoupling can only be speculated upon, however, during PPV it may be due to increased flow (and rapid shortening of diaphragm for same Edi), and during NPV, it may be due to the load removal for the same activity.
There are several potential mechanisms by which the NeoVest unloads the diaphragm. First, the “normal” abdominal load against which the diaphragm would contract is reduced by the applied negative pressure. Less load to contract against implies less force-generating ability. In fact, D’angelo18 have demonstrated in rabbits switched from supine to upright position (removing gravitational load and hence abdominal pressure) that supramaximal twitch pressure is reduced. Second, Yoshida et al.12 demonstrated more improved recruitability of the dorsal (dependent) lung units when continuous abdominal pressure was applied, suggesting less pressure required to distend the lungs, and more homogeneity. Previously, investigators have demonstrated that with positive pressure NAVA, there is preferential recruitment of the dependent regions of the lung (during synchronized and proportional assist) compared with standard pressure support ventilation.19 Therefore, perhaps the unloading we observed is due to an “easier” lung recruitment caused by the simultaneous action of the synchrony and proportionality combined with the negative pressure application.
The mean negative pressure swings applied in the NeoVest never exceeded −13 cm H2O (observed after ALI with RES) and are within the pressures that have been applied during previous studies in infants. Note that the efficiency of transmission from the NeoVest to the gastric compartment was roughly 70% (range 61–77%, depending on the condition). In adults, Levy et al.20 used negative pressures up to −30 cm H2O to the chest wall (including upper abdomen) using a whole-body interface (“pneumosuit”) to evaluate apnea in COPD patients during sleep studies. Papers describing CNEP have reported values in the range of −4 to −15 cm H2O without evidence of changes in BP, except for one study after 2 h CNEP vs CPAP the BP decreased,21 although it is difficult to compare studies due to differences in patient population, and interfaces. In the present pre-clinical study, blood pressure was not significantly different between NPV and PPV, similar to other investigators’ results in a piglet model of lung injury.22 It should be kept in mind that NPV may be advantageous in some physiological states, by improving central venous return and reducing right ventricular afterload (in part by reducing pulmonary hypertension)23,24; however, since our interface is placed abdominally, and is not entirely extra-thoracic, it is not possible to compare these concepts, and a future study with detailed hemodynamic measurements would be useful.
One of the limitations about our study is the fact that we used no PEEP during the PPV conditions.. During NPV, we used continuous NAVA, which could offer “neurally adjusted negative PEEP,” which in theory could affect the breathing pattern, inspiratory drive, and hemodynamics.6 The largest amount of NEEP observed was ~−4 cm H2O during the ALI condition. The amount of tonic Edi and the neural respiratory rate did not seem to be affected by the different modes (Table 1). In rabbits ventilated with continuous-NAVA vs. triggered-NAVA, we previously demonstrated respiratory muscle unloading, a reduction of inspiratory effort, and a preservation of end-expiratory lung volume, with minimal over-distension.6,7 Regardless of continuous or triggered-NAVA, in the present study, we standardized the application of pressure during both NPV and PPV by doing a titration to the point of 50% reduction in Edi. In both modes, we were able to match this amount of de-activation from baseline. Notably PPV via ET tube was more efficient as indicated by the slightly less positive pressures required to unload the diaphragm. However, both modes were efficient in unloading the diaphragm as the relative changes in Pdi swings were always more than the relative changes in the Edi.
Regarding our model, in the present study, it was not possible to compare oxygenation between NPV and PPV since the O2 was delivered through a leaky interface during NPV. Previous reports in infants have demonstrated improved oxygenation during NPV (CNEP),25,26 but the present study cannot draw any conclusions. The fact that we had tracheal intubation during the entire study (open to air/O2 during NPV and connected to ventilator during PPV) also implies that the upper airways were not free to participate in any regulation of end-expiratory lung volume. However, the tube was present for both modes of ventilation and presumably influenced our results similarly. Similar to our conclusion of paradoxing, we cannot verify whether or not application of NPV resulted in upper airway closure/collapse (because the endo-tracheal tube was in place). To resolve this in a true non-invasive situation, one could apply constant nasal positive pressure either by nasal CPAP, or high flow nasal cannula to maintain upper airway patency.
Lastly, we cannot ignore the fact that our model is an anesthetized (not moving), yet spontaneously breathing rabbit with quite a regular breathing pattern. Whether or not application of the NeoVest in an awake infant (where the breathing pattern is more variable and the neural response to sub-diaphragmatic unloading may be different) provides the same results remains to be investigated.
Conclusion
This is the first study to demonstrate the feasibility of using the Edi to control and apply synchronized and proportional NPV using an abdominal interface. In our pre-clinical model, we showed the ability of NPV to downregulate Edi, and to unload the diaphragm, similarly to PPV in the NAVA mode.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Newnam, K. M. et al. An integrative review of skin breakdown in the preterm infant associated with nasal continuous positive airway pressure. J. Obstet. Gynecol. Neonatal Nurs. 42, 508–516 (2013).
Thomson, A. The role of negative pressure ventilation. Arch. Dis. Child. 77, 454–458 (1997).
Takahashi, D., Liu, L., Sinderby, C. & Beck, J. Feasibility of neurally synchronized and proportional negative pressure ventilation in a small animal model. Physiol. Rep. 8, e14499 (2020).
Heldt, G. P. & McIlroy, M. B. Distortion of chest wall and work of diaphragm in preterm infants. J. Appl. Physiol. 62, 164–169 (1987).
Guslits, B. G., Gaston, S. E., Bryan, M. H., England, S. J. & Bryan, A. C. Diaphragmatic work of breathing in premature human infants. J. Appl. Physiol. 62, 1410–1415 (1987).
