Abstract
Background
Oxylipins are metabolites derived from fatty acids such as arachidonic acid (AA) and are key mediators in inflammation, host defense, and tissue injury. Serum oxylipins increase in adults after cardiopulmonary bypass (CPB) but tissue-level changes are poorly defined. The objective of this study was to characterize pulmonary tissue oxylipins in an infant porcine model of CPB with deep hypothermic circulatory arrest (DHCA).
Methods
Infant pigs underwent CPB with DHCA. Controls received anesthesia only. Right upper and lower lobes of the lung underwent oxylipin analysis via liquid chromatography–tandem mass spectrometry. One-way ANOVA was utilized to assess differences in oxylipin concentrations across groups, followed by pairwise comparisons.
Results
AA and multiple AA metabolites via cytochrome P450 (CYP450), lipoxygenase (LOX), and cyclooxygenase (COX) pathways were significantly increased in the upper and lower lobe of pigs exposed to CPB/DHCA as compared to controls. Multiple prostaglandin metabolites produced via COX were also significantly elevated in the lower lobes of control animals.
Conclusions
CPB/DHCA induces a significant increase in pulmonary tissue AA, with subsequent metabolism via COX, LOX, and CYP450 pathways. Interestingly, prostaglandins were also elevated in the lower lobes of the controls, suggesting a mechanism separate from CPB/DHCA. Future oxylipin studies are needed to better understand CPB-induced acute lung injury.
Impact
-
CPB/DHCA and, to a lesser extent, lung region influence pulmonary tissue-level AA metabolite production.
-
Inflammatory mediator AA metabolites have been noted in previous studies to increase following CPB; however, this is the first study to look at pulmonary tissue-level differences following CPB/DHCA.
-
Increases in many AA metabolites, including LOX- and CYP450-derived products, were seen in both upper and lower lobe of piglets following CPB/DHCA.
-
COX-derived prostaglandin metabolites were increased not only in CPB upper and lower lobe but also in mechanically ventilated control lower lobe, suggesting an additional, separate mechanism from CPB/DCHA.
Similar content being viewed by others
Introduction
Congenital heart disease (CHD) affects up to 13.7 per 1000 live births in the United States with 1 in 150 adults living with some form of CHD.1 About 25% of children born with CHD need a procedure or surgery within the first year of life often requiring cardiopulmonary bypass (CPB) with deep hypothermic circulatory arrest (DHCA) or selective cerebral perfusion for these corrective and palliative surgeries.2 Unfortunately, CPB/DHCA is independently associated with significant postoperative morbidity due to systemic and organ-specific physiologic derangements leading to inflammation and oxidative stress.3,4,5,6,7,8,9,10
Acute lung injury (ALI) secondary to CPB/DHCA is one of the least well-studied postoperative morbidities. This is likely due to the unique physiologic characteristics of CHD, which makes ALI difficult to define clinically in the postoperative time frame. The diagnosis of pediatric ALI focuses on the presence of infiltrates on the chest x-ray and the amount of airway pressure and FiO2 required to oxygenate a patient.11 Cyanotic heart disease, left ventricular dysfunction, and fluid overload are common to children with CHD undergoing surgery and are all asterisks in the definition of pediatric ALI.11 Therefore, a large animal model of CPB/DHCA is invaluable to understanding the pathophysiology of organ-specific injury, particularly ALI where there is no good clinical phenotype in this population.
The lungs are uniquely primed for injury following CPB/DHCA due to the complete cessation of blood flow through the pulmonary artery (ischemia) and subsequent return of pulmonary artery perfusion after separation from CPB (reperfusion). Despite adequate perfusion pressures of the bronchial artery, ischemia of the lungs with CPB may still occur.12 Ischemia–reperfusion (IR) injury in the lungs is due to the production of reactive oxygen species (ROS), sequestration and activation of leukocytes in the pulmonary circulation, and endothelial cell injury.4 These reactions lead to many downstream effects including the release of polyunsaturated fatty acids (PUFAs) from the phospholipid cellular membrane and their subsequent metabolism via the cytochrome P450 (CYP450), lipoxygenase (LOX), and cyclooxygenase (COX) pathways into oxylipins. Oxylipins are an umbrella term for oxygenated compounds formed from PUFAs and are key mediators in inflammation, pulmonary vascular tone, platelet function, host defense, and tissue injury.13,14
Arachidonic acid (AA) and its metabolites are the most potent and well-studied oxylipins regarding oxidative stress, inflammation, and cardiopulmonary disease.13,15,16,17,18,19,20 With advances in metabolomic technology, large metabolite panels can be efficiently evaluated from serum and tissue.21 Regarding CPB/DHCA-associated oxylipin metabolite profiles, there is a paucity of research with limited clinical data focused on serum samples and no correlation with tissue-specific metabolite production.19,22
In this study, we used our established infant porcine model of CPB/DHCA to profile pulmonary tissue oxylipins with liquid chromatography–tandem mass spectrometry. This study was performed as a planned secondary study under a previously published parent study examining the effect of bovine intestinal alkaline phosphatase (BiAP) infusion on post-bypass AKI.23,24 We hypothesized that the lungs of animals exposed to CPB/DHCA would have a different oxylipin profile compared to control animals receiving anesthesia and mechanical ventilation alone. Furthermore, based on our recent findings of significant regional differences in global metabolism in our model,25 we hypothesized that there would be variation in oxylipin production between the upper and lower lobes due to regional differences in ventilation and perfusion. As a secondary aim, we examined whether BiAP infusion modulated oxylipin production in the lung. Analyzing pulmonary tissue oxylipin profiles after CPB/DHCA will allow us to investigate activation of these immune-modulating molecules, determine regional responses, and set the initial groundwork for future development of BiAP or other targeted therapeutics to mitigate or prevent CPB/DHCA-associated ALI.
