Abstract
Background
Neurodevelopmental abnormalities are prevalent in children with tetralogy of Fallot. Our aim was to investigate the structural brain alterations of preschool-aged children with tetralogy of Fallot and its correlation with neurodevelopmental outcome.
Methods
T1-weighted structural images were obtained from 25 children with tetralogy of Fallot who had undergone cardiopulmonary bypass surgery and from 24 normal controls. Cortical morphological indices including gray matter volume, cortical thickness, sulcal depth, gyrification, and cortical surface complexity were compared between the two groups. Neurodevelopmental assessments of the children with tetralogy of Fallot were performed with the Wechsler Preschool and Primary Scale of Intelligence.
Results
Cortical morphological differences between groups were distributed throughout the right caudal middle frontal gyrus, right fusiform gyrus, right lateral occipital gyrus, right precuneus, and left inferior parietal lobule. Among children with tetralogy of Fallot, altered cortical structures were correlated with the visual spatial index, working memory index, and perioperative variables.
Conclusion
Our results suggested that abnormal cortical structure in preschool-aged children with tetralogy of Fallot may be the persistent consequence of delayed cortical development in fetuses and cortical morphology can be used as an early potential biomarker to capture regional brain abnormalities that are relevant to neurodevelopmental outcomes.
Impact
-
Altered cortical structures in preschool-aged children with ToF were correlated with both neurodevelopmental outcomes and clinical risk factors.
-
Cortical morphology can be used as an effective tool to evaluate neuroanatomical changes and detect underlying neural mechanisms in ToF patients.
-
Abnormal cortical structure may be the continuous consequence of delayed fetal brain development in children with ToF.
Similar content being viewed by others
Introduction
Congenital heart disease (CHD) is the most common congenital malformation in children.1 With improvements in surgical techniques and perioperative care, the long-term survival rate of children with CHD has been significantly increased.2 Previous studies have demonstrated neurodevelopmental deficits in children with CHD, including impaired executive function, memory, language, and social interaction, which reduce the quality of life and impose financial pressures on families and society.2 Tetralogy of Fallot (ToF) is the most common cyanotic cardiac defect and is characterized by pulmonary artery stenosis, aortic straddling, right ventricular hypertrophy, and ventricular septal defect.3 Knowledge of neurodevelopmental deficits in survivors with ToF is of great significance for optimizing the long-term treatment of patients.
Many magnetic resonance imaging (MRI) studies have found that children with CHD have a high prevalence of brain abnormalities. Delayed cortical development, impaired microstructural integrity, abnormal cerebral perfusion, and metabolism in CHD populations have been reported.4,5,6,7 Other functional MRI studies have observed connectivity disorders involving multiple brain regions in this population.8 Studies have provided evidence that brain abnormalities are associated with unfavorable neurodevelopmental outcomes in children with CHD. Andropoulos et al. found that preoperative brain injuries (white matter injury, infarction, and hemorrhage) were associated with impaired motor and language function in early infancy.9,10 Furthermore, studies have demonstrated that brain volume in infants and adolescents with CHD correlates with impaired cognition, motor function, and language development.11,12,13 In addition, diffusion tensor imaging studies have revealed that delayed maturation of white matter microstructure in CHD adolescents poses risks for impairments in working memory, attention, and learning.14,15 The preschool period is the critical period of brain and intelligence development in children. However, studies on the brain cortical characteristics and neurodevelopmental status of preschool-aged children with ToF remain rare.
The analysis of cortical morphology is an important method of observing brain diseases, such as schizophrenia, Alzheimer’s disease, and attention-deficit hyperactivity disorder.16,17,18 Surface-based morphometry (SBM) and voxel-based morphometry (VBM) based on T1-weighted high-resolution structure images can provide a comprehensive assessment of cortical structures, such as gray matter volume, cortical thickness, sulcal depth, gyrification, and cortical surface complexity. Since previous studies have found delayed cortical development in fetuses with CHD,19,20,21 we hypothesized that cortical structure abnormalities may still exist in preschool-aged children with ToF and are relevant to neurocognitive outcomes. In order to test this hypothesis, we aimed to evaluate the cortical structural changes in preschool-aged children with ToF using SBM and VBM and its correlations with both neurodevelopmental outcomes and clinical risk factors.
