Abstract
Background
The role of left ventricular (LV) diastolic pressure in the pathophysiology of bronchopulmonary dysplasia (BPD) is unclear. We evaluated the trajectory of echocardiographic parameters of LV diastolic function and the association with respiratory outcomes in preterm infants.
Methods
We retrospectively analysed measurements of LV diastolic function (E, e’, A, Ee’ and E/A ratios) in infants below 32 weeks’ gestation (GA). We compared infants with and without BPD by two-way RM ANOVA. We considered Ee’ ratio as a proxy of LV filling pressure and identified a cut-off value using ROC analysis. We divided infants using such threshold and compared respiratory outcomes between groups by Mann–Whitney or Chi-square tests.
Results
We included 72 infants. Ee’ ratio at 28 days was significantly associated with the duration of respiratory support (beta (std. error) = 5.32 (1.82), p = 0.005) and BPD (beta = 0.27 (0.10), p = 0.008). Infants with Ee’ ratio > 12 at 28 days had longer respiratory support, oxygen requirement, and higher BPD rates than infants with Ee’ ratio ≤ 12.
Conclusion
LV diastolic function associated with elevated LV filling pressure may contribute to the pathophysiology of BPD. Serial echocardiographic measurements could identify infants at risk of worse respiratory outcomes.
Impact
-
In very preterm infants, we assessed the trajectory of left ventricular diastolic function by serial echocardiographic evaluations and evaluated its association with respiratory outcomes.
-
On average, infants who developed bronchopulmonary dysplasia had higher Ee’ at 28 postnatal days and 36 weeks postmentrual age than infants who did not develop the disease.
-
Infants with elevated Ee’ at 28 postnatal days, suggestive of elevated left atrial pressure, required longer respiratory support.
Similar content being viewed by others
Introduction
Bronchopulmonary dysplasia (BPD) represents a continuum of different diseases, including airway obstruction, alveolar and pulmonary vascular underdevelopment. The appraisal of the relative contribution of BPD traits could guide therapeutic interventions. Elevated pulmonary vascular resistance and right ventricular dysfunction are well-recognised contributors to pulmonary vascular disease.1,2,3 By contrast, the contribution of the systemic circulation, left heart function and pulmonary venous pressure to the pathophysiology of BPD is less well appreciated and understood.
A few investigators have recently focused on the contribution of left-sided cardiac and vascular changes to BPD pathophysiology and treatment.4,5,6 Mourani et al. first reported that left ventricular (LV) diastolic dysfunction contributes to the pathophysiology of severe BPD and may play a significant role in disease morbidity.7 In BPD complicated by LV diastolic dysfunction, conventional pulmonary vasodilators (e.g., iNO and sildenafil) may be ineffective or contraindicated. By contrast, afterload reduction agents may result in a significant hemodynamic and clinical improvement in these patients.
In the cases reported by Mourani and colleagues, cardiac catheterisation identified elevated postcapillary pulmonary hypertension. Echocardiographic techniques, including Doppler tissue imaging, allow for the non-invasive assessment of LV diastolic function. Mitral E/A and Ee′ ratios proved to be sensitive indicators of impaired LV diastolic function in BPD infants.8 Increased mitral E-wave deceleration time (EDT) and E/A ratio, isovolumetric relaxation time, and LV myocardial performance index were also reported in BPD patients.5 Even though echocardiography is a non-invasive bedside tool that might provide useful insights regarding the LV diastolic function, limited data exist about echocardiographic parameters of LV diastolic function in preterm infants and their relationship with lung disease.
The mitral Ee’ ratio, in particular, is a highly feasible and reproducible parameter for estimating LV filling pressure.9,10 Therefore, we hypothesised that Ee’ ratio could be a key parameter for evaluating the contribution of LV diastolic dysfunction to the pathophysiology of BPD. Indeed, elevated mitral Ee’ ratio values are consistent with elevated LV filling pressure and postcapillary pathology, which may lead to pulmonary venous congestion and pulmonary oedema, affecting compliance and prolonging the need for respiratory support. The present study aims to (1) describe the trajectory of echocardiographic parameters of LV filling pressure in very preterm infants and (2) evaluate the relationship between such parameters and respiratory morbidity.