Materials and methods
CPB/DHCA piglet model
Our animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Colorado (Protocol Number: 107715(02)1D). Seventeen female infant specific pathogen-free piglets (5–10 kg) were included in our study. The animals utilized for this study were part of a larger cohort in a pilot study investigating the effect of alkaline phosphatase infusion on CPB/DHCA-induced organ injury.23 Three main groups were analyzed, CPB/DHCA without BiAP infusion (n = 5), CPB/DHCA with BiAP infusion (75 U/kg/h bolus followed by 25 U/kg/h infusion)23 (n = 5), and anesthesia-only controls (n = 7). All subjects were supine, mimicking human surgical conditions. CPB/DHCA was initiated with peripheral cannulation via the external jugular vein and internal carotid artery utilizing a pediatric oxygenator (Sorin Group, Arvada, CO) and a standard roller pump. Anesthesia and analgesia were provided with isoflurane, fentanyl, and propofol throughout the case. The animals were cooled to 22 °C, underwent 75 min of DHCA, and were then rewarmed to 36 °C over approximately 30 min. During DHCA, the ventilator maintained a positive end expiratory pressure (PEEP) of 5 with a rate of 10 and 100% FiO2. After rewarming, they were separated from CPB and a goal mean arterial pressure of 45–65 mmHg was maintained with a combination of milrinone, epinephrine, dopamine, and vasopressin. Invasive mechanical ventilation continued with a PEEP of 5, 100% FiO2, and minute ventilation titrated for normocapnia. Pentobarbital was administered 4 h after separation from bypass for euthanasia. Anesthesia control pigs were similarly ventilated for a total of 7 h (similar duration of mechanical ventilation as the CPB/DHCA arm) with the same anesthesia and euthanasia medications. A pre-specified secondary aim of our study was to characterize ALI and markers of inflammation/oxidative stress in pulmonary tissue.
Clinical monitoring
For continuous cardiovascular monitoring, all animals underwent femoral venous and arterial cannulation. Hourly vitals were recorded. Arterial blood gas measurements were performed at cannulation, rewarming, and every hour after rewarming for titration of ventilator until the time of euthanasia using iSTAT point-of-care testing (Abbott, Princeton, NJ).