Methods
Participants
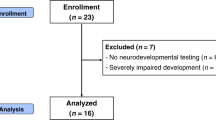
In this study, 31 ToF children who had undergone open-heart surgery requiring cardiopulmonary bypass (CPB) and 25 normal controls with comparable sex, education, and age were recruited in our hospital. The inclusion criteria for children with ToF were as follows: (1) age from 3 to 6 years; (2) no congenital disease or metabolic disease except ToF with pulmonary stenosis; (3) no central nervous system disease, such as tumor or trauma; (4) no history of mental illness or psychiatric medication; (5) cardiopulmonary bypass surgeries were performed before 3 years of age; and (6) right-handedness. Normal controls were recruited in these ways: (1) outpatients with transient fever (they are healthy after follow-up); (2) children who underwent regular physical examinations at the child health care clinic; and (3) volunteers from the society. The inclusion criteria for normal controls were as follows: (1) no congenital disease or metabolic disease; (2) no history of mental illness or psychiatric medication; (3) no central nervous system disease; (4) no history of surgery; and (5) right-handedness. The exclusion criteria were as follows: (1) contraindicated for MRI examination and (2) refusal to participate in this study. Informed consent was obtained from the legal guardian of the children, and the protocol was approved by the Institutional Ethics Committee of Children’s Hospital of Nanjing Medical University.
In this study, 4 ToF children who did not meet the inclusion criteria were excluded. Another 2 ToF children and 1 control were excluded because of poor MRI image quality. Thus, the cortical structures of 25 ToF children and 24 controls were analyzed.
Clinical data
At the study visit, ToF participants underwent measurement of height and weight. The educational background of each participant and the economic status and educational background of the parents were obtained through a questionnaire. Clinical data relevant for this study were extracted from the medical records of ToF participants, including McGoon index, age at intervention, time of surgery, CPB time, aortic cross-clamp (ACC) time, length of stay in the intensive care unit (ICU), length of hospital stay and duration of ventilation.
Neurodevelopmental assessment
The Chinese version of the Wechsler Preschool and Primary Scale of Intelligence—fourth edition (WPPSI-IV) was used to assess the neurodevelopmental outcome, which can test the verbal comprehension index, the visual spatial index, the working memory index, and the full-scale intelligence quotient (an IQ score with an expected mean of 100). The verbal comprehension index reflects language processing, including comprehension, reasoning, and expression. The visual spatial index assesses nonverbal skills to analyze and organize visual patterns. The working memory index shows the ability to temporarily store, manipulate and process information.
In this study, 20 ToF children completed the neurodevelopmental assessment, while normal controls lacked.
MR imaging
All participants underwent brain MRI scanning on a clinical 3.0 Tesla MRI system (Ingenia 3.0, Philips Healthcare, Best, the Netherlands) using a 16-channel head coil in the radiology department of our hospital. Before scanning, all participants were asked to keep awake for 8 h. MRI scanning was performed at night during natural sleep or with chloral hydrate sedation (1 ml/kg) with parental consent. Earplugs and foam were used to decrease the noise of scanning and head motion, respectively. Three-dimensional T1-weighted high-resolution structural images were obtained using the following parameters: echo time (TE) = 3.5 ms, repetition time (TR) = 7.9 ms, field of view (FOV) = 200 × 200 × 200 mm, slice thickness 1 mm, and acquisition time = 4 min 24 s. Conventional axial T2-weighted images were acquired to exclude brain lesions using the following parameters: TE = 110 ms, TR = 4000 ms, FOV = 200 × 200 × 119 mm, slice thickness 5 mm, and acquisition time = 1 min 28 s. Then, two experienced pediatric neuroradiologists blinded to the details of each participant’s medical history reviewed the images. If there was a difference of opinion, a consensus was reached through discussion.
Image analysis
Cortical morphology was evaluated by SBM analysis based on Statistical Parametric Mapping (SPM12) and the Computational Anatomy Toolbox (CAT12) on the MATLAB 8.2 platform (R2013b). First, the original DICOM data were converted into 3D NIfTI format. Next, the converted images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) and normalized. Then, the normalized images were used for the evaluation of cortical thickness and reconstruction of the central surface. Subsequently, cortical surface complexity, gyrification, and sulcal depth were calculated. Finally, the cortical thickness data were smoothed using a 15.0 mm full-width-at-half-maximum (FWHM) Gaussian kernel, whereas sulcal depth, gyrification, and cortical surface complexity data were smoothed using a 20.0 mm FWHM Gaussian kernel.
