Abstract
Background
Autonomic nervous system (ANS) dysregulation has been described in congenital central hypoventilation syndrome (CCHS). The objectives were to describe heart rate variability (HRV) analyses in children suffering from CCHS both while awake and asleep and their relationships with both ambulatory blood pressure (BP) and ECG monitoring results.
Methods
This retrospective study enrolled children with CCHS (n = 33, median age 8.4 years, 18 girls) who had BP and ECG monitored during the same 24 h. From the latter, HRV analyses were obtained during daytime and nighttime.
Results
The prevalences of hypertension and sinus pauses were 33% (95% confidence interval [CI]: 18–52) and 18% (95% CI: 7–35), respectively. The decrease in systolic BP at night negatively correlated with an increase in very low frequency (VLF) and LF powers at night, and the longest RR interval positively correlated with daytime VLF and LF powers. Among the three groups of children (polyalanine repeat expansion mutation [PARM], moderate [20/25 and 20/26], severe [20/27 and 20/33], and non-PARMs), the prevalence of elevated BP or hypertension was different: in PARM subjects: 6/18 moderate, 7/9 severe versus 0/6 in non-PARM (p = 0.002).
Conclusion
Modifications of cardiac ANS are associated with systemic hypertension and the occurrence of sinus pauses in CCHS.
Impact
-
Children with congenital central hypoventilation syndrome (CCHS) exhibit an increased prevalence of hypertension and sinus pauses that are linked to cardiac autonomic nervous system dysfunction.
-
Sinus pauses are the main manifestation of sinus nodal dysfunction in children with CCHS.
-
The increased prevalence of hypertension, especially at nighttime, is a new finding in CCHS.
-
Sinus nodal dysfunction can be due to the sole impairment of the cardiac autonomic nervous system.
-
Ambulatory blood pressure and ECG monitoring are mandatory in patients with CCHS.
Similar content being viewed by others
Introduction
Congenital central hypoventilation syndrome (CCHS) is a rare lifelong and life-threatening disorder that affects the autonomic nervous system (ANS) that controls many of the automatic functions in the body such as heart rate (HR), blood pressure (BP), sensing of oxygen and carbon dioxide levels in the blood, temperature, bowel and bladder control, and more. Thus, CCHS is defined as an alveolar hypoventilation due to a deficient autonomic central control of breathing and a global autonomic dysfunction.1 Almost all individuals with the comprehensive CCHS phenotype are heterozygous for a mutation in the PHOX2B gene: polyalanine repeat expansion mutations (PARMs) or non-PARMs or PHOX2B deletion.1
ANS dysregulation has been well described in CCHS1,2,3,4,5 with a positive correlation between the number of ANS dysfunction symptoms and PARM in PHOX2B gene.6 A similar relationship has further been confirmed with an objective measure of ANS, pupillometry.7 All the studies are consistent with a predominant vagal dysfunction with signs of vagal withdrawal and baroreflex failure.8,9,10,11 Trang et al. suggested a relative preservation of the cardiac and vascular sympathetic function10 while Diedrich et al., in a preliminary report, suggested that CCHS leads to generalized abnormalities in parasympathetic and sympathetic control of HR as well as sympathetic control of the peripheral vasculature.11 This sympathovagal imbalance has also been demonstrated during sleep. Patients with CCHS showed virtually no high-frequency activity of overall heart rate variability (HRV) due to parasympathetic withdrawal during sleep.8 The low-frequency/high-frequency ratios in most patients with CCHS increased during sleep but decreased in healthy subjects.8 Overall, there is no doubt that ANS dysfunction is present in CCHS, but the cardiovascular consequences of this dysfunction remain debated.
The arterial baroreflex contributes importantly to the short-term regulation of BP and HRV.12 Many central neural structures are involved in the regulation of the cardiovascular system and contribute to the integrity of the baroreflex. Consequently, brain injuries, as observed in CCHS, may induce baroreflex impairment and deranged cardiovascular variability.4,13 In addition, early depression of the baroreceptor sensitivity during the onset of hypertension has been demonstrated.14 Altered baroreflex has repeatedly been demonstrated in CCHS.9,10,15 Moreover, Trang et al. showed that children with CCHS had lower BP while awake and higher BP while asleep as compared to control subjects. Nocturnal BP dipping was abnormally low in patients with CCHS.16 ANS dysfunction could also contribute to sinus nodal dysfunction. Silvestri et al. showed that children with CCHS have more arrhythmias than healthy subjects, specifically an increased severity and frequency of bradyarrhythmias such as sinus pauses.17 Consequently, one may wonder whether ANS dysfunction of CCHS contributes to the abnormalities of BP and to sinus pauses (transient asystole). The primary objectives of our study were to describe HRV analyses in children suffering from CCHS during both wakefulness and sleep, and the relationships with both ambulatory blood pressure monitoring (ABPM) results (normal BP, elevated BP, hypertension, and normal versus reduced nocturnal dipping) and ambulatory ECG monitoring results (sinus pauses). The effect of CCHS genotype on the results of ABPM and ambulatory ECG monitoring was assessed as a secondary objective. We hypothesized that the abnormal nocturnal dipping and the sinus pauses could be associated with abnormal HRV indices, especially those that have previously been associated with baroreflex sensitivity.18,19
Methods
Design
This was a retrospective study based on the analysis of recordings obtained according to the French national guidelines in the routine follow-ups with children suffering from CCHS (https://www.has-sante.fr/jcms/c_2829809/fr/syndrome-d-ondine).