Oxylipin analysis
Tissue samples were obtained from standardized areas based on gross anatomy (apical vs dorsocaudal along the diaphragmatic surface). These areas corresponded to the significant differences seen visually between upper and lower (dorsocaudal) lobes. One hundred and fifty milligrams of flash frozen tissue was ground on liquid nitrogen and solubilized in 1:1 phosphate-buffered saline/methanol (high-performance liquid chromatography (HPLC) grade), homogenized with electric homogenizer, and sonicated on ice for 5 min. The homogenate was centrifuged, and the supernatant was transferred and stored. The pellet was dissolved in 500 μL of methanol, sonicated on ice for 5 min, centrifuged, and supernatant was combined with the first fraction. Speedvac was used to pellet the samples without heat. Dried samples were reconstituted in 1 mL of 80:20 water (v/v) containing 100 ng/mL mixture of internal standard solutions (see below). Samples were centrifuged at 16,000 rpm for 20 min and transferred into HPLC vials. In all, 500 µL of supernatants were injected onto a 3.0 × 5 mm guard column (Halo, C8, 2.7 μM; Advanced Materials Technology, Wilmington, DE) and, after clean-up, back flushed with 100% acetonitrile/methanol (1:1, v/v) onto a 3.0 × 1000 mm analytical column (Halo C8, 2.7 μM, Advanced Materials Technology). For HPLC separation, the starting mobile phase consisted of 40% water supplemented with 0.1% formic acid (buffer A) and 60% buffer B (acetonitrile/methanol, 1:1, v/v) with a flow of 0.8 mL/min for the first minute. After 2.5 min, the gradient increased to 53% B and further to 70% B within 8.5 min. At 11.5 min, buffer B was at 95% and was held for 1 min. The column was re-equilibrated for 2 min to starting conditions. The API5500 mass spectrometer (AB Sciex, Concord, ON, Canada) was run in the negative electrospray ionization in the multiple reaction monitoring mode. The following oxylipins were quantified: prostaglandin E2 (PGE2), delta-12 prostaglandin D2 (d12-PGD2), prostaglandin D2 (PGD2), 15-deoxy-prostaglandin D2 (15d-PGD2), 13,14-dihydro-15-keto prostaglandin D2 (DK-PD2), prostaglandin J2 (PGJ2), 15-deoxy-prostaglandin J2 (15d-PGJ2), prostaglandin F2 alpha (PGF2α), 8-iso-PGF2α/8-isoprostane (8-IsoP), AA, 5-hydroxyeicosatetraenoic acid (5-HETE), 8-HETE, 9-HETE, 11-HETE, 12-HETE, 15- HETE, 20-HETE, (±)8(9)-epoxy-5Z,11Z,14Z-eicosatrienoic acid (±8(9)-EET), (±)11(12)-epoxy5Z,8Z,14Z-eicosatrienoic acid (±11(12)-EET), (±)14(15)-epoxy-5Z,8Z,11Z-eicosatrienoic acid (±14(15)- EET), 9-hydroxyoctadecadienoic acid (9-HODE), 13-HODE, 5-hydroxy-6E,8Z,11Z,14Z,17Z-eicosapentaenoic acid (5-HEPE), 9-HEPE, 15-HEPE, 18-HEPE, 17(S)-hydroxy-docosahexaenoic acid (17S-HDHA), and leukotriene (L) B4. All quantifications were performed using freshly prepared calibration curves; the performance of the assay was monitored by inclusion of multiple quality-control samples. The following internal standards were used: PGD2-d9, 13,14-dihydro-15-keto PGD2-d4, 15- deoxy-Δ12, 14-PGJ2-d4, 11β-PGF2α-d4, PGE2-d9, PGF2α-d9, 8-Isop-d4, 5-HETE-d8, 12-HETE-d8, 20-HETE-d6, (±)8(9)-EET-d11, (±)11(12)-EET-d11, (±)14(15)-EET-d11, LB-d4, LC-d5, LD-d5, and LE-d5. All compounds were purchased from Cayman Chemicals. No chiral analysis of hydroxylated fatty acids was performed, thus no information about the enzymatic source can be determined using this method.
Statistical analysis
Oxylipin levels are presented in ng/mL except for prostaglandins and LB4, which are presented in pg/mL. All statistical analyses and graphic production were performed using MetaboAnalyst 5.0 (Montreal, Quebec, Canada).26 Values were log transformed and auto-centered (mean centered and divided by the standard deviation of each variable) consistently resulting in a normal distribution for subsequent analyses. One-way analysis of variance (ANOVA) was performed to assess for differences in individual oxylipin levels among groups. Statistical significance was defined as p < 0.05 with a false discovery rate <0.1. When indicated, subsequent pairwise comparisons using Fisher’s least significant difference were then used to identify differences between specific groups. In addition, global differences in lung oxylipin profiles between the CPB/DHCA animals with and without BiAP infusion were analyzed using partial least squares discriminant analysis (PLS-DA).
Results
The gross appearance of lung tissue following CPB/DHCA demonstrated a hyperemic, atelectatic, and edematous pattern focused in the dorsal–caudal regions of the lungs as previously published (Fig. 1a, b).25 In addition, a single animal also showed evidence of severe injury in the upper lobes (Fig. 1c). This prompted further regional evaluation of lung tissue oxylipin metabolites across all groups (CPB/DHCA upper and lower lobes with and without BiAP and mechanically ventilated control upper and lower lobes) to see whether significant differences existed. Because we had not yet recognized the consistent regional differences in gross lung injury at the beginning of the study, one control and two bypass animals (the earliest ones performed) did not have an apical sample taken.
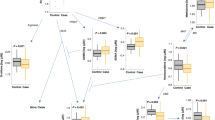
Individual oxylipins that differed by group are demonstrated in Table 1. Quantified values of all measured molecules are presented in Supplemental Table 1. AA, the initial product following cleavage of the phospholipid membrane, was the first oxylipin metabolite analyzed. Using one-way ANOVA, AA levels were significantly different across groups (Table 1). On post hoc pairwise comparison using Fisher’s least significant difference, AA metabolites followed a general pattern of higher levels in all lobes of CPB/DHCA animals (with and without BiAP) compared to mechanically ventilated controls (Fig. 2). Most pairwise comparisons reached statistical significance (Table 1) despite the small sample size.
