Abstract
Obese youth with sleep-disordered breathing are treated with positive airway pressure to improve sleep and cardiovascular status. While improvements in sleep parameters have been confirmed, a study by Katz et al. showed no major improvement in ambulatory blood pressure. The aim of this ancillary study was to analyze short-term blood pressure variability, following positive airway pressure treatment, as a more sensitive marker of cardiovascular health. We analyzed 24-h blood pressure variability data in 17 children, taken at baseline and after 12 months of treatment. These data were derived from an already published prospective, multicenter cohort study conducted in 27 youth (8–16 years) with obesity who were prescribed 1-year of positive airway pressure for moderate–severe sleep-disordered breathing. Significant decreases were found in 24 h systolic blood pressure (p = 0.040) and nighttime diastolic blood pressure (p = 0.041) average real variability, and diastolic blood pressure (p = 0.035) weighted standard deviation. Significant decreases were noted in nighttime diastolic blood pressure time rate variability (p = 0.007). Positive airway pressure treatment resulted in a significant decrease in blood pressure variability, suggesting a clinically significant improvement of sympathetic nerve activity in youth with obesity and sleep-disordered breathing.
Impact
-
Cardiovascular variability, as measured by blood pressure variability, is improved in children following positive airway pressure treatment.
-
Our novel findings of improved blood pressure time rate variability are the first described in the pediatric literature.
-
Future studies aimed at analyzing target organ damage in this patient population will allow for a better understanding as to whether alterations in blood pressure variability translate to decreasing target organ damage in children, as seen in adults.
Similar content being viewed by others
Introduction
Children and adolescents with obesity and sleep-disordered breathing (SDB) have increased activity of the sympathetic nervous system (SNS).1 Clinical manifestations of SNS activation include nighttime hypertension and absence of nocturnal blood pressure (BP) dip with eventual development of daytime or 24-h hypertension.2 It is known that positive airway pressure (PAP) treatment can improve SDB, with an expected effect on cardiovascular risk. Improvement in metabolic disturbances with PAP treatment have been demonstrated by Katz et al.;3 however, the only clinically significant effect on BP was a decrease in the systolic BP (SBP) load after 12 months of PAP treatment.
More sensitive markers of SNS activity include elevated BP variability (BPV) and altered heart rate variability (HRV).4,5,6 HRV was recently analyzed by Kirk et al.,7 who evaluated the effect of PAP treatment on adolescents with obesity and SDB using electrocardiogram tracings obtained during polysomnography. Measurements of HRV included the standard deviation of the N–N interval, the low frequency (LF) and high frequency (HF) power, and the LF/HF ratio, which are conventional measures of HRV.7 A reduction in HR and an improvement in HR variability to arousal events were observed in these children with obesity following 12 months of PAP treatment. Kirk et al. analyzed beat-to-beat variability, which is considered very short-term variability.
Increased BPV is observed in patients with SDB.2 BPV assessment methods include average real variability,4 standard deviation,8 weighted SD, and time rate variability.9 In contrast to beat-to-beat variability assessing very short-term variability, methods analyzing short-term variability can be obtained from ambulatory 24-h BP monitoring (ABPM). ABPM is relatively easy to perform and is part of routine assessment of hypertensive children with obesity and SDB. It also allows for a longer recording period (24 h for ABPM versus ~8 h of cardiovascular recordings during polysomnography).
The objective of our study was to determine if children with obesity and SDB showed clinically meaningful improvements in short-term BPV 12 months following prescribed PAP treatment, as surrogates of decreasing SNS and cardiovascular risk. Additionally, we assessed HRV using non-conventional methods (i.e., ABPM) as an exploratory analysis. Lastly, we evaluated correlations between BPV, exploratory HRV, and sleep parameters.