According to these guidelines, these children had a 48-h ambulatory ECG monitoring and a 24-h ABPM every 1–2 years. We selected the 24-h ambulatory ECG monitoring obtained during the same 24-h of ABPM. Then, when children had multiple visits, we selected the 24-h ECG recording with the longest RR interval as previously done by Gronli et al.20 An additional selection criterion used was the age between 5 and 16 years due to the set of normative ABPM values.21
Ethical approval was obtained from our Ethical Committee for the assessment of this cohort. The parents were informed of the collection of the prospective data for research purposes, and they could request that their child be exempted from this study in accordance with French law (noninterventional observational research).
Ambulatory ECG monitoring (ECG Holter)
Electrocardiographic recording was acquired with the Holter recorder SpiderView (ELA Medical, SORIN Group, Clamart, France). Analog data were edited on a SyneScope station and further exported in ASCII files. The files were then processed using the HRV analysis software 1.1 downloaded from https://anslabtools.univ-st-etienne.fr and validated by Pichot et al.,22 as previously done.23
The 24-h recordings were split into daytime (8 AM–9 PM) and nighttime (11 PM–6 AM) as previously done by others to determine normal values of daytime and nighttime HRV indices,24 since our aim was to assess whether modifications of ABPM and ECG Holter occur at daytime or nighttime.
ECG Holters were all interpreted by an expert cardiologist (I.D.). Sinus node dysfunction was defined by sinus pause >3 s.25 The longest RR value was systematically retrieved from the 24-h recording with its time of occurrence. HR characteristics were expressed as mean value, skewness (a measure of the symmetry of probability distribution), and kurtosis (a measure of tailedness of probability distribution).
Linear analyses
Time-domain variables included the mean sinus HR, the standard deviation of the RR intervals (SDNN), and the root mean of squared successive differences (RMSSD). After fast Fourier transform, the power spectrum indices were calculated as recommended.26 The spectrum was calculated using Welch’s periodogram algorithm with a Hamming window of 256 points, an overlap of 50%, and a precision of 256 points/Hz.22
The frequency-domain measurements were the total power (Ptot), very low frequencies (VLF: 0–0.04 Hz), low frequencies (LF: 0.04–0.15 Hz), and high frequencies (HF: 0.15–0.40 Hz). LF and HF were also expressed as normalized values, LFnu = 100 × LF/(Ptot) and HFnu = 100 × HF/(Ptot), and the LF/HF ratio was calculated.
In accordance with Billman,27 LF/HF = (0.50 parasympathetic + 0.25 sympathetic nerve activity)/(0.90 parasympathetic + 0.10 sympathetic nerve activity). Thus, when HR fluctuations mediated by autonomic nerves are observed in the HF band (>0.15 Hz), it is considered an index of parasympathetic tone. A decrease in the LF/HF ratio together with a decrease in LF was considered indicative of a possible reduction in sympathetic tone, as previously done.28
Since HR variations per se influence HRV responses, frequency data (power spectra) were corrected by division with the corresponding mean RR interval in seconds,27 giving LFcorrected and HFcorrected, as previously done.23
Nonlinear analyses18
Beat-to-beat regulation is under the influence of sympathovagal balance, central oscillators, reflexes circuits such as baroreflex and chemoreflex control, sympatho-sympathetic reflexes, molecular, and hormonal regulation. All these mechanisms are responsible for HRV complexity. Thus, nonlinear analyses of HRV were computed using methods whose indices had previously been linked to the baroreflex.