Liu, L. et al. Feasibility of neurally adjusted positive end-expiratory pressure in rabbits with early experimental lung injury. BMC Anesthesiol. 15, 124 (2015).
Brander, L. et al. Neural control of ventilation prevents both over-distension and de-recruitment of experimentally injured lungs. Respir. Physiol. Neurobiol. 237, 57–67 (2017).
Rochon, M. E. et al. Continuous neurally adjusted ventilation: a feasibility study in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 105, 640–645 (2020).
Chernick, V. & Vidyasagar, D. Continuous negative chest wall pressure in hyaline membrane disease: one year experience. Pediatrics 49, 753–760 (1972).
Bancalari, E., Gerhardt, T. & Monkus, E. Simple device for producing continuous negative pressure in infants with IRDS. Pediatrics 52, 128–131 (1973).
Fanaroff, A., Cha, C., Sosa, R., Crumrine, R. S. & Klaus, M. H. Controlled trial of continuous negative external pressure in the treatment of severe respiratory distress syndrome. J. Pediatr. 82, 921–928 (1973).
Yoshida, T. et al. Continuous negative abdominal pressure recruits lungs at lower distending pressures. Am. J. Respir. Crit. Care Med 197, 534–537 (2018).
Smith, I. E., King, M. A. & Shneerson, J. M. Choosing a negative pressure ventilation pump: are there any important differences? Eur. Respir. J. 8, 1792–1795 (1995).
Stahlman, M. T. et al. Negative pressure assisted ventilation in infants with hyaline membrane disease. J. Pediatr. 76, 174–182 (1970).
Linton, D. M. Cuirass ventilation: a review and update. Crit. Care Resusc. 7, 22–28 (2005).
Stern, L., Ramos, A. D., Outerbridge, E. W. & Beaudry, P. H. Negative pressure artificial respiration: use in treatment of respiratory failure of the newborn. Can. Med. Assoc. J. 102, 595–601 (1970).
Hassinger, A. B., Breuer, R. K., Nutty, K., Ma, C. X. & Al Ibrahim, O. S. Negative-pressure ventilation in pediatric acute respiratory failure. Respir. Care 62, 1540–1549 (2017).
D’Angelo, E., Pecchiari, M., Acocella, F., Monaco, A. & Bellemare, F. Effects of abdominal distension on breathing pattern and respiratory mechanics in rabbits. Respir. Physiol. Neurobiol. 130, 293–304 (2002).
Blankman, P., Hasan, D., van Mourik, M. S. & Gommers, D. Ventilation distribution measured with EIT at varying levels of pressure support and neurally adjusted ventilatory assist in patients with ALI. Intensive Care Med. 39, 1057–1062 (2013).
Levy, R. D., Bradley, T. D., Newman, S. L., Macklem, P. T. & Martin, J. G. Negative pressure ventilation. Effects on ventilation during sleep in normal subjects. Chest 95, 95–99 (1989).
Alexander, G., Gerhardt, T. & Bancalari, E. Hyaline membrane disease. Comparison of continuous negative pressure and nasal positive airway pressure in its treatment. Am. J. Dis. Child 133, 1156–1159 (1979).
Easa, D., Mundie, T. G., Finn, K. C., Hashiro, G. & Balaraman, V. Continuous negative extrathoracic pressure versus positive end-expiratory pressure in piglets after saline lung lavage. Pediatr. Pulmonol. 17, 161–168 (1994).
Mahmood, S. S. & Pinsky, M. R. Heart-lung interactions during mechanical ventilation: the basics. Ann. Transl. Med. 6, 349 (2018).
Shekerdemian, L. & Bohn, D. Cardiovascular effects of mechanical ventilation. Arch. Dis. Child. 80, 475–480 (1999).
Bancalari, E., Garcia, O. L. & Jesse, M. J. Effects of continuous negative pressure on lung mechanics in idiopathic respiratory distress syndrome. Pediatrics 51, 485–493 (1973).
Cvetnic, W. G., Cunningham, M. D., Sills, J. H. & Gluck, L. Reintroduction of continuous negative pressure ventilation in neonates: two-year experience. Pediatr. Pulmonol. 8, 245–253 (1990).
Acknowledgements
The authors wish to thank M. Norman Comtois for his help with the analysis software and M. Greg Phillips for his work on the 3D printing of the NeoVest.
Funding
The study was supported by the St. Michael’s Hospital Foundation (Angels Den Award), The Global Health Innovation Award, and the RS McLaughlin Fund.
Author information
Authors and Affiliations
Contributions
J.B. and C.S. came up with the concept and designed the experimental protocol; J.B. analyzed the data, interpreted the data, and prepared the manuscript; H.-L.L. and C.L. carried out the experiments. C.S., D.M.C., H.L., and C.L. revised the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.B. and C.S. have made inventions related to neural control of mechanical ventilation that are patented. The patents are assigned to the academic institution(s) where inventions were made. The license for these patents belongs to Maquet Critical Care. Future commercial uses of this technology may provide financial benefit to D.B. and C.S. through royalties. D.B. and C.S. each own 50% of Neurovent Research Inc. (NVR). NVR is a research and development company that builds the equipment and catheters for research studies. NVR has a consulting agreement with Maquet Critical Care. St. Michael’s Hospital has a research agreement with Maquet Critical Care AB (Solna, Sweden) and receives royalty and overhead from this agreement. The remaining authors have no competing interests to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Beck, J., Li, HL., Lu, C. et al. Synchronized and proportional sub-diaphragmatic unloading in an animal model of respiratory distress. Pediatr Res 93, 878–886 (2023). https://doi.org/10.1038/s41390-022-02238-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02238-x