Methods
This study was an ancillary, sub-analysis of a larger, recently published multicentre, prospective cohort study, which aimed to evaluate the effects of PAP treatment on BP and metabolic markers of obesity-related disease in children with obesity and SDB.3 The data from the original study were reanalyzed, focusing on ABPM measurements to derive variability measures. Methodology for this larger study has been previously reported.3
Briefly, youth aged 8–16 years, with obesity and moderate–severe SDB (obstructive sleep apnea or obesity hypoventilation, diagnosed on polysomnography), and who had been prescribed PAP, were recruited from four tertiary centers in Canada. Most children had not undergone adenotonsillectomy. This study was undertaken between June 2011 and July 2014. Youth in this study were followed for 1 year. For the purposes of our study, we evaluated outcomes at two time points: baseline and 12-month follow-up. Exclusion criteria included craniofacial abnormalities, central nervous system lesions; neuromuscular, neurological, or genetic syndromes; congenital cardiac disease or ventricular dysfunction; chronic respiratory conditions (aside from asthma); use of corticosteroids within the past 3 months; regular compliant use of PAP; use of sleep medications; and/or known diabetes (either type 1 or 2).
SDB, including OSA and/or obesity hypoventilation, was confirmed in this patient population prior to study enrollment using overnight laboratory diagnostic polysomnograms which were performed and scored by sleep technologists, according to the American Academy of Sleep Medicine guidelines.10 SDB was classified as moderate (apnea–hypopnea index (AHI) of 5–9.9 events/h) or severe (AHI of ≥10 events/h).3 These criteria were decided on by a consensus group comprising pan-Canadian pediatric sleep medicine experts. Obesity hypoventilation was defined as CO2 > 50 mmHg for >25% of total sleep time events/h. Because some patients had obesity hypoventilation, not all had elevated obstructive apnea–hypopnea index (OAHI). Height and weight were collected on the night of the polysomnography. Following SDB diagnosis, children were prescribed PAP therapy by their treating physician.
Within 3 months of the polysomnography, all participants underwent 24-h ABPM monitoring. For this study, we focused exclusively on the analysis of short-term BPV and the exploratory analysis of HRV, using unique methods deriving variability measures from ABPM. ABPM measurements were recorded on a portable electronic device (Spacelabs monitor Model # 90207- IQ, Spacelabs Healthcare, Issaquah, Washington, United States) worn by participants. BP measurements were obtained from the right arm, using an appropriate-sized cuff, every 15 min until midnight, every 30 min from midnight until 6:00 am and then every 15 min thereafter, over a 24-h sampling period. All ABPM recordings were converted into.csv files (raw data) for further analysis. All recorded BP and HR readings were analyzed for three separate intervals: 24-h, daytime, and nighttime periods separately. In addition, as per ABPM guidelines, the interval from 8.00 AM to 8.00 PM was considered daytime, and the interval from midnight to 6.00 AM was considered nighttime.11 The time interval between 8.00 PM and midnight was not included in the daytime or nighttime analysis; however, when analyzing the 24-h component, all BP readings at all time intervals were included. This is an accepted, published protocol for assessment of ABPM. HR is expressed in beats per minute (bpm) and BP is expressed in millimeters of mercury (mmHg).
Adherence to PAP treatment was regularly assessed by participants’ PAP usage diaries, self-reporting at clinic visits, and corroborated with downloaded information from PAP machines’ internal data-loggers. PAP adherence was defined as use of PAP therapy for an average of ≥ 4 h/night and >50% of nights. In line with our previous study,3 adherence status was determined at the 6-month mark. While we categorized some youth as non-adherent if they did not meet these stringent criteria, we opted to include all youth in our analysis as all of the patients reported some use of PAP.
At the 1-year follow-up visit, children underwent polysomnography and ABPM recording again for comparison to baseline. This was done on two separate nights as ABPM recording may interrupt the participant’s sleep, and thus affect polysomnography indices. Children wore PAP during the follow-up polysomnography and ABPM recordings; however, PAP was not worn during the baseline polysomnography or ABPM assessment.
Outcome measures
For assessment of BPV and HRV, we used four different methods, derived from data obtained solely from ABPM:
-
1.
Average real variability (ARV): average of absolute changes between consecutive HR or BP readings.4
-
2.
Standard deviation (SD): standard deviation of HR or BP readings.
-
3.