Detrended fluctuation analysis (DFA) extracts the correlations between successive RR intervals over different time scales. DFA is a method that quantifies the long-term correlations and fractal structure in HR dynamics. This analysis results in slope DFAα1, which describes brief fluctuations related to the baroreceptor reflex,19 and slope DFAα2, which describes long-term fluctuations related to regulatory mechanisms that limit fluctuation of the beat cycle.18
A Poincaré plot is graphed by plotting every RR interval against the prior interval, creating a scatter plot. We analyzed Poincaré plot by fitting an ellipse (curve that resembles a squashed circle) to the plotted points. After fitting the ellipse, we derived two nonlinear measurements, SD1 and SD2 that have been linked to the baroreflex.18 The ratio of SD1/SD2, which measures the unpredictability of the RR time series, was used to measure autonomic balance when the monitoring period is sufficiently long and there is sympathetic activation.
Ambulatory blood pressure monitoring (ABPM)
Each subject underwent 24-h ABPM using an oscillometric device (SpaceLabs 90207; SpaceLabs Medical; Redmond, WA). The cuff was chosen to match arm size and placed around the nondominant arm, as recommended.21 The monitor was programmed to read BP every 20 min during daytime (8 am to 10 pm) and every 30 min during nighttime (10 pm to 8 am). BP measurements were compared to normative values of children ABPM,21 and elevated BP (≥90th percentile and <95th percentile) and hypertension (≥95th percentile) were defined according to current recommendations for children.29 Nondipping status was defined as a mean reduction in systolic BP or diastolic BP lower than 10% during nighttime.30 The percentage of daytime and nighttime with BP <50th percentile was also recorded. All the ABPMs were interpreted by a single investigator (C.D.).
Statistical analyses
The results were expressed as median (25th–75th percentiles) since most indices did not follow a normal distribution (HRV indices for instance). Comparisons between daytime and nighttime recordings were performed using Wilcoxon signed-rank test. Comparisons of the continuous variables between the three groups of children were performed using the Kruskal–Wallis test and two-group comparisons were further performed using the Mann–Whitney U test. Categorical variables were compared using the χ2 test (Fisher’s exact test when necessary) and correlations were evaluated using Pearson’s correlation coefficient. A p value <0.05 was deemed significant. No correction for multiple testing was done due to the pathophysiological design of the study.31 All statistical analyses were performed with StatView 5.0 software (SAS Institute, Cary, NC, United States).
Results
Thirty-three participants suffering from CCHS, 18 females and 15 males, were enrolled; their median age was 8.4 years [6.4; 11.1]. Their somatic growth was normal: median height of 131 cm [119; 142], 48th percentile [24; 80], median weight of 27 kg [22; 34], 49th percentile [16; 73] and median body mass index of 16.45 kg.m–2 [14.77; 18.67], 46th percentile [13; 80]. All participants were heterozygous for a mutation in the PHOX2B gene: PARMs, 20/25, n = 10; 20/26, n = 8; 20/27, n = 7; 20/33, n = 2, or non-PARMs, n = 6. Twenty-nine children received night ventilation and four required 24/24 h ventilation. The distribution of mask ventilation and tracheostomy ventilation was equivalent (16 and 17, respectively). None required a respiratory or cardiac pacemaker. The results of ABPM and ECG Holters are described in Table 1 as well, while the results of HRV analyses are described in Table 2.
Prevalence of ABPM abnormalities
The prevalence of abnormalities was very high with only seven children depicting normal results. Thirteen children had isolated abnormal nocturnal dipping, either diastolic (n = 1) or systolic (n = 6) or both (n = 6). Two children had an elevated BP and 11/33 children (33%, 95% confidence interval [CI]: 18–52) had hypertension (two 5-year-old children already had hypertension; nocturnal hypertension only, n = 6). Age, sex and body mass index of children with elevated BP or hypertension were not significantly different from those with normal BP (p = 0.580, p = 0.284, and p = 0.173, respectively).
Prevalence of ECG Holter abnormalities
As shown in Table 1, the sinus pauses were encountered in 6/33 children: 18%, 95% CI: 7–35. No child had symptoms that were easily attributable to the sinus pauses, and recorded pauses were asymptomatic. Also, no child had permanent pacing.
HRV analyses, daytime versus nighttime (Table 2)
In these children with CCHS the RMSSD, the HFcorrected power, and the HFnu decreased at nighttime as compared to the daytime, which was associated with a significant increase in LF/HF ratio. Skewness and kurtosis also decreased at night. The positive skew (right tail [longer RR] is longer) during the daytime is consistent with the occurrence of bradyarrhythmias during the daytime in CCHS.
Relationships between HRV analyses and ABPM indices
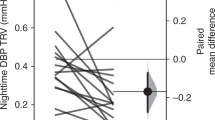
At night, there was a significant decrease in both mean systolic BP (p < 0.001) and mean diastolic BP (p < 0.001) as compared to the daytime. The decrease in mean systolic BP and mean diastolic BP significantly correlated (r2 = 0.59, p < 0.001). A negative correlation was evidenced between systolic dipping at night and LF change at night. The smallest decreases in mean systolic dipping at night were associated with increases in both LF and VLF at night (Fig. 1).