AA metabolites followed a general pattern of higher levels in all lobes of CPB/DHCA animals (with and without BiAP) compared to mechanically ventilated controls. 1 = control upper lobe, 2 = control lower lobe, 3 = CPB/DHCA upper lobe, 4 = CPB/DHCA lower lobe, 5 = CPB/DHCA with BiAP upper lobe, 6 = CPB/DHA with BiAP lower lobe.
AA metabolites produced through CYP450 were the next group of metabolites analyzed and multiple metabolites (8-HETE, 9-HETE, 12-HETE, 15-HETE) were found to be significantly different across groups as well (Table 1). These four metabolites followed a similar pattern to AA, with generally higher levels in animals undergoing CPB/DHCA (with or without BiAP) compared to controls (Fig. 3). Pairwise comparisons that reached statistical significance are shown in Table 1. Of note, a few of the CYP450 metabolites (8-HETE, 12-HETE, 15-HETE) can also be produced through the LOX pathway.
CYP450 metabolites (8-HETE, 9-HETE, 12-HETE, 15-HETE) followed a similar pattern to AA, with generally higher levels in animals undergoing CPB/DHCA (with or without BiAP) compared to controls. 1 = control upper lobe, 2 = control lower lobe, 3 = CPB/DHCA upper lobe, 4 = CPB/DHCA lower lobe, 5 = CPB/DHCA with BiAP upper lobe, 6 = CPB/DHA with BiAP lower lobe.
Three AA metabolites produced through the LOX pathway (17S-HDHA, 15-HEPE, 13-HODE) were also significantly different across groups, again following the general pattern of higher levels in the lungs of CPB/DHCA animals (with or without BiAP) compared to controls (Fig. 4). Pairwise comparisons that reached statistical significance are shown in Table 1.
LOX metabolites (17S-HDHA, 15-HEPE, 13-HODE) followed the general pattern of higher levels in the lungs of CPB/DHCA animals (with or without BiAP) compared to controls. 1 = control upper lobe, 2 = control lower lobe, 3 = CPB/DHCA upper lobe, 4 = CPB/DHCA lower lobe, 5 = CPB/DHCA with BiAP upper lobe, 6 = CPB/DHA with BiAP lower lobe.
Prostaglandin metabolites of AA via the COX pathway (15d-PD2, PF2a, total PJ2, PD2, DK-PD2) were also significantly different across groups (Table 1) but followed a unique pattern compared to the metabolites from the other pathways (Fig. 5). On pairwise comparison, the general pattern showed lower levels of COX metabolites in the upper lobe of control animals with higher levels in the control lower lobe, even compared to CPB/DHCA animals (with and without BiAP) (Fig. 5). Again, multiple pairwise comparisons reached statistical significance despite the small samples size (Table 1).
COX metabolites (15d-PD2, PF2a, total PJ2, PD2, DK-PD2) followed a unique pattern compared to the metabolites from the other pathways, with lower levels in the upper lobe of control animals with higher levels in the control lower lobe, even compared to CPB/DHCA animals (with and without BiAP). 1 = control upper lobe, 2 = control lower lobe, 3 = CPB/DHCA upper lobe, 4 = CPB/DHCA lower lobe, 5 = CPB/DHCA with BiAP upper lobe, 6 = CPB/DHA with BiAP lower lobe.
Overall, we found minimal evidence that BiAP infusion at the tested dose significantly impacted lung tissue oxylipin levels in animals exposed to CPB/DHCA. By PLS-DA, there was no global difference in tissue levels of all measured oxylipins in CPB/DHCA + BiAP animals compared to CPB/DHCA animals without BiAP (R2 = 0.1; Q2 = −0.71). At the level of the individual metabolites, only two significant pairwise differences were identified: higher 15-HEPE and 13-HODE in the lower lobes of CPB/DHCA with BiAP animals compared to CPB/DHCA animals without BiAP.
Discussion
Key findings
In this study, we present the first report of pulmonary tissue-level changes in oxylipins induced by CPB/DHCA. Based on our gross tissue findings of a consistent injury pattern focused in the lower lobe regions of both CPB/DHCA animals and controls, we analyzed the lower lobe tissue oxylipin levels separately from the upper lobe tissue. Our key findings show an increase in AA metabolites, including multiple CYP450 and LOX derivatives, in CPB/DHCA-exposed animals compared to controls that appeared to be independent of lung region. COX metabolites showed a slightly different pattern, with higher levels of PGE metabolites in both CPB/DHCA-exposed animals and the lower lobe of mechanically ventilated control animals compared to the upper lobe of control animals.