Weighted SD (wSD): mean of day and night SD values for HR or BP readings, corrected for the number of hours included in each of these subperiods.8
-
4.
Time rate variability (TRV): Given a number of recordings N, of BP, across a 24-h window (unless subdivided into daytime or nighttime), we calculated the N−1 values for the rate of BP change at N−1 time points. Thereafter, using an algorithm published by Zakopoulos et al.,9 we described the magnitude of BP increases/decreases with a time component. We used the mean of the absolute rate of BP change during the whole 24-h period, and we therefore refer to the 24-h rate of BP change in mmHg/min.9
While ARV and weighted SD are well established markers of short-term BPV, TRV is a relatively new and under-investigated marker. ARV estimates the variability by analyzing the difference between consecutive readings irrespective of time, whereas TRV adds in the time factor. High BP TRV is considered an independent risk factor for cardiovascular disease in adults;9 however, to the best of our knowledge, TRV has not been assessed in children.
Conventional measures12 of HRV allow for a variety of assessments, including the fast-changing component (HF, 0.15–0.4 Hz), which reflects parasympathetic activity. Contrast this to the slow-changing component (LF, 0.04–0.15 Hz), which represents both the sympathetic and parasympathetic activities. When combined into a ratio, the LF/HF, it represents the sympatho-vagal balance. When using our non-conventional methods, we are not able to differentiate these components, which means at this time, they are non-comparable to conventional methods. Conventional methods of HRV analysis were not employed in this study.
Statistical analysis
All variables were tested for normal distribution using Shapiro–Wilk normality testing. As some parameters were not normally distributed, we opted to present all data as medians (ranges) for better comparability. Differences in BPV and HRV parameters before and after PAP treatment were analyzed using paired Wilcoxon tests. Additionally, relationships between changes in HRV/BPV and sleep parameters (including AHI, OAHI), lowest O2 saturation, sleep efficiency, arousal index, and obstructive apnea, and obstructive hypopnea events were assessed using correlation analysis.
In addition to null hypothesis significance testing, we used estimation statistics. These analyses focused on the magnitude of the effect size, and its precision. We used permutation testing with 1000 resampling events, and bootstrapping with bias-corrected accelerated confidence intervals.13, 14 Effect size of the difference between variables was assessed using bias-corrected accelerated Cohen d, allowing us to compare effect sizes of all the variables regardless of their distribution.13 Thresholds for Cohen d effect size include 0.2 (small effect), 0.5 (medium effect). and 0.8 (large effect). All statistical analyses were done with R version 1.1.456, Python version 3.8.3, and the Dabest package version 0.3.1.13 P values <0.05 were considered statistically significant.
Results
Of the 27 children enrolled in this study,3 17 had ABPM done at baseline and after 1 year of treatment with PAP. Two out of 17 children did not have a complete nighttime ABPM record, and therefore their nighttime period could not be analyzed. Demographics are shown in Table 1.
The median age was 13.9 (8.0–17.0) at baseline and 15.6 (9.2–18.3) years at follow-up. Median baseline BMI was 37.3 kg/m2 (median Z-score 2.6 (1.9–3.0)) and increased to 40.2 kg/m2 (median Z-score 2.7 (1.5–3.1)) after 1 year. Twelve out of the 17 patients had an increase in BMI over the 1-year period. Sleep parameters are also outlined in Table 1. We noted a decrease in the arousal index, the AHI, and the OAHI (all in events/h), while there was an increase in the lowest O2 saturation (%). Sleep efficiency (%) was unchanged following PAP treatment. Five out of 17 children were non-adherent to PAP treatment according to our stringent cut-offs; however, according to the self-reported measures and PAP downloads, “non-adherent” participants reported some usage on 10, 80, 97.5, and 100% of nights. Most children would report inability to wear PAP for the full night, necessitating removal before reaching 4 h.