Relationships between HRV analyses and sinus pauses
The comparison of HRV analyses among the children with sinus pauses (n = 6) to those with normal ECG Holter (n = 27) is provided in Table 3. Overall, children with sinus pauses during the daytime were characterized by an increased HRV at both daytime and nighttime. Similarly, their positive skewness was also higher at both daytime and nighttime.
Finally, positive correlations were evidenced between the longest RR of ECG Holter recordings and Ptot during the daytime (R = 0.46, p = 0.010) that was related to both VLF and LF (Fig. 2). The skewness at daytime also positively correlated with the longest RR (R = 0.60, p < 0.001).
Influence of CCHS genotype
Among the children in the three groups based on genotype (PARMs, moderate [20/25 and 20/26], severe [20/27 and 20/33], and non-PARMs32) the prevalence of elevated BP or hypertension was different in the three groups of children: 6/18 moderate, 7/9 severe versus 0/6 non-PARMs (p = 0.002). Two-group comparisons demonstrated that elevated BP or hypertension was more frequent in the severe PARM group as compared to the non-PARM group (p = 0.007).
The prevalence of the sinus pauses was not significantly different in the three groups of children: 3/18 moderate PARMs, 2/9 severe PARMs, and 1/6 non-PARMs (p = 0.934). The longest RR values were not significantly different in the three groups: moderate PARMs, 1277 ms [1080; 2804] versus severe PARMs, 1120 ms [997; 2466] versus non-PARMs, 1155 ms [940; 1175], p = 0.347.
One may wonder whether HRV indices could be modified by the genotype. We restricted the analyses to indices of HRV that had been linked to the baroreflex (nonlinear indices). Daytime DFAα1 was significantly different in the three groups: in severe PARMs, 1.08 [1.04; 1.11]); in moderate PARMs (1.31 [1.15; 1.37]), and in non-PARMs (1.29 [1.23; 1.39]) (p = 0.041). Two-group comparisons demonstrated that DFAα1 was lower in severe PARMs as compared to the two other groups (moderate: p = 0.040 and non-PARMs: p = 0.035). At night, DFAα2 was different in the three groups: in severe PARMs (1.23 [1.22; 1.26]); in moderate PARMs (1.09 [1.00; 1.14]) and in non-PARMs (1.14 [0.94; 1.17]) (p = 0.006). Two-group comparisons demonstrated that DFAα2 was higher in severe PARMs as compared to the two other groups (moderate: p = 0.004 and non-PARMs: p = 0.003). The other nonlinear indices obtained from Poincaré plot (see Table 2) were not significantly different (data not shown).
Discussion
The main results of this retrospective observational study were that the prevalence of hypertension was high in children with CCHS (33%) and that the prevalence of sinus pauses was similar to that of previous studies (18%). We further show that the dysfunction of cardiac ANS is associated with the abnormalities of BP and the occurrence of sinus pauses. The HRV indices linked to these abnormalities are those previously related to the baroreflex function and/or the sympathetic overdrive. The genotype of the children with CCHS (severe PARMs) influenced the prevalence of elevated BP and hypertension.
Correlations with structural abnormalities, pathophysiological plausibility
Impaired neural structure and function contributing to autonomic symptoms has been demonstrated in CCHS.4 Injury or thinning of the insula may reduce its modulatory role on the hypothalamus and contribute to the enhanced sympathetic outflow. Both, the left and the right insula, are injured in CCHS. The hippocampal injury found in patients with CCHS may lead to autonomic impairments; perhaps from the role of this structure as part of a ventral medial frontal cortex-hippocampal BP regulatory circuit. Thus, these injuries related to CCHS may lead to both impaired BP regulation and increased sympathetic flow that have been associated with arrhythmia.