Gross lung injury
Initial differences in appearance of gross anatomic lung specimens were noted in our animal models, prompting further investigation into oxylipin metabolites. Gross appearance of the lungs demonstrated a hyperemic and edematous pattern focused in the lower lobe regions of the lung. These dependent areas are at higher risk of atelectasis and injury. This has been described in acute respiratory distress syndrome (ARDS) literature, noting that, in supine position, the dorsal lung unit is exposed to higher pleural pressures and is more likely to become atelectatic.27,28 Porcine models of respiratory physiology demonstrate posture-mediated regional ventilation heterogeneity in the caudocranial axis and blood flow heterogeneity in the ventrodorsal axis, leading to the greatest reduction in alveolar oxygen tension in the dorsocaudal lung.29 This combination of physiologic factors places the lower lobe at high risk for injury during cardiac surgery with CPB, where supine positioning is required both during surgery and in the immediate postoperative period.
Oxylipins in lung disease
Despite the clear risk to the lung during cardiac surgery with CPB, the tissue biochemical changes associated with this abnormal perioperative lung physiology are poorly characterized. Tissue oxylipin production may be an important contributor. Non-CPB ALI is consistently accompanied by increased oxylipin production.30,31 Neutrophils are thought to be the primary source of this oxylipin production. Endothelial cells of the lung vasculature and airway epithelial cells have also been shown to be sites of production, release, and breakdown in non-CPB models.10,32,33 AA is released from cell membranes by cytosolic phospholipase A2 and then metabolized via three primary pathways (CYP450, COX, or LOX) resulting in biologically active lipid mediators.31
The effects of AA metabolites on lung tissue have been described in non-CPB models. AA metabolites are generally thought to be pro-inflammatory via cytokine production and activation of the innate and adaptive immune system.34,35 Prostaglandins and leukotrienes are often produced at the onset of inflammation, promoting the induction of edema and leukocyte recruitment.36 PGE2 specifically is implicated in hyperalgesia and fever. Leukotrienes such as LTB4 are associated with neutrophil recruitment and vascular leakage.37 Kidney models have also suggested association between PGE2 and development of glomerulonephritis.34
Many metabolites also possess dual pro-/anti-inflammatory properties leading to counter-regulatory effects. For example, pro-inflammatory prostanoids PGE2 and prostacyclin, implicated in maintaining low pulmonary vascular resistance, are thought to have some anti-inflammatory properties as well.14,36,38,39 CYP450 metabolites (such as epoxyeicosantrienoic acids or EETs) have also been shown to have anti-inflammatory properties, especially in vascular inflammation, with some suggestion of similar properties in the lungs.13 PGD2, PGF2a, and LOX-derived 5-HETE, proposed anti-inflammatory oxylipins, were found to be increased in hypoxic rabbit lung homogenates compared to normoxic samples.40 Elevated prostaglandins have also previously been seen in bronchoalveolar lavage fluid of patients with ARDS and PGD2 has reported anti-inflammatory effects in endotoxin-mediated ALI.41 Data has been mixed as to whether COX inhibition alone mitigates ARDS, suggesting a possible benefit of the anti-inflammatory effect provided by some prostaglandins following acute inflammation.42,43,44
Oxylipin activation in CPB
Systemic AA release also occurs following cardiac surgery with CPB.10,45,46 AA metabolite production during CPB is thought to be driven by the concomitant activation of the complement, fibrinolytic, and coagulation cascades secondary to the global inflammatory response that occurs during CPB.5,10 This ultimately leads to endothelial cell activation of PLA2 and COX.10 Increased circulating serum prostaglandins (thromboxane, PGE2, prostacyclin) following CPB have been described in both human and animal models. The effect of these increased prostanoids is not well described. There is speculation that prostacyclin is protective to pulmonary vasculature by aiding in smooth muscle relaxation. PGE2, a potent vasodilator, may also play a role in modulating pulmonary vascular resistance.44,46,47 While the existing literature demonstrates elevated circulating oxylipins following CPB, to our knowledge no prior study has evaluated tissue-specific concentrations following CPB or DHCA.