Methods
Study design
This study was conducted in the tertiary neonatal intensive care unit of Fondazione Monza e Brianza per il Bambino e la sua Mamma Onlus. In our unit, we routinely perform serial echocardiographic screening in all infants born <32 weeks gestation at 7 (±1), 14 (±1) and 28 (±2) postnatal days and 36 (±1) weeks postmenstrual age (PMA). The current study is a retrospective analysis of the left heart diastolic function data collected between January 2020 and October 2021. The local ethical committee approved the study (protocol no. 3804, September 2021).
Subjects
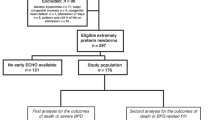
We included in the analysis infants born <32 weeks gestation without genetic disorders, congenital malformations, or incomplete data.
Echocardiography
Detailed echocardiography was performed with the Philips Affiniti 70 system using 4–2-MHz probe. We performed pulse wave Doppler measurements of ventricular inflow through the mitral valve and measured the peak of the early (E) and late diastole (A) waves, and calculated the E/A ratio.11 We assessed tissue Doppler velocities in an apical four-chamber view, measured the early peak diastolic velocity (e’) at the medial and lateral annulus of the mitral valve, and calculated the Ee′ ratio.12 Pulse wave doppler measurements were calculated from the average of two cardiac cycles. We focused our analysis on the measurements obtained on the lateral side of the mitral valve because the medial side might be affected by the right heart function, particularly in early life. We also calculated the average between the measurements obtained on the two sides, as suggested in consensus recommendations for the adult population.13 We also evaluated LV systolic function, pulmonary hypertension (PH), and the patent ductus arteriosus (PDA) to rule out possible factors that might affect the interpretation of LV diastolic function parameters. We considered LV systolic function normal if the fractional shortening was higher than 35 %. We classified a PDA as hemodynamically significant when the internal diameter was >1.5 mm, the flow pattern was pulsatile,14 and one of the following conditions was present: (i) end-diastolic blood flow velocity in the left pulmonary artery >20 cm/s, (ii) systemic hypoperfusion, or (iii) diastolic flow reversal in the descending aorta.
Clinical data and outcomes
Demographics and clinical data were extracted from the electronic medical records. The primary outcome was the difference in Ee’ ratio at different time points between infants with vs. without BPD; secondary outcomes were the associations between Ee’ ratio and the duration of respiratory support and oxygen requirement. We defined BPD as oxygen dependency for >28 days, according to the 2001 National Institute of Health (NIH) definition.15 To evaluate the effect of LV diastolic function on respiratory outcomes regardless of gestational age (GA), we stratified our population into infants below and above 28 weeks gestation.
Statistical analysis
Aiming for a 95% power at the 5% significance level, we calculated a minimum required sample size of 50 to compare LV diastolic function in infants with and without BPD, using two-way ANOVA with four repeated measurements assuming an effect size of 0.25 and a correlation between repeated measurements of 0.2.5,16 We assessed the significance of differences between infants with and without BPD using two-way ANOVA for repeated measurements, with BPD classification and time as factors and Holm-Sidak as correction method.
We assessed the association between LV diastolic function parameters and the simultaneous presence of a hemodynamically significant PDA using linear mixed-effects models. We built separate models for each echocardiographic parameter, intercepts were allowed to vary for each participant, and all models were adjusted for GA and PNA age.
We assessed the association between Ee’ ratio and respiratory outcomes by linear regression (duration of respiratory support) and logistic regression (BPD). We performed univariable regressions and multivariable regressions adjusting for GA. We investigated the ability of Ee’ ratio to discriminate between infants with and without BPD using receiver operating characteristic (ROC) analysis and identified an Ee’ ratio cut-off value by the Youden index method. We divided the population based on the identified cut-off value and compared clinical data between groups using the Mann–Whitney test for continuous variables and Chi-square test for dichotomous variables. p-values < 0.05 were considered statistically significant. Data were analysed using SigmaPlot v11 (Systat Software, Inc., San Jose, CA) and Matlab R2019b (MathWorks, Natick, MA).