Daytime versus nighttime HRV
In considering all of the organ systems affected by the ANS, individuals with CCHS will have variable manifestations with varying severity.5 Cardiac ANS has been extensively studied in CCHS. It has been demonstrated that the LF/HF ratios in most patients with CCHS increased during sleep but decreased in healthy subjects.8 We show a similar result in our children with CCHS that was mainly related to a decrease in HF power at nighttime, which contrasts with the increase in HF power at nighttime evidenced in healthy children. It is well known that during non-REM sleep, breathing becomes more regular as it switches to an involuntary mode. Because respiration modulates HR and generates HF component at respiratory frequency, the power of the HF component concentrates on a narrower frequency band as the regularity of the respiratory cycle increases. One may wonder whether mechanical ventilation of children with CCHS is responsible for these changes in HF power. The respiratory frequency of mechanical ventilation used in our children (around 0.40 Hz, in the HF band) rules out this hypothesis. Hemodynamic oscillations during normal (negative pressure) respiration are predominantly due to changes in intrathoracic pressure. The effects of augmented positive intrathoracic pressure, which occurs with positive airway pressure ventilation, on cardiovascular autonomic control have been evaluated in healthy subjects.33 This study showed that positive airway pressure induced a decrease in the total power of HRV despite the mean RR interval remaining unchanged. The overall decrease in HRV was accompanied by a reduction across all frequency bands, which was not observed in our study, ruling out the sole effect of mechanical ventilation on HRV indices. Finally, higher nonlinear behavior has been evidenced at night in healthy subjects,24 which is similar in our children with CCHS (at least for SD2 and DFAα1).
Blood pressure changes in CCHS and relationships with ANS
Throughout the overnight sleep period, the arterial baroreflex acts to buffer surges of sympathetic activation by means of rapid changes in cardiac vagal circuits.34 Baroreflex function has been studied in CCHS. Trang et al. showed that BP levels were preserved at rest, but the maneuvers demonstrated a limited capacity to elevate BP.10 Lin et al. suggested that sympathetic tone was markedly elevated in CCHS,9 and recently Vu et al. showed a greater hypotension observed in CCHS consequent to orthostatic provocation.15 From these results, one may conclude that the baroreflex is altered in CCHS.
Few studies have evaluated long-term BP changes in CCHS. Trang et al. showed that children with CCHS had lower BP while awake and higher BP while asleep as compared to the control subjects. Consequently, nocturnal BP dipping was abnormally low in patients with CCHS.16 These results were obtained in a restricted sample of children with CCHS (n = 11) and before genetic characterization. In our study, systolic BP was <50th percentile for 63% of daytime in the 33 children, and 13/33 (39%) children demonstrated an isolated reduced nocturnal dipping, which confirm some trends previously evidenced.
Importantly, we show for the first time that elevated BP and hypertension are prevalent in childhood CCHS, especially at night. Our definitions of BP abnormalities are consistent with recent guidelines29 and use gold standard ABPM normative values, as recommended.21 The observed prevalence of hypertension (33%) is higher than that expected (5% prevalence above the 95th percentile), suggesting that ABPM should be performed in all patients with CCHS, as recommended in France.
We also show correlations between systolic BP change at night and VLF or LF changes at night. The VLF component may closely relate to the body temperature regulation, fluctuations in the activity of the rennin-angiotensin system, and peripheral chemoreceptor activity. Cevese et al. concluded that in supine rest conditions, the oscillation of RR at low frequency (0.1 Hz: LF band) is almost entirely accounted for by the baroreflex mechanism.35
Thus, altered baroreflex at night, suggested by an absence of increase in VLF and LF powers, and relative sympathetic overdrive at night (increased LF/HF ratio with decreased HF) may be responsible for the absence of normal nocturnal dipping and even elevated BP or hypertension at night in children with CCHS. Along this line, it has been shown in hypertensive subjects that the reverse dipping state is characterized by a sympathetic activation of a greater magnitude than that seen in the other conditions displaying abnormalities in nighttime BP pattern.36 Furthermore, patients with primary autonomic failure and very low sympathetic and parasympathetic activities also have a high incidence of nondipping.37 In the context of altered baroreflex, cerebral blood flow depends essentially on BP driven mechanism in patients with CCHS.15 A study that followed patients with CCHS over several years showed that brain injury progresses with advancing age.13 Whether the absence of nocturnal dipping is an adaptative or deleterious mechanism in CCHS remains to be elucidated. Nevertheless, it has been shown in the late middle-aged community-dwelling adults that reverse dipping in the presence of hypertension is associated with small vessel cerebrovascular disease, which, in turn, mediates memory functioning.38 This latter finding is important since it has been shown that CCHS increased the risk to develop neurocognitive deficiencies, affecting particularly speed of processing and working memory.39
Prevalence of arrhythmia and relationships with ANS
The prevalence of sinus node dysfunction in our study (18%, 95% CI: 7–35) was similar to that observed in a recent study in patients with CCHS (16/72, 22%, 95% CI: 13–34).40 Among individuals with a prolonged RR interval, development of asystoles is a potential cause of sudden death.20 Prolonged sinus pauses in individuals with CCHS have consistently occurred when the person is awake,41 as observed in our study. A challenging issue is whether sinus pauses are responsible for enhanced HRV and/or whether ANS dysfunction is responsible for enhanced HRV and sinus pauses. It has been stated that the pauses occur suddenly and unpredictably.20 We show a correlation of skewness during the daytime with the longest RR values and a correlation between the longest RR values and daytime VLF and LF powers. These correlations suggest that sinus pauses are associated with repeated events of low frequency (bradycardia). Whether these events are related to enhanced sympathetic flow and/or altered baroreflex remains open to debate. At night, children with the sinus node dysfunction had enhanced HRV and increased complexity of HRV, which has previously been demonstrated in adults42 and children.43 The sinus node dysfunction is usually due to degenerative fibrosis of the sinus node, affecting older subjects. Sinus bradycardia attributable to autonomic denervation, surgical trauma, ischemia, rejection, and prior amiodarone use is common after the heart transplant.25 Our study suggests a link between the dysfunctions of both ANS and sinus node, which remains to be demonstrated.