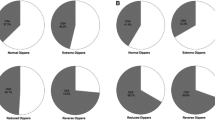
The HR, BP, and variability results are shown in Tables 2 and 3. Regarding the BP Z-scores, we observed no significant change in daytime or nighttime systolic or diastolic BP Z-scores between baseline and 12 months, as previously reported.3 We however did observe decreases in 24 h SBP ARV following PAP treatment (paired mean difference = −1.43 (95% CI = −2.48, −0.39); Wilcoxon p = 0.04; permutation p = 0.02; Cohen d = 0.85). No difference in 24 h DBP ARV was detected; however, the DBP weighted SD improved (Table 2). Daytime SBP ARV showed a decrease (Table 2) with medium-to-large effect size (Cohen d = 0.65). There were no notable differences in nighttime ARV, except for nighttime DBP ARV (Table 2). In contrast, the nighttime DBP TRV decreased by a paired mean difference of −0.17 (95% CI −0.27, −0.07) (Wilcoxon p = 0.007; permutation p = 0.007; Cohen d = 0.80) (Table 3); only 3 of 15 children had increases in nighttime DBP TRV (Fig. 1). Here again, these three children were reportedly adherent to PAP treatment.
The non-weighted SD did not show meaningful differences (data not shown). None of the changes in HRV or BPV correlated with change in BMI over time.
Differences in Cohen d effect sizes are summarized in Fig. 2. Twenty-four-hour SBP and HR ARV were associated with a large effect size (Cohen d > 0.8). Medium-to-large effect sizes were noted for nighttime DBP TRV (0.8), nighttime SBP TRV (0.76), nighttime HR TRV (0.70), DBP weighted SD (0.70), daytime HR ARV (0.68), daytime SBP ARV (0.65), and 24 h HR TRV (0.57).
Thresholds for Cohen d effect size include 0.2 (small effect), 0.5 (medium effect), and 0.8 (large effect). HR heart rate, SBP systolic BP, DBP diastolic BP, 24 24 h, D daytime, N nighttime, W weighted, ARV absolute real variability, SD standard deviation, TRV time rate variability (i.e., SD_DBP_W weighted standard deviation for DBP).
Clinically relevant sleep parameters at baseline and after 12 months of PAP treatment are summarized in Table 1. Additionally, we analyzed correlations between the change in variability (delta variability) and the change in some sleep parameters (delta sleep parameters). While some sleep variables seemed to correlate with BPV on initial analysis, we noted the significant influence of outliers. The only significant correlation was between arousal index at nighttime and the change in SBP TRV (r = 0.76).
The HRV analysis was considered exploratory. Regarding the HR and HRV, the median HR did not change significantly between baseline and 12 months after PAP treatment (Table 2). However, the median nighttime HR did decrease significantly (p = 0.048; permutation p = 0.046; Cohen d = 0.30) (Table 2). Furthermore, the 24 h HR ARV also decreased following PAP treatment (paired mean difference = −2.38 (95% CI = −4.07, −1.26); Wilcoxon p = 0.001; permutation p = 0.002; Cohen d = 0.82). Two of 17 children, both reportedly adherent to PAP treatment, had increases in 24 h HR ARV after 1 year. Daytime HR ARV showed a decrease (Table 2), with medium-to-large effect size (Cohen d = 0.68). Nighttime HR TRV decreased (paired mean difference −0.15 (95% CI −0.24, −0.05); Wilcoxon p = 0.03; permutation p = 0.01; Cohen d = 0.70) (Table 3).
Discussion
We observed improvements in short-term BPV parameters over 24-h, day, and night periods, 1 year after PAP treatment in children with obesity and SDB. This was most notable in 24-h SBP ARV and nighttime DBP TRV. Our results suggest a decrease in sympathetic activity with PAP treatment, despite an increase in BMI in 12 of 17 children. An exploratory analysis of HRV using non-conventional techniques revealed a notable, but clinically unclear difference in 24-h HR ARV and nighttime HR TRV following the 12 months of PAP treatment.