VBM analysis was performed to measure gray matter volume using the VBM8 toolbox in SPM8 on the MATLAB 8.2 platform. Each structural image was segmented into GM, WM, and CSF and subsequently normalized to the Montreal Neurological Institute (MNI) space template using the diffeomorphic anatomical registration through exponentiated lie algebra approach. An example of a segmented image is presented in Supplementary Fig. 1. The normalized images were then smoothed (FWHM = 8 mm) for statistical analysis.
Statistical analysis
SPSS 25.0 software was used to analyze the differences in demographic data and clinical data between the two groups. Continuous variables were analyzed by unpaired two-sample t test and are shown as the mean ± standard deviation (m ± sd) or median and range, whereas categorical variables were analyzed by the chi-square test and are expressed as numbers and percentages. The differences in cortical structures between the two groups were compared using unpaired two-sample t tests adjusting for age at MRI and sex and corrected for multiple comparisons using family-wise error (FWE) at the cluster level. Pearson correlation analysis and multiple linear regression analysis were used to investigate the associations between cortical structure changes and both clinical variables and neurodevelopmental outcomes by using SPSS 25.0. p < 0.05 was considered to be statistically significant.
Results
Individual and clinical characteristics
There was no significant difference in age, sex, and education between the ToF children and controls. The major comprehensive scores of WPPSI were <100 in the ToF children. The characteristics of the participants are presented in Table 1.
Cortical structure changes
Compared with the control group, the ToF group exhibited decreased cortical surface complexity in the right caudal middle frontal gyrus and decreased sulcal depth in the right fusiform gyrus and right lateral occipital gyrus; furthermore, cortical thickness in the right precuneus and gyrification index in the left inferior parietal lobule were increased in the ToF group relative to the control group (p < 0.05, FWE corrected) (Fig. 1 and Table 2). GM volume in the left medial frontal gyrus was larger in the ToF group than in the control group (p < 0.05, FWE corrected; Fig. 2 and Table 3). Furthermore, we extensively analyzed relative volumes normalized by total intracranial volume (TIV) and found no significant differences in GM/TIV, WM/TIV, CSF/TIV, GM/WM, and GM/(GM + WM) between the two groups (Table 4).
Increased thickness in the right precuneus (a) and gyrification in the left inferior parietal lobule (b), decreased cortical surface complexity in the right caudal middle frontal gyrus (c), sulcal depth in the right fusiform gyrus (d), and sulcal depth in the right lateral occipital gyrus (d) were observed in ToF group (p < 0.05, FWE corrected).
Relationships between cortical structure and both clinical variables and neurodevelopmental outcomes
In the ToF group, negative correlations were detected between sulcal depth in the right lateral occipital gyrus and time of ACC (r = −0.664, p = 0.001), time of CPB (r = −0.677, p = 0.001), and ICU stay (r = −0.486, p = 0.022) (Fig. 3a–c). Sulcal depth in the right fusiform gyrus was positively correlated with the visual spatial index (r = 0.477, p = 0.039), and gyrification in the left inferior parietal lobule was negatively correlated with the working memory index in the ToF group (r = −0.481, p = 0.037) (Fig. 3d, e). There were negative correlations between the visual spatial index and both time of ACC (r = −0.523, p = 0.031) and time of CPB in the ToF group (r = −0.560, p = 0.019) (Fig. 3f, g). Furthermore, the ToF group showed a negative correlation between the working memory index and time of CPB (r = −0.485, p = 0.048) (Fig. 3h). In addition, WM volume was positively correlated with sulcal depth in the right fusiform gyrus (r = 0.520, p = 0.009) and right lateral occipital gyrus (r = 0.595, p = 0.002) in the ToF group. There was no statistical significance in multiple linear regression analysis. Details about all statistical results were presented in Supplementary Tables 1 and 2.