In the study of Gronli et al., 501 ECG Holters were obtained in 39 patients with CCHS, which is the largest study published.20 Overall, the first ECG Holter recording was obtained at 5.5 ± 4.9 years, and the Holter recording with the longest RR interval was obtained at 7.8 ± 5.8 years (this is quite similar to the results in our study). Thus, the occurrence of sinus pauses worsens with age, which is consistent with the fact that about 20% of adults with CCHS in France have permanent pacing (Dr Maxime Patout, unpublished observation), whereas no child had permanent pacing in our study. The mechanisms leading to the worsening of the sinus nodal function remain to be established. Nevertheless, the normal changes in the ANS function from infancy to adulthood44 may contribute to these alterations. The changes in the baroreflex function with age are also characterized by a reduced baroreflex control of HR, which favors cardiac sympathetic tone and parasympathetic withdrawal, combined with the decreased ability of the baroreflex to buffer changes in BP.45
In the study of Laifman et al.,40 patients with 20/27 PARMs presented with symptoms at a mean age of 12.4 ± 8.8 years, whereas those with 20/25 PARMs and non-PARMs had symptoms at a mean age of 15.0 ± 6.0 years, which may explain why our younger children with CCHS had no symptoms. In patients with isolated sinus bradycardia without symptoms due to cerebral or systemic hypoperfusion, there is no minimum HR or maximum pause duration where permanent pacing is recommended. Establishing a temporal correlation between symptoms and age-related bradycardia is of importance when determining whether permanent pacing is needed.25 Thus, permanent pacing was not performed in our children with CCHS, which seems in contrast with the practice in the USA where pacemaker implantation is often recommended in presence of pauses ≥3 s.20
Influence of CCHS genotype
Hypertension was mainly observed in the children with severe PARMs, which is an original finding. Furthermore, daytime DFAα1 was lower in these children, which could be related to an impaired baroreflex19 in children with hypertension. The specific increase in nighttime DFAα2 remains unexplained. Nevertheless, a slight increase in the α2 scaling exponent has been demonstrated in mice with sinoaortic denervation,19 which suggests that this condition also changes the long-term fractal structure. Finally, we did not find an influence of genotype, on the opposite of previous results,20,40 on the prevalence of sinus nodal dysfunction. This could be related to the restricted sample size of children with severe PARMs (n = 9) and restricted follow-up in our study.
Clinical perspectives
The modifications of HRV associated with abnormal BP or bradyarrythmia seem both related to an increased sympathetic drive, at nighttime for BP and at daytime for sinus pauses. Once sinus pauses are observed, the occurrence of symptoms is a major issue to indicate permanent pacing.
Since BP abnormalities were not observed in the non-PARM subgroup, which need to be confirmed in a larger sample, one may wonder if ABPM is mandatory in this subgroup of children. For the PARM subgroup, annual ABPM could be recommended, as done in our French guidelines. Finally, the long-term cardiovascular consequences of BP abnormalities remain to be assessed.
Limitations
We did not include healthy children since our aim was to assess the correlations of different measurements in a diseased population. Furthermore, several studies have already described HRV modifications in children with CCHS as compared to healthy children.8,10,11,17,20,46,47 Finally, this retrospective study shows correlations that do not make causation.
In conclusion, autonomic system dysfunction of CCHS is associated with the risk of both hypertension and sinus pauses in children.
Data availability
The data that support the findings of this study are available from C.D. but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of C.D.
References
Trang, H. et al. Guidelines for diagnosis and management of congenital central hypoventilation syndrome. Orphanet J. Rare Dis. 15, 252 (2020).
Gaultier, C., Trang, H., Dauger, S. & Gallego, J. Pediatric disorders with autonomic dysfunction: what role for PHOX2B? Pediatr. Res. 58, 1–6 (2005).