Data on the effect of PAP on short-term BPV are limited in children. We believe our study is one of the first to show differences in ABPM-derived BPV following PAP treatment and to the best of our knowledge, this analysis represents the first time that TRV was analyzed in children. We observed significant improvements related to 24-h SBP ARV, daytime SBP ARV (based on Cohen’s d), and DBP weighted SD. With respect to TRV, the benefits of PAP treatment were most apparent for nighttime DBP (Fig. 1). Change in SBP TRV at night was not statistically significant using Wilcoxon testing and was borderline using permutation testing (p = 0.06) but had a near-large effect size (Cohen’s d = 0.76), suggesting a possibly meaningful difference not apparent using point-estimate significance testing. The daytime SBP was also improved with PAP therapy (Table 2). Our results therefore suggest a clinically significant, positive effect of PAP treatment on sympathetic activity in children with obesity and SDB.
It is well described that OSA can lead to autonomic dysregulation.15 The combination of apneas, hypoxia, and hypercapnia leads to dysregulation of the autonomic system, favoring increased sympathetic activity, and decreased parasympathetic activity.16 Very short-term variability was analyzed by Shi et al.17 who observed an increase in nighttime systolic and 24-h diastolic BPV in already hypertensive adults with OSA. This was also seen for very short-term BPV, where increased variability was related to the severity of OSA.18 Ke et al.19 showed that adults newly diagnosed with OSA had higher 24-h SBP SD compared to those without, and this was linked to an increased prevalence of cardiovascular disease. Lastly, El Mokadem et al.20 reviewed 100 adults with controlled hypertension for markers of BPV, including SD, ARV, and coefficient of variance. Those with evidence of target organ damage (TOD) had increased markers of BPV. In pediatric patients, a recent study by Wang et al.21 revealed that higher SD or ARV of BP was independently associated with a higher probability of high BP in a large Chinese pediatric cohort.
Of clinical interest, the results of our study may be in line with the results seen in adult studies by Zakopoulos et al. looking specifically at TOD and TRV. The absolute differences in 24 h SBP and DBP TRV before and after PAP treatment in our cohort were 0.12 and 0.04 mmHg/min, respectively, which is very similar to the adult studies discussed.9, 22, 23 Our observed differences in TRV of this magnitude could have a significant impact on vascular stiffness as documented by Zakopoulos et al.,9 who showed that a 0.1 mmHg/min increase in 24 h SBP TRV was associated with a 0.029 mm increase in carotid intima-media thickness (CIMT). Zakopoulos et al.23 also showed that an increase in daytime SBP TRV was associated with a concomitant increase in the left ventricular mass (LVM) by 7.087 g. Our results showed decreases of daytime SBP and DBP TRV of 0.10 and 0.05 mmHg/min. We therefore believe that our results are clinically significant, and hypothesize that the decrease in TRV may have a positive impact on LVM and CIMT in our patients and may lead to a decreased risk of TOD, as seen in these adult studies.9, 22, 23
To date, no studies have compared our ABPM-derived variability measures with conventional measures of variability, at least to the best of our knowledge. While specifically discussing HRV, the conventional measurements of analysis can be broken down into the HF and LF components, as well as their ratio, LF/HF.12 Additionally, the time scales are of importance when looking at conventional methods of HRV, specifically the short-term versus long-term, where long-term looks at measures derived from 24-h recordings and includes the SDNN (standard deviation of all R–R intervals) and SDANN (mean of the standard deviations of all R–R intervals for all 5-min segments).24 It is therefore clear that the time scale is of significant importance when referring to conventional measures of HRV. However, the time scale used for our ABPM approach (minutes) is not comparable with the time scale used for these conventional measurements (seconds). When using non-conventional methods (ABPM), one is also unable to separate the sympathetic from the parasympathetic components. Regarding ABPM-derived HRV in the literature, in adults with type 2 diabetes, increased ABPM-derived HRV during sleep was associated with increased cardiovascular disease risk.25 On the contrary, pediatric literature showed that decreased HRV was found in hypertensive patients compared to non-hypertensive patients.26 It is therefore not clear in the literature how to compare conventional methods of HRV analysis with ABPM-derived HRV.
