Abstract
Background
Infants with single ventricle heart disease (SVHD) suffer morbidity from insufficient pulmonary blood flow, which may be related to impaired arginine metabolism. No prior study has reported quantitative mapping of arginine metabolites to evaluate the relationship between circulating metabolite levels and outcomes.
Methods
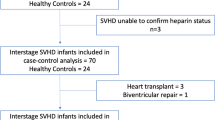
Prospective cohort study of 75 SVHD cases peri-Stage 2 and 50 healthy controls. We targeted pre- and post-op absolute serum quantification of 9 key members of the arginine metabolism pathway by tandem mass spectrometry. Primary outcomes were length of stay (LOS) and post-Stage 2 hypoxemia.
Results
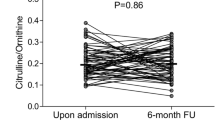
Pre-op cases showed alteration in 6 metabolites including decreased arginine and increased asymmetric dimethyl arginine (ADMA) levels compared to controls. Post-op cases demonstrated decreased arginine and citrulline levels persisting through 48 h. Adjusting for clinical variables, lower pre-op and 2 h post-op concentrations of multiple metabolites, including arginine and citrulline, were associated with longer post-op LOS (p < 0.01). Increased ADMA at 24 h was associated with greater post-op hypoxemia burden (p < 0.05).
Conclusion
Arginine metabolism is impaired in interstage SVHD infants and is further deranged following Stage 2 palliation. Patients with greater metabolite alterations experience greater post-op morbidity. Decreased arginine metabolism may be an important driver of pathology in SVHD.
Impact
-
Interstage infants with SVHD have significantly altered arginine-nitric oxide metabolism compared to healthy children with deficiency of multiple pathway intermediates persisting through 48 h post-Stage 2 palliation.
-
After controlling for clinical covariates and classic catheterization-derived predictors of Stage 2 readiness, both lower pre-operation and lower post-operation circulating metabolite levels were associated with longer post-Stage 2 LOS while increased post-Stage 2 ADMA concentration was associated with greater post-op hypoxemia.
-
Arginine metabolism mapping offers potential for development using personalized medicine strategies as a biomarker of Stage 2 readiness and therapeutic target to improve pulmonary vascular health in infants with SVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Hoffman, J. I. E. & Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 39, 1890–1900 (2002).
Ohye, R. G., Schranz, D. & D’Udekem, Y. Current therapy for hypoplastic left heart syndrome and related single ventricle lesions. Circulation 134, 1265–1279 (2016).
Caspi, J., Pettitt, T. W., Mulder, T. & Stopa, A. Development of the pulmonary arteries after the Norwood procedure: comparison between Blalock-Taussig shunt and right ventricular-pulmonary artery conduit. Ann. Thorac. Surg. 86, 1299–1304 (2008).
Huffmyer, J. L. & Groves, D. S. Pulmonary complications of cardiopulmonary bypass. Best. Pr. Res Clin. Anaesthesiol. 29, 163–175 (2015).
Kogon, B. E. et al. The bidirectional Glenn operation: A risk factor analysis for morbidity and mortality. J. Thorac. Cardiovasc Surg. 136, 1237–1242 (2008).
Brown, D. W. et al. Cardiac magnetic resonance versus routine cardiac catheterization before bidirectional Glenn anastomosis: long-term follow-up of a prospective randomized trial. J. Thorac. Cardiovasc Surg. 146, 1172–1178 (2013).
Lee, T. M. et al. Risk factor analysis for second-stage palliation of single ventricle anatomy. Ann. Thorac. Surg. 93, 614–618 (2012).
Malhotra, S. P. et al. Performance of cavopulmonary palliation at elevated altitude: midterm outcomes and risk factors for failure. Circulation 118, S177–S181 (2008).
Rashid, J. et al. Therapeutic potential of citrulline as an arginine supplement: A clinical pharmacology review. Paediatr. Drugs 22, 279–293 (2020).