Ogren, J. A., Macey, P. M., Kumar, R., Woo, M. A. & Harper, R. M. Central autonomic regulation in congenital central hypoventilation syndrome. Neuroscience 167, 1249–1256 (2010).
Harper, R. M., Kumar, R., Macey, P. M., Harper, R. K. & Ogren, J. A. Impaired neural structure and function contributing to autonomic symptoms in congenital central hypoventilation syndrome. Front. Neurosci. 9, 415 (2015).
Weese-Mayer, D. E. et al. Case/control family study of autonomic nervous system dysfunction in idiopathic congenital central hypoventilation syndrome. Am. J. Med. Genet 100, 237–245 (2001).
Weese-Mayer, D. E. et al. Idiopathic congenital central hypoventilation syndrome: analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2b. Am. J. Med. Genet A 123A, 267–278 (2003).
Patwari, P. P. et al. Pupillometry in congenital central hypoventilation syndrome (CCHS): quantitative evidence of autonomic nervous system dysregulation. Pediatr. Res. 71, 280–285 (2012).
Woo, M. S. et al. Heart rate variability in congenital central hypoventilation syndrome. Pediatr. Res. 31, 291–296 (1992).
Lin, Z., Chen, M. L., Keens, T. G., Ward, S. L. D. & Khoo, M. C. K. Noninvasive assessment of cardiovascular autonomic control in congenital central hypoventilation syndrome. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2004, 3870–3873 (2004).
Trang, H., Girard, A., Laude, D. & Elghozi, J.-L. Short-term blood pressure and heart rate variability in congenital central hypoventilation syndrome (Ondine’s curse). Clin. Sci. 108, 225–230 (2005).
Diedrich, A. et al. Vagal and sympathetic heart rate and blood pressure control in adult onset PHOX2B mutation-confirmed congenital central hypoventilation syndrome. Clin. Auton. Res. 17, 177–185 (2007).
Lanfranchi, P. A. & Somers, V. K. Arterial baroreflex function and cardiovascular variability: interactions and implications. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R815–R826 (2002).
Kumar, R., Woo, M. S., Macey, P. M., Woo, M. A. & Harper, R. M. Progressive gray matter changes in patients with congenital central hypoventilation syndrome. Pediatr. Res. 71, 701–706 (2012).
Moreira, E. D., Ida, F., Oliveira, V. L. & Krieger, E. M. Early depression of the baroreceptor sensitivity during onset of hypertension. Hypertension 19, II198–II201 (1992).
Vu, E. L. et al. Cerebral autoregulation during orthostatic challenge in congenital central hypoventilation syndrome. Am. J. Respir. Crit. Care Med. 205, 340–349 (2022).
Trang, H., Boureghda, S., Denjoy, I., Alia, M. & Kabaker, M. 24-hour BP in children with congenital central hypoventilation syndrome. Chest 124, 1393–1399 (2003).
Silvestri, J. M. et al. Cardiac rhythm disturbances among children with idiopathic congenital central hypoventilation syndrome. Pediatr. Pulmonol. 29, 351–358 (2000).
Shaffer, F. & Ginsberg, J. P. An overview of heart rate variability metrics and norms. Front. Public Health 5, 258 (2017).
Silva, L. E. V., Rodrigues, F. L., de Oliveira, M., Salgado, H. C. & Fazan, R. Heart rate complexity in sinoaortic-denervated mice. Exp. Physiol. 100, 156–163 (2015).
Gronli, J. O., Santucci, B. A., Leurgans, S. E., Berry-Kravis, E. M. & Weese-Mayer, D. E. Congenital central hypoventilation syndrome: PHOX2B genotype determines risk for sudden death. Pediatr. Pulmonol. 43, 77–86 (2008).
Flynn, J. T. et al. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension 63, 1116–1135 (2014).
Pichot, V., Roche, F., Celle, S., Barthélémy, J.-C. & Chouchou, F. HRVanalysis: a free software for analyzing cardiac autonomic activity. Front. Physiol. 7, 557 (2016).
Bokov, P. et al. Salbutamol worsens the autonomic nervous system dysfunction of children with sickle cell disease. Front. Physiol. 11, 31 (2020).
Beckers, F., Verheyden, B. & Aubert, A. E. Aging and nonlinear heart rate control in a healthy population. Am. J. Physiol. Heart Circ. Physiol. 290, H2560–H2570 (2006).
Kusumoto, F. M. et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 140, e382–e482 (2019).
Anonymous. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93, 1043–1065 (1996).
Billman, G. E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 4, 26 (2013).
Bokov, P. et al. Cross-sectional case-control study of the relationships between pharyngeal compliance and heart rate variability indices in childhood obstructive sleep apnoea. J. Sleep. Res. 30, e13337 (2021).