The impact of PAP treatment on very short-term, conventional HRV was investigated in our recent multicenter, prospective primary study.7 An absolute decrease in HR was observed following PAP treatment; however, using the parameters HF and LF, and their ratio, there was no observed improvement in very short-term variability. Our current analysis corroborated the finding of improved absolute HR with PAP treatment and went on to show a change in the HRV using non-conventional parameters (ABPM derived). The interpretation of these findings is challenging as there are no studies comparing conventional methods of HRV analysis with our exploratory approach. Importantly, Kirk et al.7 evaluated only very short-term variability (conventional), limited to an 8-h observation during polysomnography whereas we analyzed a 24-h time window. It is therefore possible that using non-conventional, but more protracted testing using ABPM, may allow for meaningful interpretation as it pertains to HRV; however, given that we have no comparison to conventional methods of HRV analysis, we are unable to draw conclusions at this time.
Lastly, there were observed differences in sleep parameters before and after PAP treatment. In this study, we confirmed that only one change in variability (nighttime SBP TRV) correlated with changes in arousal index, as outlined in the “Results” section. This is not surprising, as arousal events are associated with SNS activation in children,27 and this would account for an increasing SBP TRV with increasing arousal events. As the focus of this analysis was on BPV, we refer readers to the previously published material3, 7 for full details on sleep parameter analyses.
Our study has several limitations. Specifically, having echocardiographic and CIMT data would have allowed us to draw a more robust conclusion as to whether PAP treatment improved TOD. We are also aware that our study has a small number of enrolled patients limiting generalizability; however, we confirmed our results using both null hypothesis significance testing and estimation statistics with bootstrapping. Approximately 30% of the children enrolled reported non-adherence to our stringent criteria, thus limiting our ability to assess the true effect of PAP treatment; however, this is more in keeping with a real-world scenario, where patients are not always adherent to treatment. Also, because we used a binary measure of adherence, we were unable to assess whether there was a continuous relationship between hours of PAP use and changes in BPV and HRV. Lastly, we are unable to account for the possible impact of PAP treatment on the night of the second ABPM taken at the 12-month mark, in addition to the expected positive effect of the preceding 12 months of treatment. Strengths of our study include analysis of novel BPV, and exploratory HRV parameters from raw ABPM recordings, and the use of estimation statistical analyses.
In conclusion, we observed significant improvements in BP short-term variability with PAP treatment in children with SDB and obesity. Our novel findings suggest PAP treatment can improve BPV in obese children with SDB, despite an increase in BMI.
References
Van Eyck, A. et al. Sleep disordered breathing and autonomic function in overweight and obese children and adolescents. ERJ Open Res. 2, 1–8 (2016).
Feber, J. & Litwin, M. Primary Hypertension. in Hypertension in Children and Adolescents. Updates in Hypertension and Cardiovascular Protection (eds. Lurbe, E. & Wühl, E.) 95–110 (Springer Nature Switzerland, 2019).
Katz, S. L. et al. Insulin resistance and hypertension in obese youth with sleep-disordered breathing treated with positive airway pressure: a prospective multicenter study. J. Clin. Sleep. Med. 13, 1039–1047 (2017).
Mena, L. J., Felix, V. G., Melgarejo, J. D. & Maestre, G. E. 24-hour blood pressure variability assessed by average real variability: a systematic review and meta-analysis. J. Am. Heart Assoc. 6, 1–10 (2017).
Villareal, R. P., Liu, B. C. & Massumi, A. Heart rate variability and cardiovascular mortality. Curr. Atheroscler. Rep. 4, 120–127 (2002).
Chadachan, V. M., Ye, M. T., Tay, J. C., Subramaniam, K. & Setia, S. Understanding short-term blood-pressure-variability phenotypes: from concept to clinical practice. Int. J. Gen. Med. 11, 241–254 (2018).
Kirk, V. G. et al. Cardiovascular changes in children with obstructive sleep apnea and obesity after treatment with noninvasive ventilation. J. Clin. Sleep. Med. 16, 2063–2071 (2020).
Bilo, G. et al. A new method for assessing 24-h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J. Hypertens. 25, 2058–2066 (2007).
Zakopoulos, N. A. et al. Time rate of blood pressure variation is associated with increased common carotid artery intima-media thickness. Hypertension 45, 505–512 (2005).