Lewis, G. D. et al. Metabolic profiling of right ventricular-pulmonary vascular function reveals circulating biomarkers of pulmonary hypertension. J. Am. Coll. Cardiol. 67, 174–189 (2016).
Chao, Y. K. et al. Pulmonary perfusion with L-arginine ameliorates post-cardiopulmonary bypass lung injury in a rabbit model. J. Surg. Res. 167, e77–e83 (2011).
Barr, F. E. et al. Pharmacokinetics and safety of intravenously administered citrulline in children undergoing congenital heart surgery: potential therapy for postoperative pulmonary hypertension. J. Thorac. Cardiovasc Surg. 134, 319–326 (2007).
Hassinger, A. B. et al. Elevated preoperative serum asymmetrical dimethylarginine (ADMA) is associated with poor outcomes after pediatric cardiac surgery. Intensive Care Med 38, 1697–1704 (2012).
Navaei, A. H. et al. Derangement of arginine and related amino acids in children undergoing surgery for congenital heart disease with cardiopulmonary bypass. Crit. Care Explor 2, e0150 (2020).
Frank B. S. et al. Interstage single ventricle heart disease infants show dysregulation in multiple metabolic pathways: Targeted metabolomics analysis. JACC Adv. 2, 100169 (2023).
Frank, B. S. et al. Proteomic profiling identifies key differences between inter-stage infants with single ventricle heart disease and healthy controls. Transl. Res. 229, 24–37 (2021).
Klepacki, J., Klawitter, J., Klawitter, J., Thurman, J. M. & Christians, U. A high-performance liquid chromatography-tandem mass spectrometry-based targeted metabolomics kidney dysfunction marker panel in human urine. Clin. Chim. Acta 446, 43–53 (2015).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach ot multiple testing. J. R. Stat. Soc. 57, 289–300 (1995).
Wu, G. & Morris, S. Arginine metabolism: Nitric oxide and beyond. J. Biochem 336, 1–17 (1998).
Zuckerbraun, B. S., George, P. & Gladwin, M. T. Nitrite in pulmonary arterial hypertension: therapeutic avenues in the setting of dysregulated arginine/nitric oxide synthase signalling. Cardiovasc. Res. 89, 542–552 (2011).
Lavallee, M. et al. Crosstalk between endothelin and nitric oxide in the control of vascular tone. Heart Fail. Rev. 6, 265–276 (2011).
Xia, Y. & Zweier, J. Superoxide and peroxynitrite generation from inducible nitric oxide synthase. Macrophages. PNAS 94, 6954–6958 (1997).
Murakami, K. et al. L-arginine attenuates acute lung injury after smoke inhalation and burn injury in sheep. Shock 28, 477–483 (2007).
Kielstein, J. T. et al. Asymmetrical dimethylarginine in idiopathic pulmonary arterial hypertension. Arterioscler Thromb. Vasc. Biol. 25, 1414–1418 (2005).
Pearson, D. et al. Urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N. Engl. J. Med. 344, 1832–1838 (2001).
Xu, W. et al. Integrative proteomics and phosphoproteomics in pulmonary arterial hypertension. Sci. Rep. 9, 18623 (2019).
Luneburg, N., Harbaum, L. & Hennigs, J. K. Low plasma citrulline levels are associated with acute respiratory distress syndrome in patients with severe sepsis. Crit. Care 17, 1–8 (2013).
O’Connor, M. G. et al. Pulmonary hypertension in the premature infant population: Analysis of echocardiographic findings and biomarkers. Pediatr. Pulmonol. 53, 302–309 (2018).
Motoki, N. et al. Identification of metabolomic profile related to adult Fontan pathophysiology. Int J. Cardiol. Heart Vasc. 37, 100921 (2021).
Barr, F. E. et al. Effect of cardiopulmonary bypass on urea cycle intermediates and nitric oxide levels after congenital heart surgery. J. Pediatr. 142, 26–30 (2003).