Flynn, J. T. et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140, e20171904. https://doi.org/10.1542/peds.2017-1904 (2017).
Lopez-Sublet, M. et al. Nondipping pattern and cardiovascular and renal damage in a population-based study (The STANISLAS Cohort Study). Am. J. Hypertens. 32, 620–628 (2019).
Rothman, K. J. No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46 (1990).
Bachetti, T. & Ceccherini, I. Causative and common PHOX2B variants define a broad phenotypic spectrum. Clin. Genet 97, 103–113 (2020).
Valipour, A., Schneider, F., Kössler, W., Saliba, S. & Burghuber, O. C. Heart rate variability and spontaneous baroreflex sequences in supine healthy volunteers subjected to nasal positive airway pressure. J. Appl. Physiol. (1985) 99, 2137–2143 (2005).
Iellamo, F. et al. Baroreflex buffering of sympathetic activation during sleep: evidence from autonomic assessment of sleep macroarchitecture and microarchitecture. Hypertension 43, 814–819 (2004).
Cevese, A., Gulli, G., Polati, E., Gottin, L. & Grasso, R. Baroreflex and oscillation of heart period at 0.1 Hz studied by alpha-blockade and cross-spectral analysis in healthy humans. J. Physiol. 531, 235–244 (2001).
Grassi, G. et al. Adrenergic, metabolic, and reflex abnormalities in reverse and extreme dipper hypertensives. Hypertension 52, 925–931 (2008).
Mann, S., Altman, D. G., Raftery, E. B. & Bannister, R. Circadian variation of blood pressure in autonomic failure. Circulation 68, 477–483 (1983).
Chesebro, A. G. et al. White matter hyperintensities mediate the association of nocturnal blood pressure with cognition. Neurology 94, e1803–e1810 (2020).
Trang, H., Bourgeois, P. & Cheliout-Heraut, F. Neurocognition in congenital central hypoventilation syndrome: influence of genotype and ventilation method. Orphanet J. Rare Dis. 15, 322 (2020).
Laifman, E., Keens, T. G., Bar-Cohen, Y. & Perez, I. A. Life-threatening cardiac arrhythmias in congenital central hypoventilation syndrome. Eur. J. Pediatr. 179, 821–825 (2020).
Weese-Mayer, D. E. et al. Congenital central hypoventilation syndrome. In GeneReviews® (eds Adam, M. P. et al.) (University of Washington, Seattle (WA), accessed 13 Dec 2021). http://www.ncbi.nlm.nih.gov/books/NBK1427/ (1993).
Buttà, C. et al. Heart rate variability in sick sinus syndrome: does it have a diagnostic role? Minerva Cardioangiol. 67, 464–470 (2019).
Dahlqvist, J. A. et al. Sinus node dysfunction in patients with Fontan circulation: could heart rate variability be a predictor for pacemaker implantation? Pediatr. Cardiol. 40, 685–693 (2019).
Eyre, E. L. J., Duncan, M. J., Birch, S. L. & Fisher, J. P. The influence of age and weight status on cardiac autonomic control in healthy children: a review. Auton. Neurosci. 186, 8–21 (2014).
La Rovere, M. T. & Pinna, G. D. Beneficial effects of physical activity on baroreflex control in the elderly. Ann. Noninvasive Electrocardiol. 19, 303–310 (2014).
Princi, T., Accardo, A. & Peterec, D. Linear and non-linear assessment of heart rate variability in congenital central hypoventilation syndrome. Biomed. Sci. Instrum. 42, 434–439 (2006).
Macey, P. M. et al. Temporal trends of cardiac and respiratory responses to ventilatory challenges in congenital central hypoventilation syndrome. Pediatr. Res. 55, 953–959 (2004).
Acknowledgements
B.D. thanks Dr. Ha Trang for providing the list of children who underwent ambulatory ECG monitoring. The authors also thank the children and parents who have consented to their data being used for research purposes; they also thank the Association Française du Syndrome d’Ondine (AFSO) for their valuable support for years.
Author information
Authors and Affiliations
Contributions
The authors completed each of the following criteria: substantial contributions to conception and design (B.D., P.B., C.D.), acquisition of data (B.D., I.D., C.D.), or analysis and interpretation of data (all authors); drafting the article or revising it critically for important intellectual content (all authors); and final approval of the version to be published (all authors).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dudoignon, B., Denjoy, I., Patout, M. et al. Heart rate variability in congenital central hypoventilation syndrome: relationships with hypertension and sinus pauses. Pediatr Res 93, 1003–1009 (2023). https://doi.org/10.1038/s41390-022-02215-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02215-4