Pulmonary oxylipins in CPB and mechanically ventilated controls
Our study comparing oxylipin levels of CPB/DHCA and mechanically ventilated control animals showed two distinct patterns. The first pattern was elevated lung tissue AA, CYP450, and LOX metabolites seen with exposure to CPB/DHCA. These metabolites were elevated in both upper and lower lobes of CPB/DHCA animals suggesting a relatively uniform physiologic insult. Due to the circulatory changes caused by CPB and DHCA, the lungs are at significant risk of IR injury. This increase in oxylipins is thought to be secondary to the systemic inflammation caused by CPB/DHCA, leading to activation of AA metabolites via COX and PLA2.10 Cellular damage from ROS following IR injury may also promote activation of AA metabolites, causing endothelial injury and in turn complement activation.4 Further studies are needed to understand the exact mechanisms of AA metabolite production in the lungs and assess whether targeted modulation of the lung oxylipin production seen in our study could mitigate post-CPB/DHCA injury. Furthermore, pulmonary IR injury may also contribute to the systemic effects of CPB, releasing bioactive mediators, including oxylipins, and resulting in remote effects on the heart, kidneys, and liver through organ crosstalk.4,48 Although our study did not address crosstalk following IR injury, if pro-inflammatory AA metabolites produced in the lung could be therapeutically modulated in a targeted fashion, it could reduce or prevent not only lung injury but also systemic sequelae following CPB.
The COX-derived metabolites (15d-PD2, PF2a, total PJ2, PGD2, DK-PD2) followed a slightly different pattern, in that tissue levels were higher in both CPB/DHCA-exposed lung tissue and mechanically ventilated control lower lobes. This pattern introduces the possibility of factors outside of CPB-induced inflammation leading to higher levels of lung oxylipins. Hypoxic lung homogenate from mechanically ventilated rabbits has previously been shown to have higher levels of PGD2 and PFA2a compared to lung from normoxic ventilated controls.40 PFA2a is a vasoconstrictor that contributes to hypoxic pulmonary vasoconstriction, while PGD2 is a vasodilator that acts in a counter-regulatory fashion. Our data suggest that dysregulation of COX-derived AA metabolites may contribute to the cycle of vasoconstriction and ischemia occurring in the high-risk dorsocaudal region of the lung and may be targets for mitigation of regional lung injury.49
Limitations
In this study, several limitations existed. The first limitation was the small number of animals. The limited number of animals in our study does introduce the possibility of a Type II error, such that, with a larger sample size, we may have seen significant differences across more metabolites or more subtle differences across lung regions. Despite the small sample size, significant differences were seen in many AA metabolite groups across upper and lower lobe CPB/DHCA animals demonstrating the substantial effect size of CPB/DHCA on lung oxylipin production. It is also possible that more minor effects of BiAP infusion on oxylipin production could have been missed due to the limited sample size. Second, because a clear regional differences of regional lung injury had not yet been identified, we did not obtain apical tissue in one control, one CPB/DHCA animal, and one CPB/DHCA with BiAP animal. Another limitation of our study is that the study was designed to assess CPB/DHCA effects and regional effects rather than the effects of anesthesia and mechanical ventilation. All animals were exposed to supine positioning, anesthesia, and mechanical ventilation and we did not include healthy control animals with no ventilator exposure or alternate positioning strategies. Further studies are needed to understand the impact of anesthesia, mechanical ventilation, and positioning on pulmonary AA metabolite production. We acknowledge that there is a substantial amount of within-group variation, and it is unclear whether that is due to true differences among individual animals or due to variation imposed by limited sampling in the setting of grossly heterogeneous lung disease. In future studies, it would be beneficial to obtain samples from multiple lung sites to further address this question. Ideally, human lung tissue samples would be used to compare oxylipin production rather than porcine models. However, obtaining human lung tissue after pediatric cardiac surgery is ethically untenable and similarities between human and pig lung anatomy have helped to establish pigs as a relevant translational respiratory model.50
Conclusion
In conclusion, our findings show that both CPB/DHCA and, to a lesser extent, lung region influence tissue-level AA metabolite production. We did not find substantial evidence for an effect of BiAP infusion on pulmonary oxylipin production. Given that this was one of the first studies of its kind, there are still unanswered questions regarding the mechanisms of lung oxylipin regulation and the impact of oxylipin dysregulation on tissue injury and outcomes following CPB/DHCA. The finding of differential regional COX/prostaglandin metabolite production in both CPB/DHCA animals and controls also suggests a need to further assess both regional oxylipin metabolism with mechanical ventilation alone and the potential effects of patient positioning on oxylipin production.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
19 July 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41390-022-02189-3
References
Benjamin, E. J. et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137, e67–e492 (2018).
Centers for Disease Control and Prevention. Congenital heart defects. www.cdc.gov/ncbddd/heartdefects (2022).
Christen, S. et al. Oxidative stress precedes peak systemic inflammatory response in pediatric patients undergoing cardiopulmonary bypass operation. Free Radic. Biol. Med. 38, 1323–1332 (2005).
Ferrari, R. S. & Andrade, C. F. Oxidative stress and lung ischemia-reperfusion injury. Oxid. Med. Cell Longev. 2015, 590987 (2015).