Trittmann, J. K. et al. Plasma asymmetric dimethylarginine levels are increased in neonates with bronchopulmonary dysplasia-associated pulmonary hypertension. J. Pediatr. 166, 230–233 (2015).
Fang, Z. F. et al. Asymmetric dimethyl-l-arginine is a biomarker for disease stage and follow-up of pulmonary hypertension associated with congenital heart disease. Pediatr. Cardiol. 36, 1062–1069 (2015).
Dimitroulas, T. et al. Asymmetrical dimethylarginine in systemic sclerosis-related pulmonary arterial hypertension. Rheumatol. (Oxf.) 47, 1682–1685 (2008).
Goldberg, D. J. et al. Results of the FUEL trial. Circulation 141, 641–651 (2020).
Goldberg, D. J. et al. Impact of oral sildenafil on exercise performance in children and young adults after the fontan operation: a randomized, double-blind, placebo-controlled, crossover trial. Circulation 123, 1185–1193 (2011).
Goldberg, D. J. et al. Results of a phase I/II multi-center investigation of udenafil in adolescents after fontan palliation. Am. Heart J. 188, 42–52 (2017).
Rosenkranz, S. et al. Riociguat for pulmonary arterial hypertension associated with congenital heart disease. Heart 101, 1792–1799 (2015).
Ghofrani, H. A. et al. Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 369, 330–340 (2013).
Smith, H. A. et al. Nitric oxide precursors and congenital heart surgery: a randomized controlled trial of oral citrulline. J. Thorac. Cardiovasc. Surg. 132, 58–65 (2006).
Douglass, M. et al. Folic acid, either solely or combined with L-citrulline, improves NO signaling and ameliorates chronic hypoxia-induced pulmonary hypertension in newborn pigs. Physiol. Rep. 9, e15096 (2021).
Dikalova, A. et al. Combined l-citrulline and tetrahydrobiopterin therapy improves NO signaling and ameliorates chronic hypoxia-induced pulmonary hypertension in newborn pigs. Am. J. Physiol. Lung Cell Mol. Physiol. 318, L762–L772 (2020).
Douglass, M. S., Zhang, Y., Kaplowitz, M. R. & Fike, C. D. L-citrulline increases arginase II protein levels and arginase activity in hypoxic piglet pulmonary artery endothelial cells. Pulm. Circ. 11, 20458940211006289 (2021).
Vadivel, A. et al. L-citrulline attenuates arrested alveolar growth and pulmonary hypertension in oxygen-induced lung injury in newborn rats. Pediatr. Res. 68, 519–525 (2010).
Fike, C. D. et al. Rescue treatment with L-citrulline inhibits hypoxia-induced pulmonary hypertension in newborn pigs. Am. J. Respir. Cell Mol. Biol. 53, 255–264 (2015).
Funding
This study was supported by the American Heart Association - AHA 20CDA35310498 (Frank) and AHA18IPA34170070 (Davidson), the National Institutes of Health - NIH/NCATS Colorado CTSA, No. UL1 TR001082 and UL1 TR002535, NIH/NHLBI K23HL123634 (Frank), and R01HL156936 (Davidson), and the Jayden DeLuca Foundation. There was no relationship with industry associated with this manuscript.
Author information
Authors and Affiliations
Contributions
B.F. wrote the first draft. B.F. and J.D. created the original study design. D.N. performed the statistical analysis. D.N., L.K., M.M., G.M., M.T., M.D. and J.D. assisted with study operations, analyzed the data, and edited the manuscript. All authors have approved the final version and agree to take accountability for the submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent Statement
Written informed consent was obtained from the study subjects’ parents in all cases.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frank, B.S., Niemiec, S., Khailova, L. et al. Arginine-NO metabolites are associated with morbidity in single ventricle infants undergoing stage 2 palliation. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03162-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03162-y