The top panel shows relationships between cortical structure and clinical variables (a-c), the middle panel shows relationships between cortical structure and neurodevelopmental outcomes (d, e), and the bottom panel shows relationships between clinical variables and neurodevelopmental outcomes (f-h).
Discussion
This study comprehensively evaluated cortical morphology changes in preschool-aged children with ToF based on structural MRI techniques. We found abnormal cortical morphology in cortical thickness, sulcal depth, gyrification, cortical surface complexity and gray matter volume. In addition, relationships between abnormal cortical structures and both poor neurodevelopmental outcomes and clinical risk factors were found.
The sulcus is the structure that undergoes the most obvious morphological change during cortex development. The development of the sulcus can be used as a criterion for judging the maturity of the brain.22 In this study, the reduced sulcal depth in the right fusiform gyrus and right lateral occipital gyrus in the children with ToF suggested a lack of brain maturity in ToF, which supports the finding that delayed cortical development is often seen in children with CHD.20,23 In addition, the lateral occipital gyrus and fusiform gyrus are important parts of the human visual pathway.24 The correlation between sulcal depth in the fusiform gyrus and visual spatial ability in this study further suggested visual pathway impairments in ToF children. A previous study revealed visual spatial memory deficits in adolescents with ToF, which is consistent with our study.25 Moreover, time of ACC, time of CPB, and ICU stay were negatively correlated with sulcal depth in the right lateral occipital gyrus and visual spatial ability in the ToF group, which indicated that these clinical variables may be risk factors for brain development and neurologic deficits, which may be the clue to improve postoperative neurodevelopment for survivors of CHD. Based on previous findings that altered cortical development may be secondary to white matter abnormalities in CHD, we also found WM volume was positively associated with sulcal depth in the right fusiform gyrus and right lateral occipital gyrus in our study. The underlying neuroanatomic relationship may be further confirmed by advanced diffusion MRI techniques which have been used to study WM and cortical microstructure.
Cortical surface complexity, also known as fractal dimension, can be used to characterize the architectural pattern of the cortex and is a measure of the complexity of cortical folding described by the gyrus.26 The middle frontal gyrus is a recognized secondary language area that is related to language expression, such as semantics, grammar, language fluency, and language working memory, among other cognitive functions.27 The reduced cortical surface complexity in the right middle frontal gyrus observed in this study may indicate language impairment in children with ToF, which supports the hypothesis that disorders of verbal expression may be linked to the right middle frontal cortex dysfunction in school-aged children with ToF.28 These results suggest that clinicians should pay more attention to the language development of ToF participants during long-term follow-up.
Interestingly, we also found increased cortical thickness in the right precuneus, increased gyrification index in the left inferior parietal lobule, and increased GM volume in the left medial frontal gyrus in ToF children. Cortical thickness is used to assess volumetric growth, and the gyrification index is used as a measure of maturation.23 MRI studies of typical development suggest cortical thickness, gyrification and volume increased in early childhood.29 In addition, volume ratio is used to provide information on brain maturation at brain scale,30 and has been reported in fetuses and neonates with CHD.31,32 In this study, there are no significant differences in brain volume ratios between children with ToF and normal controls. Taken together, we speculated that the discrepancy in cortical morphology in our cohort may derive from disturbances in typical development. Peyvandi et al. proposed that brain structure alterations may be the result of the complex interaction between heart disease, brain injury, and brain growth.33 Furthermore, we also found a negative correlation between gyrification index in the inferior parietal lobule and working memory index in the current study, which may indicate worse working memory performance in children with ToF. Since CPB time was negatively correlated with working memory index, we speculated that a greater time of CPB may increase the risk of adverse neurodevelopmental outcomes in preschool-aged children with ToF.
In addition, we did not find any association between cortical structure and neurodevelopmental outcomes in multiple linear regression analysis, controlled for sex, age at MRI, time of ACC, time of CPB, ICU stay, family annual income, and parental education. Socioeconomic status, time of ACC, time of CPB, and ICU stay are well-known risk factors of adverse neurodevelopmental outcome of children with CHD.34 In our study, these risk factors seem to be stronger predictors of adverse neurodevelopmental outcome than altered cortical structure. Perhaps, larger sample size may better determine the association between cortical structure and neurodevelopmental outcomes in children with ToF.