Iber, C., Ancoli-Israel, S., Chesson, A. & Quan, S. The AASM Manual for Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (American Academy of Sleep Medicine, 2007).
Wühl, E., Witte, K., Soergel, M., Mehls, O. & Schaefer, F. Distribution of 24-h ambulatory blood pressure in children: Normalized reference values and role of body dimensions. J. Hypertens. 20, 1995–2007 (2002).
Shaffer, F. & Ginsberg, J. P. An overview of heart rate variability metrics and norms. Front. Public Heal. 5, 1–17 (2017).
Ho, J., Tumkaya, T., Aryal, S., Choi, H. & Claridge-Chang, A. Moving beyond P values: data analysis with estimation graphics. Nat. Methods 16, 565–566 (2019).
Halsey, L. G. The reign of the p-value is over: what alternative analyses could we employ to fill the power vacuum? Biol. Lett. 15, 1–8 (2019).
DelRosso, L. M., Mogavero, M. P. & Ferri, R. Effect of sleep disorders on blood pressure and hypertension in children. Curr. Hypertens. Rep. 22, 1-7 (2020).
Bonsignore, M. R. & Zito, A. Metabolic effects of the obstructive sleep apnea syndrome and cardiovascular risk. Arch. Physiol. Biochem. 114, 255–260 (2008).
Shi, J. et al. Obstructive sleep apnea increases systolic and diastolic blood pressure variability in hypertensive patients. Blood Press. Monit. 22, 208–212 (2017).
Marrone, O. & Bonsignore, M. R. Blood-pressure variability in patients with obstructive sleep apnea: current perspectives. Nat. Sci. Sleep. 10, 229–242 (2018).
Ke, X. et al. Association of 24 h-systolic blood pressure variability and cardiovascular disease in patients with obstructive sleep apnea. BMC Cardiovasc. Disord. 17, 287 (2017).
El Mokadem, M., Boshra, H., Abd el Hady, Y., Kasla, A. & Gouda, A. Correlation between blood pressure variability and subclinical target organ damage in patients with essential hypertension. J. Hum. Hypertens. 34, 641–647 (2020).
Wang, J. et al. School-based surveillance on visit-to-visit blood pressure variability and high blood pressure in children and adolescents. BMC Cardiovasc. Disord. 21, 1–10 (2021).
Manousopoulos, K. et al. Association of home and ambulatory blood pressure variability with left ventricular mass index in chronic kidney disease patients. Hypertens. Res. 44, 55–62 (2021).
Zakopoulos, N. A. et al. Impact of the time rate of blood pressure variation on left ventricular mass. J. Hypertens. 24, 2071–2077 (2006).
Aydin, M. et al. Cardiac autonomic activity in obstructive sleep apnea. Tex. Hear. Inst. J. 31, 132–136 (2004).
Eguchi, K. et al. Increased heart rate variability during sleep is a predictor for future cardiovascular events in patients with type 2 diabetes. Hypertens. Res. 33, 737–742 (2010).
Barletta, G.-M. et al. Heart rate and blood pressure variability in children with chronic kidney disease: a report from the CKiD study. Pediatr. Nephrol. 29, 1059–1065 (2014).
O’Driscoll, D. M. et al. Increased sympathetic activity in children with obstructive sleep apnea: cardiovascular implications. Sleep. Med. 12, 483–488 (2011).
Acknowledgements
We would like to thank the children and families who participated in the study.
Funding
This work was supported in part by the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception, design, acquisition of data, and interpretation of data. All authors contributed to drafting the article and revising it critically for important intellectual content. All authors have confirmed final approval of this version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
All patients were appropriately consented. This study was approved by all research ethics boards at all institutions.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Myette, R.L., Feber, J., Blinder, H. et al. Blood pressure variability in children with obesity and sleep-disordered breathing following positive airway pressure treatment. Pediatr Res 92, 810–815 (2022). https://doi.org/10.1038/s41390-021-01841-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01841-8
This article is cited by
-
The ongoing impact of obesity on childhood hypertension
Pediatric Nephrology (2024)