Limiting the risk of bias
We estimated an adequate sample size to limit random errors. To limit the risk of selection bias, we performed the measurements prospectively even though the analysis was run retrospectively. To minimise the effect of gestational age, we performed a subgroup analysis stratifying the patients by GA.
Results
Subjects characteristics
Measurements were performed between January 2020 and October 2021. We included in the analysis 72 patients out of 81 screened for eligibility. One infant was not eligible because of congenital malformations, three were transferred to other hospitals, one died within the observation period, and four had missing data due to unavailability of staff. Table 1 summarises the characteristics of the study participants. The median (IQR) GA of the study population was 29.7 (28.2, 31.0) weeks, and birth-weight (BW) was 1245 (983, 1493) g. Twenty-two infants (31 %) developed BPD. Table 2 summarises the respiratory support mode of infants with and without BPD at each time point.
Trajectory of left ventricular diastolic function
Figure 1 shows the trajectories of echocardiographic parameters of LV diastolic function in infants with and without BPD. Ee’ ratio did not change significantly over time in infants who did not develop BPD. By contrast, Ee’ ratio increased significantly compared to 7 postnatal days in infants who developed BPD and was significantly higher in infants with than without BPD at 28 postnatal days and 36 weeks PMA. We did not observe major differences in results using Ee’ ratio calculated using lateral e’ or the average between lateral and septal e’. E and A increased over time in both groups. E was significantly higher in BPD patients at 36 weeks PMA, while A did not differ between groups at any time. e’ increased over time in infants without BPD, while it increased much slower in BPD patients, leading to significantly lower values in the BPD group at 28 postnatal days. E/A was significantly lower at seven postnatal days in infants with BPD and increased over time in both groups.
Closed circles, solid lines: infants without BPD, open circles, dashed lines: infants with BPD. Data are expressed as mean and standard deviation. §: p < 0.05 vs. 7 days within BPD; + : p < 0.05 vs. 36 weeks PMA within BPD; #: p < 0.05 vs. 7 days within no BPD; *: p < 0.05 between infants with and without BPD.
Left ventricular systolic function was normal in all patients at all time points. Table 2 summarises other relevant hemodynamic parameters in infants with and without BPD at each time point. Infants with BPD had lower systolic blood pressure than infants without BPD at 7 and 14 postnatal days. The presence of a hemodynamically significant PDA was significantly associated with higher E (p < 0.001), A (p = 0.007) and Ee’ ratio (p = 0.049 and p = 0.005 for lateral and average Ee’ ratio, respectively).
Association between Ee’ ratio at 28 postnatal days and respiratory outcomes
Since Ee’ ratio was significantly higher in infants with than without BPD at 28 postnatal days and 36 weeks PMA, we focused our analysis on the measure at 28 days to identify a possible effect of LV filling pressure on pulmonary conditions as early as possible. Table 3 summarises the results of the associations between Ee’ ratio at 28 days and respiratory outcomes. Ee’ ratio was significantly associated with the duration of respiratory support (beta = 5.32 (1.82), p = 0.005), and BPD (beta = 0.27 (0.10), p = 0.008). As expected, GA was strongly associated with respiratory outcomes. The significance of the relationships between Ee’ ratio at 28 days and respiratory outcomes did not persist after adjusting for GA.
The area under the ROC curves to discriminate between patients with and without BPD based on Ee’ ratio at 28 days was 0.69 (p = 0.012). The optimal cut-off point was 12. Such cut-off value was associated with 50% sensitivity and 84% specificity for BPD. We divided our population into infants with Ee’ ratio at 28 days ≤ or > 12. Infants with Ee’ ratio at 28 days > 12 had lower GA and BW. The proportion of infants with BPD was higher, and the total duration of respiratory support and oxygen therapy was longer in infants with Ee’ ratio at 28 days > 12 (Table 4).