The present study has several limitations. First, the sample size is intermediate. However, to the best of our knowledge, the sample size is large relative to those of other brain structure studies in children with CHD, with the participants representing a homogeneous ToF population. In subsequent studies, we will increase the sample size to improve the reliability of the results. Second, cognitive assessment of healthy controls was lacking in the present study. The parents of the healthy controls reported that the intelligence of their children was normal, and they refused to have their children undergo cognitive assessment. Third, we used the adult template for normalization in image analysis, which may impose biases and limitations and miss important differences between populations. In a subsequent study, we will further find the appropriate age-specific template for neuroimaging analysis. Last, this study was a one-time follow-up study. It is very important that both multimode brain MRI and neurocognitive testing should be performed at different time points before and after surgery to better understand the morphologic changes and cognitive impairment processes in participants with ToF.
Conclusions
In the current study, we find cortical structure alterations and their correlations with both neurocognitive changes and clinical factors in preschool-aged children with ToF, which suggests that cortical morphology can be used as an effective tool for the research setting to evaluate neuroanatomical changes and detect underlying neural mechanisms in ToF patients. Furthermore, an abnormal cortical structure may be the continuous consequence of delayed fetal brain development in children with ToF, which suggests that longitudinal follow-up should be performed to determine whether this brain abnormality continues into adolescence and adulthood.
References
Bernier, P. L., Stefanescu, A., Samoukovic, G. & Tchervenkov, C. I. The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 13, 26–34 (2010).
Marelli, A., Miller, S. P., Marino, B. S., Jefferson, A. L. & Newburger, J. W. Brain in congenital heart disease across the lifespan: the cumulative burden of injury. Circulation 133, 1951–1962 (2016).
Karl, T. R. & Stocker, C. Tetralogy of Fallot and its variants. Pediatr. Crit. Care Med. 17, S330–S336 (2016).
Miller, S. P. et al. Abnormal brain development in newborns with congenital heart disease. N. Engl. J. Med. 357, 1928–1938 (2007).
Morton, S. U. et al. Abnormal left-hemispheric sulcal patterns correlate with neurodevelopmental outcomes in subjects with single ventricular congenital heart disease. Cereb. Cortex 30, 476–487 (2020).
Nagaraj, U. D. et al. Impaired global and regional cerebral perfusion in newborns with complex congenital heart disease. J. Pediatr. 167, 1018–1024 (2015).
Karmacharya, S. et al. Advanced diffusion imaging for assessing normal white matter development in neonates and characterizing aberrant development in congenital heart disease. Neuroimage. Clin. 19, 360–373 (2018).
King, T. Z. et al. fMRI investigation of working memory in adolescents with surgically treated congenital heart disease. Appl. Neuropsychol. Child. 6, 7–21 (2017).
Andropoulos, D. B. et al. Changing expectations for neurological outcomes after the neonatal arterial switch operation. Ann. Thorac. Surg. 94, 1250–1255 (2012).
Andropoulos, D. B. et al. The association between brain injury, perioperative anesthetic exposure, and 12-month neurodevelopmental outcomes after neonatal cardiac surgery: a retrospective cohort study. Paediatr. Anaesth. 24, 266–274 (2014).
Meuwly, E. et al. Postoperative brain volumes are associated with one-year neurodevelopmental outcome in children with severe congenital heart disease. Sci. Rep. 9, 10885 (2019).
Rollins, C. K. et al. White matter volume predicts language development in congenital heart disease. J. Pediatr. 181, 42.e2–48.e2 (2017).
Fontes, K. et al. Hippocampal alterations and functional correlates in adolescents and young adults with congenital heart disease. Hum. Brain Mapp. 40, 3548–3560 (2019).
Ehrler, M., Latal, B., Kretschmar, O., von Rhein, M. & O’Gorman Tuura, R. Altered frontal white matter microstructure is associated with working memory impairments in adolescents with congenital heart disease: a diffusion tensor imaging study. Neuroimage. Clin. 25, 102123 (2020).
Brewster, R. C., King, T. Z., Burns, T. G., Drossner, D. M. & Mahle, W. T. White matter integrity dissociates verbal memory and auditory attention span in emerging adults with congenital heart disease. J. Int. Neuropsychol. Soc. 21, 22–33 (2015).