Zakkar, M., Guida, G., Suleiman, M. S. & Angelini, G. D. Cardiopulmonary bypass and oxidative stress. Oxid. Med. Cell Longev. 2015, 189863 (2015).
Hoegl, S., Zwissler, B., Eltzschig, H. K. & Vohwinkel, C. Acute respiratory distress syndrome following cardiovascular surgery: current concepts and novel therapeutic approaches. Curr. Opin. Anaesthesiol. 29, 94–100 (2016).
Engels, G. E. & van Oeveren, W. Biomarkers of lung injury in cardiothoracic surgery. Dis. Markers 2015, 472360 (2015).
Blinder, J. J. et al. Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J. Thorac. Cardiovasc. Surg. 143, 368–374 (2012).
McElhinney, D. B. et al. Necrotizing enterocolitis in neonates with congenital heart disease: risk factors and outcomes. Pediatrics 106, 1080–1087 (2000).
Kozik, D. J. & Tweddell, J. S. Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann. Thorac. Surg. 81, S2347–S2354 (2006).
Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr. Crit. Care Med. 16, 428–439 (2015).
Schlensak, C. et al. Bronchial artery perfusion during cardiopulmonary bypass does not prevent ischemia of the lung in piglets: assessment of bronchial artery blood flow with fluorescent microspheres. Eur. J. Cardiothorac. Surg. 19, 326–331 (2001).
Lundstrom, S. L. et al. Lipid mediator profiling in pulmonary disease. Curr. Pharm. Biotechnol. 12, 1026–1052 (2011).
Roman, R. J. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 82, 131–185 (2002).
Heidel, J. R. et al. In vivo chemotaxis of bovine neutrophils induced by 5-lipoxygenase metabolites of arachidonic and eicosapentaenoic acid. Am. J. Pathol. 134, 671–676 (1989).
Moreno, J. J. Differential effects of arachidonic and eicosapentaenoic Acid-derived eicosanoids on polymorphonuclear transmigration across endothelial cell cultures. J. Pharm. Exp. Ther. 331, 1111–1117 (2009).
Van der Vusse, G. J., Reneman, R. S. & van Bilsen, M. Accumulation of arachidonic acid in ischemic/reperfused cardiac tissue: possible causes and consequences. Prostaglandins Leukot. Ess. Fat. Acids 57, 85–93 (1997).
Shishehbor, M. H. et al. Systemic elevations of free radical oxidation products of arachidonic acid are associated with angiographic evidence of coronary artery disease. Free Radic. Biol. Med. 41, 1678–1683 (2006).
Strassburg, K. et al. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis: application in cardiac surgery. Anal. Bioanal. Chem. 404, 1413–1426 (2012).
Buczynski, M. W., Dumlao, D. S. & Dennis, E. A. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J. Lipid Res. 50, 1015–1038 (2009).
Bujak, R., Struck-Lewicka, W., Markuszewski, M. J. & Kaliszan, R. Metabolomics for laboratory diagnostics. J. Pharm. Biomed. Anal. 113, 108–120 (2015).
Pillai, P. S. et al. Chemical mediators of inflammation and resolution in post-operative abdominal aortic aneurysm patients. Inflammation 35, 98–113 (2012).
Davidson, J. A. et al. Alkaline phosphatase treatment of acute kidney injury in an infant piglet model of cardiopulmonary bypass with deep hypothermic circulatory arrest. Sci. Rep. 9, 14175 (2019).
Khailova, L. et al. Tissue alkaline phosphatase activity and expression in an experimental infant swine model of cardiopulmonary bypass with deep hypothermic circulatory arrest. J. Inflamm. 17, 27 (2020).
Cooney, S. J. et al. Regional lung metabolic profile in a piglet model of cardiopulmonary bypass with circulatory arrest. Metabolomics 17, 89 (2021).
Pang, Z. et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 49, W388–W396 (2021).
Henderson, W. R., Griesdale, D. E., Dominelli, P. & Ronco, J. J. Does prone positioning improve oxygenation and reduce mortality in patients with acute respiratory distress syndrome? Can. Respir. J. 21, 213–215 (2014).
Carvalho, A. R. et al. Distribution of regional lung aeration and perfusion during conventional and noisy pressure support ventilation in experimental lung injury. J. Appl. Physiol. 2011, 1083–1092 (1985).
Altemeier, W. A., McKinney, S., Krueger, M. & Glenny, R. W. Effect of posture on regional gas exchange in pigs. J. Appl. Physiol. 2004, 2104–2111 (1985).
Bakhle, Y. S. Biosynthesis of prostaglandins and thromboxanes in lung. Bull. Eur. Physiopathol. Respir. 17, 491–508 (1981).