To disentangle the effect of GA and Ee’ ratio on respiratory outcomes, we stratified our population into infants with GA < 28 weeks (n = 16) and infants with GA ≥ 28 weeks (n = 56). The duration of respiratory support was significantly longer in infants with Ee’ ratio at 28 days > 12 in both subgroups (p = 0.044 and 0.027 for infants below and above 28 weeks GA, respectively). In infants with GA ≥ 28 weeks, the proportion of BPD was significantly higher in the group with Ee’ ratio at 28 days > 12 (p = 0.015).
Discussion
The main findings of the present study are that in very preterm infants: (1) Ee’ ratio was higher at 28 postnatal days and 36 weeks PMA in infants with than without BPD; (2) increased Ee’ ratio values at 28 postnatal age were associated with longer respiratory support and oxygen therapy requirements and, consistently, higher BPD rates; (3) higher Ee’ ratios were driven by slowed LV relaxation in infants with evolving BPD.
LV diastolic function and BPD pathophysiology
Our findings suggest that elevated LV filling pressure might contribute to the pathophysiology of BPD. Elevated LV filling pressure and postcapillary pulmonary hypertension, in addition to precapillary pulmonary arterial hypertension, are cardiovascular complications that might contribute to an increased risk of cardiopulmonary disease. The preterm heart has low contractile force, low compliance and low filling time due to high heart rates.17 In preterm infants, systemic hypertension and aortic stiffness have also been described, leading to an increased left ventricle afterload.6 Such characteristics can promote the development of LV diastolic dysfunction, which may result in elevated end-diastolic left atrial pressure and eventually, through a backpressure cascade, pulmonary venous hypertension,7 leading to pulmonary oedema.
Physiological interpretation
Ee’ ratio is a key echocardiographic parameter for estimating LV diastolic function in the adult population because of its association with mean pulmonary capillary wedge pressure.9,10 Indeed, Ee’ ratio plays a central role in current consensus recommendations13 to determine LV diastolic function severity and whether it is associated with elevated left atrial pressure. We used Ee’ ratio lateral as the primary outcome because it is less affected by the right heart function, particularly in preterm infants in early life. In our cohort, Ee’ ratio lateral and average provided similar results, likely because very few infants had pulmonary hypertension.
Ee’ ratio is a composite index, and it may be elevated due to slowed LV relaxation (reduced e’) or increased mitral inflow (elevated E). In our cohort, E increased over time in infants with and without BPD; the increase in Ee’ ratio in infants with BPD seems to be driven by low e’ values. Such results suggest that an increase in E associated with slowed LV relaxation might be the reason for a progressive increase in Ee’ ratio in BPD patients, which might be accompanied by a progressive increase in left atrial pressure. In our cohort, the presence of a hemodynamically significant PDA was associated with a higher E and Ee’ ratio. Such results confirm that exposure to a hemodynamically significant PDA volume load might increase mitral flow, resulting in increased LV filling pressure, especially when the LV relaxation is slowed. However, since only two infants had a hemodynamically significant PDA at 28 postnatal days, we speculate that the higher Ee’ ratios that we observed at that time were likely due to slowed LV relaxation rather than to the presence of PDA.
E/A was significantly lower at seven postnatal days and tended to be higher at 36 weeks PMA in infants who developed BPD. Since E/A is also a composite index, we investigated the trajectories of E and A separately. Both parameters increased over time in infants with and without BPD. At 36 weeks PMA, infants with BPD presented a significantly higher E than infants without BPD, which might explain the higher E/A values. In our cohort, the presence of a hemodynamically significant PDA was associated with higher E and A values; therefore, it did not present a significant correlation with E/A.
Since echocardiographic diastolic function parameters considered normal in preterm infants are different from those in other populations,14 leading to uncertainties regarding echocardiogram-derived definitions of elevated LV filling pressure in this population, we derived a cut-off value for Ee’ ratio at 28 postnatal days using ROC curve analysis on our dataset. Even though we based our analysis on the comparison between infants with Ee’ ratio at 28 postnatal days above vs below the identified cut-off, we observed a dose-effect relationship between Ee’ ratio and lung disease severity, as suggested by the significant association between Ee’ ratio and the duration of respiratory support.