Fumagalli, G. G. et al. Parieto-occipital sulcus widening differentiates posterior cortical atrophy from typical Alzheimer disease. Neuroimage. Clin. 28, 102453 (2020).
Si, F. F. et al. Cortical morphometric abnormality and its association with working memory in children with attention-deficit/hyperactivity disorder. Psychiatry Investig. 18, 679–687 (2021).
Otte, M. L. et al. Cortical morphology and illness insight in patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. https://doi.org/10.1007/s00406-021-01328-x (2021).
Clouchoux, C. et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb. Cortex 23, 2932–2943 (2013).
Ortinau, C. M. et al. Early-emerging sulcal patterns are atypical in fetuses with congenital heart disease. Cereb. Cortex 29, 3605–3616 (2019).
Limperopoulos, C. et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 121, 26–33 (2010).
Kline, J. E. et al. Early cortical maturation predicts neurodevelopment in very preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 105, 460–465 (2020).
Claessens, N. H. et al. Delayed cortical gray matter development in neonates with severe congenital heart disease. Pediatr. Res. 80, 668–674 (2016).
Harley, E. M. et al. Engagement of fusiform cortex and disengagement of lateral occipital cortex in the acquisition of radiological expertise. Cereb. Cortex 19, 2746–2754 (2009).
Cassidy, A. R., Newburger, J. W. & Bellinger, D. C. Learning and memory in adolescents with critical biventricular congenital heart disease. J. Int. Neuropsychol. Soc. 23, 627–639 (2017).
Sandu, A. L. et al. Structural brain complexity and cognitive decline in late life–a longitudinal study in the Aberdeen 1936 birth cohort. Neuroimage 100, 558–563 (2014).
Gohel, S. et al. Resting-state functional connectivity of the middle frontal gyrus can predict language lateralization in patients with brain tumors. AJNR Am. J. Neuroradiol. 40, 319–325 (2019).
Ma, S. et al. Changes in cortical thickness are associated with cognitive ability in postoperative school-aged children with tetralogy of Fallot. Front. Neurol. 11, 691 (2020).
Norbom, L. B. et al. New insights into the dynamic development of the cerebral cortex in childhood and adolescence: integrating macro- and microstructural MRI findings. Prog. Neurobiol. 204, 102109 (2021).
Coupé, P., Catheline, G., Lanuza, E. & Manjón, J. V. Towards a unified analysis of brain maturation and aging across the entire lifespan: a MRI analysis. Hum. Brain Mapp. 38, 5501–5518 (2017).
Claessens, N. H. P., Khalili, N., Isgum, I. & Ter Heide, H. Brain and CSF volumes in fetuses and neonates with antenatal diagnosis of critical congenital heart disease: a longitudinal MRI study. AJNR Am. J. Neuroradiol. 40, 885–891 (2019).
Olshaker, H., Ber, R., Hoffman, D. & Derazne, E. Volumetric brain MRI study in fetuses with congenital heart disease. AJNR Am. J. Neuroradiol. 39, 1164–1169 (2018).
Peyvandi, S. et al. The association between cardiac physiology, acquired brain injury, and postnatal brain growth in critical congenital heart disease. J. Thorac. Cardiovasc. Surg. 155, 291.e3–300.e3 (2018).
Kuhn, V. A. et al. Determinants of neurological outcome in neonates with congenital heart disease following heart surgery. Pediatr. Res. 89, 1283–1290 (2021).
Funding
This project is funded by the Six Talent Peaks Project in Jiangsu Province (No. WSN-192), Jiangsu Commission of Health (No. LGY2019009), and Nanjing Municipal Science and Technology Bureau (No. 202002055).
Author information
Authors and Affiliations
Contributions
Study conceptualization and design: Ming Yang, X.M., Y. Liu. Data collection: Mingwen Yang, S.M., S.W., M.F., M.Z., Y. Li, S.C., Z.F. Data analysis and interpretation: Mingwen Yang, Y. Liu. Drafting the manuscript: Mingwen Yang. All authors contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written informed consent for publication was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yang, M., Liu, Y., Ma, S. et al. Altered brain structure in preschool-aged children with tetralogy of Fallot. Pediatr Res 93, 1321–1327 (2023). https://doi.org/10.1038/s41390-022-01987-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-01987-z