Bonnans, C. & Levy, B. D. Lipid mediators as agonists for the resolution of acute lung inflammation and injury. Am. J. Respir. Cell Mol. Biol. 36, 201–205 (2007).
Eling, T. E. & Ally, A. I. Pulmonary biosynthesis and metabolism of prostaglandins and related substances. Environ. Health Perspect. 55, 159–168 (1984).
Holtzman, M. J. Arachidonic acid metabolism in airway epithelial cells. Annu. Rev. Physiol. 54, 303–329 (1992).
Wang, T. et al. Arachidonic acid metabolism and kidney inflammation. Int. J. Mol. Sci. 20, 3683 (2019).
de Paula Rogerio, A., Sorgi, C. A., Sadikot, R. & Carlo, T. The role of lipids mediators in inflammation and resolution. Biomed. Res. Int. 2015, 605959 (2015).
Ricciotti, E. & FitzGerald, G. A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 986–1000 (2011).
Dennis, E. A. & Norris, P. C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 15, 511–523 (2015); erratum 15, 724 (2015).
Pons, F., Williams, T. J., Kirk, S. A., McDonald, F. & Rossi, A. G. Pro-inflammatory and anti-inflammatory effects of the stable prostaglandin D2 analogue, ZK 118.182. Eur. J. Pharmacol. 261, 237–247 (1994).
Scher, J. U. & Pillinger, M. H. The anti-inflammatory effects of prostaglandins. J. Investig. Med. 57, 703–708 (2009).
Kiss, L. et al. Direct eicosanoid profiling of the hypoxic lung with comprehensive analysis via capillary liquid chromatography with dual online photodiode-array and tandem mass-spectrometric detection. Anal. Bioanal. Chem. 390, 697–714 (2008).
Takahisa, M. et al. Anti-inflammatory role of PGD2 in acute lung inflammation and therapeutic application of its signal enhancement. Proc. Natl Acad. Sci. USA 110, 5205–5210 (2013).
Hinshaw, L. B., Solomon, L. A., Erdös, E. G., Reins, D. A. & Gunter, B. J. Effects of acetylsalicylic acid on the canine response to endotoxin. J. Pharm. Exp. Ther. 157, 665–671 (1967).
Cuzzocrea, S. et al. Protective effects of Celecoxib on lung injury and red blood cells modification induced by carrageenan in the rat. Biochem. Pharmacol. 63, 785–795 (2002).
Bernard, G. R. et al. The Ibuprofen in Sepsis Study Group. The effects of ibuprofen on the physiology and survival of patients with sepsis. N. Engl. J. Med. 336, 912–918 (2007).
Greely, W. J. et al. Effects of cardiopulmonary bypass on eicosanoids metabolism during pediatric cardiovascular surgery. J. Thorac. Cardiovasc. Surg. 95, 842–849 (1988).
Saunders, C. R., Rittenhouse, E. A., Jaffe, B. M. & Doty, D. B. Prostaglandin E biosynthesis during cardiopulmonary bypass. J. Surg. Res. 24, 188–192 (1978).
Faymonville, M. E. et al. Prostaglandin E2, prostacyclin, and thromboxane changes during nonpulsatile cardiopulmonary bypass in humans. J. Thorac. Cardiovasc. Surg. 91, 858–866 (1986).
Esme, H., Fidan, H., Koken, T. & Solak, O. Effect of lung ischemia-reperfusion on oxidative stress parameters of remote tissues. Eur. J. Cardiothorac. Surg. 29, 294–298 (2006).
Galvin, I., Drummond, G. B. & Nirmalan, M. Distribution of blood flow and ventilation in the lung: gravity is not the only factor. Br. J. Anaesth. 98, 420–424 (2007).
Judge, E. P. et al. Anatomy and bronchoscopy of the porcine lung. A model for translational respiratory medicine. Am. J. Respir. Cell Mol. Biol. 51, 334–343 (2014).
Funding
This study was supported by Department of Defense PR152240 (PI Davidson), American Heart Association 17IRG33410724 (PI Davidson), and National Institutes of Health/National Heart, Lung, and Blood Institute K23HL123634 (PI Davidson).
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: K.G.I., J.R., L.K., J.J., R.I., S.L., S.M.O.L., J.K., J.A.D. Drafting the article or revising it critically for important intellectual content: K.G.I., J.R., L.K., J.A.D. Final approval of the version to be published: K.G.I., J.A.D.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This was an animal study and therefore did not require patient consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Iguidbashian, K.G., Robison, J., Khailova, L. et al. Changes in infant porcine pulmonary tissue oxylipins induced by cardiopulmonary bypass. Pediatr Res 92, 1274–1281 (2022). https://doi.org/10.1038/s41390-022-02125-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02125-5