The cardiovascular complications of BPD are associated with prematurity itself. Interestingly, Ee’ ratio was significantly higher in infants with than without BPD, even at the same postmenstrual age (36 weeks PMA). To disentangle the effect of immaturity and LV filling pressure on respiratory outcomes, we stratified our population according to GA at birth. In both subgroups, infants with Ee’ ratio at 28 days > 12 received significantly longer respiratory support than infants with Ee’ at 28 days ≤ 12. The BPD rate was higher in infants with Ee’ ratio at 28 days > 12 than in infants with Ee’ ratio ≤12 only among infants above 28 weeks gestation, likely because the probability of developing BPD among infants below 28 weeks gestation is very high regardless of the LV diastolic function.
Clinical relevance
Elevated LV filling pressure and postcapillary pulmonary hypertension are difficult to diagnose as their signs and symptoms often mimic primary lung disease. Our findings suggest that serial echocardiographic evaluations may help identify a potentially treatable cardiac impairment in selected BPD patients. Indeed, assessing echocardiographic parameters of LV diastolic dysfunction can help identify infants in whom conventional pulmonary vasodilators (e.g., iNO and sildenafil) may be ineffective or contraindicated. Finally, assessing LV diastolic function together with systemic blood pressure, right heart function, systolic pressure, and vascular parameters may identify BPD patients complicated by postcapillary pulmonary hypertension, in whom afterload reduction agents may reduce pulmonary vascular congestion. Mourani et al. described two cases in which the identification and treatment of LV diastolic dysfunction with milrinone, followed by Captopril, led to a significant clinical improvement.7 Sehgal et al. reported a series of cases in which angiotensin-converting enzyme inhibition led to clinical and echocardiographic improvements in infants with severe BPD unresponsive to conventional therapy (pulmonary vasodilators and diuretics).18
Strengths and limitations of the study
Strengths of our study include the longitudinal characterisation of echocardiographic parameters of LV filling pressure in the same subjects, the recruitment of preterm infants from 23 weeks gestation and the adherence to the American Society of Echocardiography guidelines, which increases generalisability.
Limitations include the retrospective study design.19 Another limitation is the single-centre study design, which may limit the generalisability of the results due to potential differences between centres in terms of populations and respiratory management. We used BPD classification as a proxy of lung disease severity, which is not specific to the underlying pathophysiology and might be a poor marker for the severity of cardiopulmonary interactions. However, to date, it remains the only readily available respiratory outcome. The relatively small number of BPD patients prevented the possibility of assessing the association between LV filling pressure and BPD severity. Finally, the study was adequately powered to compare the trajectory of the Ee’ ratio between infants with and without BPD but not for a multiple linear regression model to assess the relationship between LV filling pressure and respiratory outcomes adjusting for prematurity and other clinically relevant confounders. Our data can serve as pilot data for a larger prospective multicenter trial.
In conclusion, elevated LV filling pressure and postcapillary pulmonary hypertension may contribute to the pathophysiology of BPD in very preterm infants. Close monitoring of LV diastolic function, in addition to the assessment of pulmonary vascular resistance and right ventricular function, may identify infants at a greater risk of cardiopulmonary disease and assist with clinical management. Prospective studies are warranted to confirm our findings and investigate relevant therapeutic options for LV diastolic dysfunction and their effect on respiratory outcomes.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Bhat, R., Salas, A. A., Foster, C., Carlo, W. A. & Ambalavanan, N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 129, 682–689 (2012).
Khemani, E. et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 120, 1260–1269 (2007).
Savoia, M. et al. Pulmonary hypertension in a neonatologist-performed echocardiographic follow-up of bronchopulmonary dysplasia. Eur. J. Pediatr. 180, 1711–1720 (2021).
Sehgal, A. et al. The left heart, systemic circulation, and bronchopulmonary dysplasia: relevance to pathophysiology and therapeutics. J. Pediatr. 225, 13–22.e2 (2020).
Sehgal, A., Malikiwi, A., Paul, E., Tan, K. & Menahem, S. A new look at bronchopulmonary dysplasia: postcapillary pathophysiology and cardiac dysfunction. Pulm. Circ. 6, 508–515 (2016).
Sehgal, A., Malikiwi, A., Paul, E., Tan, K. & Menahem, S. Systemic arterial stiffness in infants with bronchopulmonary dysplasia: potential cause of systemic hypertension. J. Perinatol. 36, 564–569 (2016).
Mourani, P. M., Ivy, D. D., Rosenberg, A. A., Fagan, T. E. & Abman, S. H. Left ventricular diastolic dysfunction in bronchopulmonary dysplasia. J. Pediatr. 152, 291–293 (2008).
Bokiniec, R., Własienko, P., Borszewska-Kornacka, M. & Szymkiewicz-Dangel, J. Evaluation of left ventricular function in preterm infants with bronchopulmonary dysplasia using various echocardiographic techniques. Echocardiography 34, 567–576 (2017).
Nagueh, S. F., Middleton, K. J., Kopelen, H. A., Zoghbi, W. A. & Quiñones, M. A. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J. Am. Coll. Cardiol. 30, 1527–1533 (1997).
Ommen, S. R. et al. Clinical utility of doppler echocardiography and tissue doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous doppler-catheterization study. Circulation 102, 1788–1794 (2000).
Schmitz, L. et al. Doppler-derived parameters of diastolic left ventricular function in preterm infants with a birth weight <1500 g: reference values and differences to term infants. Early Hum. Dev. 76, 101–114 (2004).
Mori, K. et al. Pulsed wave doppler tissue echocardiography assessment of the long axis function of the right and left ventricles during the early neonatal period. Heart 90, 175 (2004).
Nagueh, S. F. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Hear J. Cardiovasc. Imaging 17, 1321–1360 (2016).
Su, B. H., Watanabe, T., Shimizu, M. & Yanagisawa, M. Echocardiographic assessment of patent ductus arteriosus shunt flow pattern in premature infants. Arch. Dis. Child Fetal Neonatal Ed. 77, F36–F40 (1997).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Hirose, A. et al. Evolution of left ventricular function in the preterm infant. J. Am. Soc. Echocardiogr. 28, 302–308 (2015).
Bensley, J. G., Moore, L., De Matteo, R., Harding, R. & Black, M. J. Impact of preterm birth on the developing myocardium of the neonate. Pediatr. Res. 83, 880–888 (2018).
Sehgal, A., Krishnamurthy, M. B., Clark, M. & Menahem, S. ACE inhibition for severe bronchopulmonary dysplasia—an approach based on physiology. Physiol. Rep. 6, e13821 (2018).
Lum, S., Hoo, H., Hulskamp, G., Wade, A. & Stocks, J. Potential misinterpretation of infant lung function unless prospective healthy controls are studied. Pediatr. Pulmonol. 45, 906–913 (2010).
Author information
Authors and Affiliations
Contributions
C.R. performed the measurements, interpreted the data, contributed to the first draft of the manuscript, and approved the final manuscript as submitted. D.D. performed the measurements and literature search, interpreted the data, and approved the final manuscript as submitted. E.Z. performed formal data analysis, drafted the first version of the manuscript, and submitted the final manuscript. A.S.A. contributed to the investigations, collected the clinical data, and approved the final manuscript as submitted. M.L.V. supervised the study, contributed to data interpretation, revised the manuscript, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Informed consent was waived due to the retrospective nature of this study and the analysis used anonymous and aggregated data.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rigotti, C., Doni, D., Zannin, E. et al. Left ventricular diastolic function and respiratory outcomes in preterm infants: a retrospective study. Pediatr Res 93, 1010–1016 (2023). https://doi.org/10.1038/s41390-022-02216-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02216-3